Seahorse protocol for islets using Xfe24 Analyzer
Nancy Smith
Abstract
Using Agilent's seahorse Xfe Analyzer with the islet capture microplates to assess whole islet bioenergentics in vitro. Agilent Seahorse XFe24 Analyzers measure the oxygen consumption rate (OCR) and extracellular acidification rate (ECAR) of live cells in a 24-well plate format.
Steps
Solution Prep - Calibrant Solution
Calibrant Solution (Agilent 100840-000, 500ml) is provided by Agilent and stored at room temperature.
Solution Prep - MA media
Use the DMEM (Agilent 103575-100, 500ml) provided by Agilent; stored at 4oC. Needs to be supplement with 1% Fetal Bovine Serum (FBS, Gibco 12483-020). Add 5ml of heat inactivated FBS to the 500ml DMEM. Filter sterilize and store at 4oC.
On the day of experiment, supplement with 2mM or 2.8mM D-glucose (Sigma G8270), 2mM sodium pyruvate (Gibco 11360-070) and 2mM L-glutamine (Gibco 25030-081). Add 0.018g or 0.025g of glucose to 50ml MA media. Pipette 1ml of (100mM) sodium pyruvate and 500ul of (200mM) L-glutamine to the 50ml MA media. Mix until glucose has dissolved.
Solution Prep - Stocks
Glucose 2M stock in Dimethyl sulfoxide (DMSO, Fisher D128-1) - A
Weigh 18.2g of glucose into 50ml DMSO. Aliquot and store at -20oC.
Oligomycin (Sigma O4876-5MG) 5mM stock in DMSO - B
Pipette 1.27ml DMSO to the 5mg Oligomycin. Aliquot and store at -20oC.
FCCP (Carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone, Sigma C2920-10MG) 1mM stock in DMSO - C
Prepare 50mM stock of FCCP by adding 787ul of DMSO to the vial containing 10mg of FCCP. To make 1mM stock, use 20ul of the 50mM FCCP into 980ul of DMSO. Aliquot the 1mM stock and store both stocks at -20oC.
Rotenone (Sigma R8875-5G) 5mM stock in DMSO - D
Weigh 1.97g of Rotenone into 1ml of DMSO. Aliquot and store at -20oC.
Antimycin A (Sigma A8674-25MG) 5mM stock in DMSO - D
Weigh 2.7mg of Antimycin A into 1ml DMSO. Aliquot and store -20oC.
Solution Prep - working stocks
Glucose (167mM) - A
Pipette 167µl of 2M glucose stock to 1833µl MA media. Working stock concentration of 167mM with an injected final concentration of 16.7mM.
Glucose (200mM) - A
Pipette 200µl of 2M glucose stock to 1800µl MA media. Working stock concentration of 200mM with an injected final concentration of 20mM. ******
Oligomycin (55uM) - B
Pipette 22µl of 5mM oligomycin stock
to 1978 µl MA media. Working stock concentration of 55uM with an injected final concentration of 5µM.
FCCP - C
Mouse (55.5µM)
Pipette 111 µl of 1mM FCCP stock to 1889 µl MA media. Working stock concentration of 55.5µM with an injected final concentration of 5µM.
Human (33.3µM)
Pipette 66.6 µl of 1mM FCCP stock to 1933.4 µl MA media. Working stock concentration of 33.3µM with an injected final concentration of 3µM.
**FCCP needs to be titrated for every lot**
FCCP cat#C2920-10MG, Sigma, lot#0000120405
optimal concentration on mouse islets = 5 µM
optimal concentration on human islets - 3 µM
Rotenone (55.2 µM)/Antimycin A (56.2 µM) - D
Pipette 22.5 µl of 5mM rotenone stock, 22.5 µl of 5mM antimycin A stock to 1955 µl MA media. Working stock concentration of 55.2 µM with an injected final concentration of 5µM
Day one Protocol
Day two Protocol
Warm MA media in waterbath to 37oC.
Place screens into a dish with MA media.
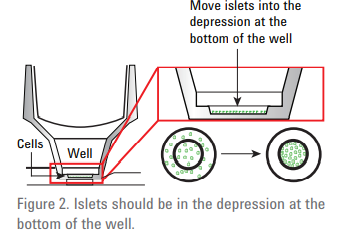
Place islets (min 70 islets per well), into tube with 500 µl of MA media. Let islets gravity settle to the bottom of the tube. Pipette islets (100µl ) and carefully dispense into center of the well of islet capture microplate. Place screen, flat side down, to well using forceps ensuring that the screens click into the well. Ensure there are no bubbles trapped under the screens. Add the remaining MA media to a total volume of 500 µl in each well (by pipetting onto the side of the well). Incubate at 37oC with NO CO2 for 1hr.

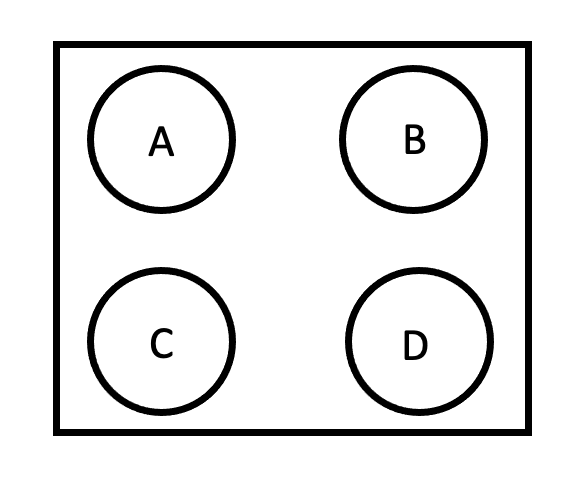
Ensure to not place islets on the wells that are designated as blanks. See template below.
| A | B | C | D | E | F |
|---|---|---|---|---|---|
| Blank | sample 1 | sample 2 | sample 3 | sample 4 | sample 5 |
| sample 6 | sample 7 | sample 8 | Blank | sample 9 | sample 10 |
| sample 11 | sample 12 | Blank | sample 13 | sample 14 | sample 15 |
| sample 16 | sample 17 | sample 18 | sample 19 | sample 20 | Blank |
While islets are incubating, turn on seahorse Xfe Analyzer, turn on computer and open Wave. Open protocol and set up plate details.
