YDV Multiplex PCR
Stephen Byrne, Virgile Ballandras, Louise McNamara
Disclaimer
DISCLAIMER – FOR INFORMATIONAL PURPOSES ONLY; USE AT YOUR OWN RISK
The protocol content here is for informational purposes only and does not constitute legal, medical, clinical, or safety advice, or otherwise; content added to protocols.io is not peer reviewed and may not have undergone a formal approval of any kind. Information presented in this protocol should not substitute for independent professional judgment, advice, diagnosis, or treatment. Any action you take or refrain from taking using or relying upon the information presented here is strictly at your own risk. You agree that neither the Company nor any of the authors, contributors, administrators, or anyone else associated with protocols.io, can be held responsible for your use of the information contained in or linked to this protocol or any of our Sites/Apps and Services.
Abstract
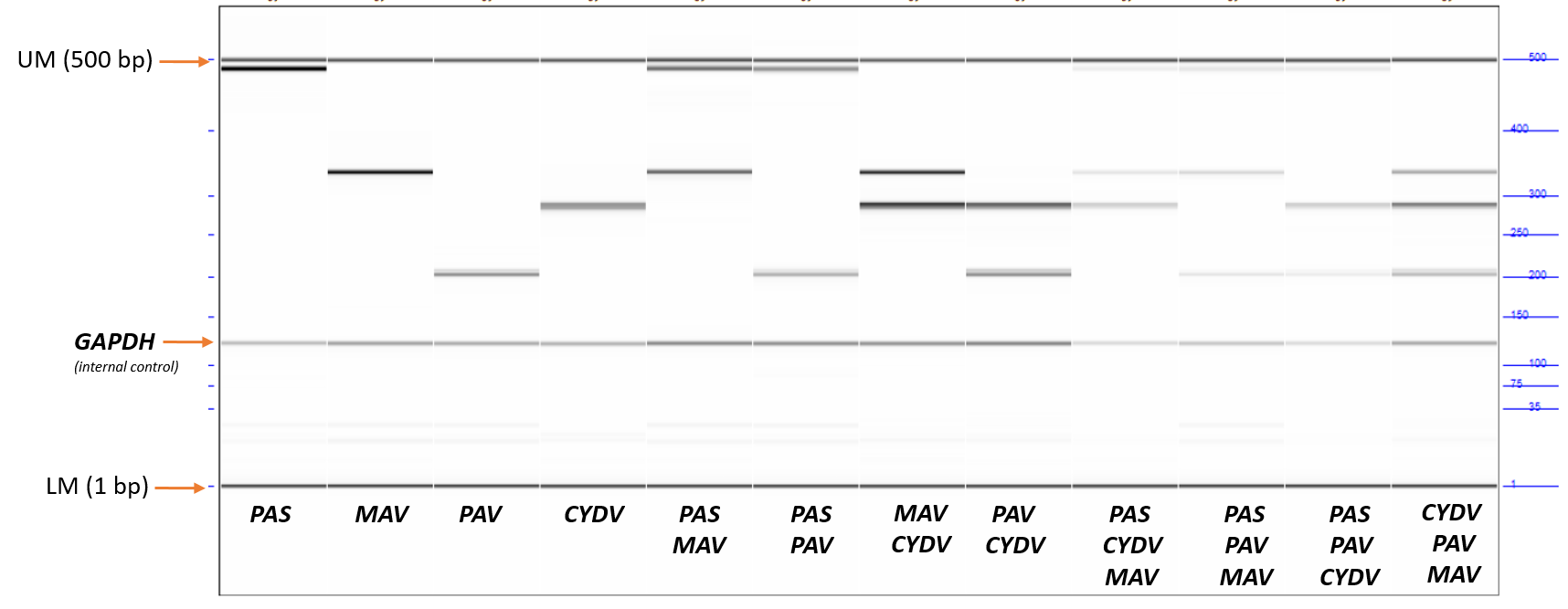
This protocol describes the process of carrying out a multiplex PCR assay followed by capillary electrophoresis to detect C/BYDV and determine species (BYDV-MAV, BYDV-PAS, BYDV-PAV, CYDV (RPS or RPV)). The starting point for this is cDNA that has been synthesized from nucleic acid extracted from single aphids or leaf material - an internal control targeting GAPDH is included and primers can be selected depending on the sample matrix (aphid or barley). The BYDV primers were taken from:
Sõmera, M., Massart, S., Tamisier, L., Sooväli, P., Sathees, K. and Kvarnheden, A., 2021. A survey using high-throughput sequencing suggests that the diversity of cereal and barley yellow dwarf viruses is underestimated. Frontiers in Microbiology , 12 , p.673218.
In-house sequence data was used to modify primers where necessary, add a primer for CYDV and an internal control for both aphid and barley samples. The CYDV primers captures both RPS and RPV (high resolution melt analysis may be required to distinguish these if required).
