Wholemount Edu Staining (Zebrafish Larvae)
Abigail Elliot, Yi Feng, Henna Myllymaki
Disclaimer
DISCLAIMER – FOR INFORMATIONAL PURPOSES ONLY; USE AT YOUR OWN RISK
The protocol content here is for informational purposes only and does not constitute legal, medical, clinical, or safety advice, or otherwise; content added to protocols.io is not peer reviewed and may not have undergone a formal approval of any kind. Information presented in this protocol should not substitute for independent professional judgment, advice, diagnosis, or treatment. Any action you take or refrain from taking using or relying upon the information presented here is strictly at your own risk. You agree that neither the Company nor any of the authors, contributors, administrators, or anyone else associated with protocols.io, can be held responsible for your use of the information contained in or linked to this protocol or any of our Sites/Apps and Services.
Abstract
Methods for evaluating cell proliferation are important for research in the field of developmental biology, cancer biology and cell biology. Recent years, Click-iT EdU Assays by Invitrogen is gaining popularity due to its fast staining speed, mild tissue treatment condition, reproducibility and specificity. However, the standard kit from Invitrogen is designed for tissue cultured cells, hence the manufactory provided protocol is optimized for cells cultured on coverslips. EdU staining became a popular method to evaluate cell proliferation in vivo in zebrafish embryos. However, the protocols that we have tested in the past often yield inconsistent results due to variable EdU incorporation efficiency. Here we describe an EdU staining protocol that we optimized for zebrafish larvae, which incorporated a crucial EdU injection step. Our protocol was used successfully by many new students in training, without any issues.
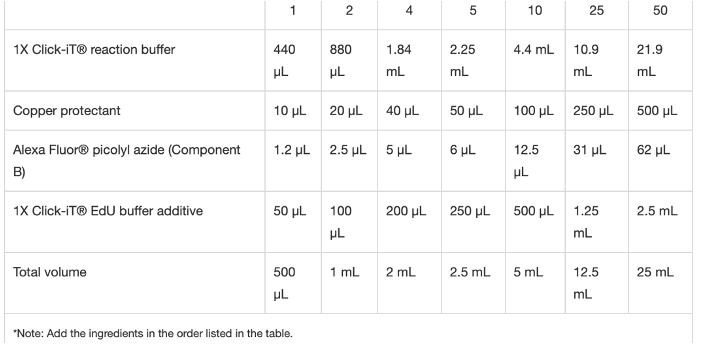
Steps
EdU staining
Inject 2nl bolus of 500uM EdU into the yolk of embryos
Incubate at 28.5°C in the dark for 2.5 hours (this can be between 2 -3 hours, but be consistent between experiments; we have done 2 hours)
Fix in 4% PFA ( 30min , Room temperature)25°C
0h 30m 0s
Wash in 0.1% PBT ( 5min , RT shaking) 30rpm
Wash in 3% BSA, Dilute in PBT ( 5min , RT shaking) -grams/100ml, i.e. 3g/100ml, 300mg in 10ml.30rpm
Block in 3% BSA, Dilute in PBT ( 1h , shaking)30rpm
Immunostaining in dark (EGFP or hRAS)
Wash in PBT ( 5min , RT shaking x3) 30rpm
Wash in 5% Goat Serum, Dilute in PBT ( 5min , RT shaking)30rpm
Block in 5% Goat Serum, Dilute in PBT ( at least 2h , RT shaking)30rpm
Incubate with anti-GFP Primary Ab 1:200, dilute in 5% Goat Serum in PBT (O/N, +4 °C shaking)
30rpm
250µL
Wash in PBT ( 25-30 min , RT shaking) x 8-10 à >4 hours in total!!! 30rpm
Incubate with Secondary Ab [] 1:250, Dilute in 3% Goat Serum in PBT (overnight at +4 °C -- prefered)30rpm
Wash in PBT ( 15 min , RT shaking) x4 30rpm
Stain with Hoechst 33258 Dilute 1 in 1000 in PBT ( 30mins , RT shaking)30rpm
Wash in PBT (15mins, PBT) x4 30rpm
Embed and Image
Incubate in AF1 solution.
Mounting with cover-slip and glass slides.