Protocol parameters:
| A | B | C | D | E |
|---|---|---|---|---|
| Cycles | Mix | Wait | Measure | |
| Basal (2 or 2.8mM Glucose) | 4 | 3:00 | 2:00 | 3:00 |
| Glucose (16.7 or 20mM) | 6 | 3:00 | 2:00 | 3:00 |
| Oligomycin (5µM) | 8 | 3:00 | 2:00 | 3:00 |
| FCCP (3 or 5µM) | 6 | 3:00 | 2:00 | 3:00 |
| Rotenone/Antimycin A (5µM) | 8 | 3:00 | 2:00 | 3:00 |
Make working stocks solutions.
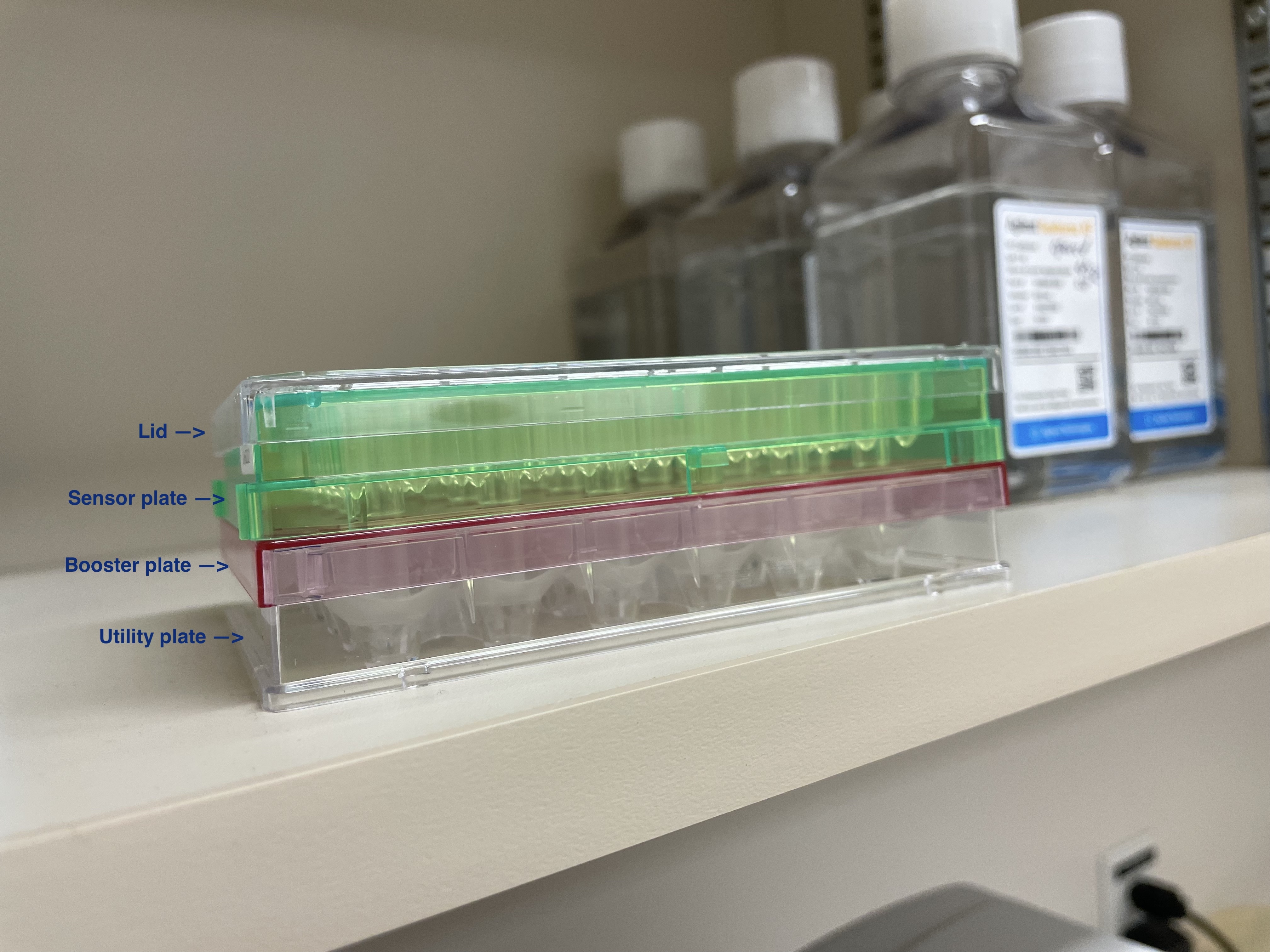
Press run on the seahorse program. Place extracellular flux assay plate (with booster plate and lid removed). Once initialization and 1hr incubation is complete remove the utility plate and replace with islet plate.
Once complete, remove plate, and collect for DNA or protein.
Collect islets from well, including scraping off islets from the capture screens into a 1.5ml tube. Use extra MA media to wash wells
Centrifuge tubes at 1000rpm for 4 minutes for protein or 1500rpm for 6 minutes for DNA.
Aspirate supernatant. Store dry pellet at -20oC until ready to run protein or DNA assay.