Before start
This protocol assumes that you have already isolated Total RNA from plant or insect material, and converted to cDNA using random hexamers. The protocol uses capillary electrophoresis for separation of PCR products; however it is also possible to separate and visualise using a high quality agarose gel electrophoresis method.
Steps
PCR protocol
Identify samples for analysis, including positive (virus infected aphid/barley) and negative controls (virus free aphid/barley), a positive control consisting of a mix of gBlocks with sequences for all targets in the multiplex PCR, and a no-template control. Details on the gBlocks can be found here: gBlocks-ordered-YDV-MULTIPLEX.txt
Prepare the primer mix for the multiplex PCR ( YDVmixHV when sample matrix is barley, or YDVmixSA when sample matrix is an aphid) using primers according to tables below. The primer mixes are prepared with each primer in the working solution at 10micromolar (µM) .
YDVmixHV:
| A | B |
|---|---|
| Primer Name | Primer Sequence |
| RpvF | CTCGTGGGCTATCGCTATGG |
| CydvR | TCATGGCGGAGCTCATGCAG |
| GAPDH-HV-F | GGAGTCCACCGGTGTTTTCA |
| GAPDH-HV-R | AGACAAACATGGGAGCGTCC |
| GavF | GTTACAAGATCACAAACGTCAAG |
| PasF | GAAGAGGGCCAAATTCTATACC |
| PavF | CTTCACAATCAGCAGGAC |
| YanR24 | TGTTGAGGRGTCTACCTATTTG |
YDVmixSA:
| A | B |
|---|---|
| Primer Name | Primer Sequence |
| RpvF | CTCGTGGGCTATCGCTATGG |
| CydvR | TCATGGCGGAGCTCATGCAG |
| GAPDH-SA-F | GGCGAAGTTTCTGTTGATGG |
| GAPDH-SA-R | CAGCACCAGCAGATCCCC |
| GavF | GTTACAAGATCACAAACGTCAAG |
| PasF | GAAGAGGGCCAAATTCTATACC |
| PavF | CTTCACAATCAGCAGGAC |
| YanR24 | TGTTGAGGRGTCTACCTATTTG |
Prepare a master-mix for the number of samples to be run so that each well will contain:
12.5µL of
10.5µL of molecular grade nuclease-free water
1µL of working solution of appropriate primer mix prepared in step 2 above
Note: use of the QIAGEN Multiplex PCR Master Mix (or equivalent) is recommended - in the case of insect/plant/crop co-infection, each target is amplified with similar efficiencies (in contrast to other polymerase options). Using different enzymes will likely require some optimisation.
Pipette 1µL of cDNA (or control) into each well of a PCR plate and keep on ice.
Pipette 24µL of master-mix from step 3 into each well, seal the plate carefully with foil lid, and centrifuge the PCR plate briefly to ensure no droplets adhere to sides of sample wells.
Place the PCR plate in a
Equipment
| Value | Label |
|---|---|
| MiniAmp Plus Thermal Cycler | NAME |
| Applied Biosystems | BRAND |
| A37835 | SKU |
| https://www.thermofisher.com/us/en/home.html | LINK |
and run the following PCR cycle:
95°C0h 5m 0s
followed by 40 cycles of
95°C0h 0m 30s60°C0h 1m 30s72°C0h 0m 30s
and a final elongation step of
68°C0h 10m 0s
Capillary Electrophoresis
Bring all reagents from dsDNA reagent kit (1-500 bp; DNF-905) to room temperature by removing from the fridge and freezer at least 0h 30m 0s prior to setting up a run.
Set up a run on the 5200 Fragment Analyser by adding 1mL of 1X Inlet Buffer into row A of the 1.2 ml deep 96-well plate (to make 12mL fresh add 2.4mL of 5X Inlet buffer to 9.6mL of molecular grade water. Inlet buffer should be changed daily. Into the same plate place 1mL of Capillary Storage solution into row H (note: the storage buffer only needs to be changed on a weekly basis). This plate needs to go into drawer "B" of the instrument.
Add 30µL of ready to use marker (1 and 500 bp) to row A of a compatible 96-well PCR plate and add a drop of miner oil (provided in kit) to each well and place plate into drawer "M" of the instrument. The marker is good for 30+ injections or 1 month.
Prepare sufficient 1X conditioning solution for the number of samples you are going to run (e.g. 45mL is sufficient for a single plate) and connect to correct fluid line.
Prepare sufficient gel for the number of samples you are going to run (e.g. 4.5µL added to 45mL of separation gel is sufficient for a single plate) and connect to correct fluid line.
The samples are prepared by adding 2µL of PCR product to 22µL of 1X TE (provided) in the well of a compatible PCR plate. Vortex the plate and centrifuge briefly to collect contents at bottom of the wells.
Update the solution levels on instrument and commence a run using method appropriate for the DNF-905-dsDNA (1 to 500 bp) kit.