Small volume viral RNA extraction using MagMAX Viral RNA Isolation Kit
Joyce Akello, Alex Shaw, Catherine Troman, Javier Martin, Nick Grassly, Erika Bujaki, khurshida, alammu, arshady
Abstract
This protocol describes the viral nucleic acid recovery and purification from stool suspensions. The method utilises the magnetic bead based MagMAX TM Viral RNA Isolation Kit to purify nucleic acid from 300 µL of sample. To perform manual extractions follow Workflow A, for automated extraction on King Fisher Duo Prime follow steps in Workflow B, and for automated extraction on King Fisher Flex follow Workflow C.
Steps
Reagent Preparation
Wash Solution 1
Add indicated volume of 100% Isopropanol to the bottle of Wash Solution 1 Concentrate.
Mix well by inverting at least 5 times and mark bottle to indicate that the alcohol was added.
Wash Solution 2
Add indicated volume of 100% ethanol to the bottle of Wash Solution 2 Concentrate.
Mix well by inverting at least 5 times and mark the bottle to indicate that ethanol was added.
Lysis/Binding Solution
Combine the components listed below in the order indicated. Prepare enough reagents for the number of samples extracted that day, including controls, with adding extra 10% for pipetting loss.
Add Carrier RNA to Lysis/Binding Solution Concentrate according to the table below, and mix briefly
| A | B |
|---|---|
| Reagent | Per sample |
| Lysis/Binding Soln. Concentrate | 300 μl |
| Carrier RNA | 1.5 μl |
Add 100% Isopropanol and mix well by vortexing.
| A | B |
|---|---|
| Component | Per sample |
| 100% Isopropanol | 300 μl |
Bead Mix
Vortex the nucleic acid binding beads well to ensure that the beads are fully resuspended.
Combine the components that are listed below:
| A | B |
|---|---|
| Component | Volume per sample |
| Nucleic Acid Binding Beads | 10 μl |
| Lysis/Binding ENHANCER | 10 μl |
| Total volume | 20 μl |
Mix well by vortexing and place the prepared bead mix on top of ice or in the fridge until it is needed, but avoid freezing as it destroys its properties.
RNA extraction - Workflows A,B,C
Manual extraction – Workflow A
Prepare the lysate:
For each sample:
-
Set up and label 1.5 ml Eppendorf DNA LoBind centrifuge tubes, then aliquot 600 μl of the lysis/binding solution (supplemented with carrier RNA and 100% Isopropanol - see Reagent preparation section) into each tube.
-
Transfer 300 μl of the stool suspension supernatant to the labelled tubes containing the Lysis/Binding Solution (supplemented with carrier RNA and 100% Isopropanol).
Bead capture and washes:
- Add 20 μl of prepared bead mix to each sample tube containing the lysed sample solution.
-
It is important to remove the lysis supernatant fully, so a brief centrifugation before collecting the remaining supernatant might be necessary.
-
Remove tubes from magnetic stand and place in tube rack for washing with Wash Solution 1.
-
Add 300 µl Wash Solution 1 to each sample and vortex at moderate speed for 30s.
-
Centrifuge briefly to collect tube content.
-
Capture beads on magnet until mixture becomes clear, indicating full capture.
-
Carefully aspirate and discard supernatant.
-
Repeat steps 9-13 one more time to complete two washes with Wash Solution 1.
-
Remove tube from magnetic stand and place in tube rack for washing with Wash Solution 2.
-
Add 450 µl Wash Solution 2 to each sample and vortex at moderate speed for 30s.
-
Capture beads on magnet for 2 mins or until mixture becomes clear, indicating full capture.
-
Carefully aspirate and discard supernatant.
-
Repeat steps 15-19 to complete two washes with Wash Solution 2.
Drying the beads and elution:
-
Centrifuge briefly and remove any residual solution with a small volume fine-tipped pipette, without disturbing the pellet.
-
Dry the beads by leaving the tube open for 2 minutes to allow any remaining alcohol to evaporate.
-
Centrifuge briefly to collect tube content.
-
Capture the beads on the magnet as before and collect supernatant containing the purified RNA in labelled containers and keep on ice for immediate use or store frozen until needed.
Automated extraction using King Fisher Duo Prime – Workflow B MVRI_DUO_SV_300ul.bdz
Read the King Fisher Flex instrument manual for installation and operating instructions in its entirety before operating the magnetic particle processor.
Please find attached the associated King Fisher Duo Prime protocol for importing into your equipment.
Plasticware for small volume sample extractions on King Fisher Duo Prime equipped with 12-tip magnetic head.
| A | B |
|---|---|
| Item | ThermoFisher product code |
| KingFisher deep-well 96 plate (50) | 95040450 |
| King Fisher Duo Combi Pack for 96 DW Plate All plasticware for extraction of 8 plates (96samples) | 97003530 |
| KingFisher Duo cap for elution strip (40) | 97003540 |
| KingFisher Duo elution strip (40) | 97003520 |
| KingFisher 96 KF plate | 97002540 |
| KingFisher 12 tip comb for 96 deep-well plate (50) | 97003500 |
Preparing 96 deep well plate:
Set up plate by loading the required reagents into the appropriate positions as shown in table below:

-
Aliquot 600 μl of the Lysis/Binding Solution supplemented with carrier RNA and 100% Isopropanol (see step 1 and 2 in Reagent preparation) to the top row (row A) of the 96 deep-well plate.
-
Transfer 300 μl of the sample to the same top row (row A) of the 96 deep-well plate containing the Lysis/Binding Solution. Mix by gently pipetting up and down in the 96 deep-well plate a few times. (Discard tube containing the punches).
-
Add 300 μl of prepared wash solution 1 to rows B and C of the 96 deep well plate.
-
Add 4500 μl of prepared wash solution 2 to rows D and E of the 96 deep well plate.
-
Place a 12-tip comb in a 96 deep well plate in Row H.
-
Add 50 μl of Elution buffer/ nuclease free water to the elution strip tube.
Setting up and running the King Fisher Duo Prime:
- Check to confirm that the KingFisher Duo Prime is set up with 12-tip magnet and heating block.
Follow the below steps to change the magnetic head on the King Fisher Duo Prime if necessary y:
- Select and start Change Magnetic Head protocol in Maintenance protocols in the device menu. (This will position the magnet to be accessible.)
- Unscrew and remove the screws holding the magnetic head in place and lift the magnetic head to take it out.
- Replace the required magnetic head and tighten the screws to hold it in place.
- Remember to also change the heating blocks as the machine doesn’t give a prompt to do so!
- Run Check 12 tip protocol with a dummy test plate containing the tip comb in the required row to ensure the right positioning. Unload the test plate.
-
Load the prepared sample plate onto the King Fisher Duo Prime as prompted, ensuring the right orientation by matching the A1 marking on the turntable. Ensure that the plate is lying completely flat.
-
Place the prepared elution strip into the device in the metal rack next to the loaded plate and use the fold over lock on the elution block to secure it in place. Use the position of the small round hole on the elution strip to match the red conical protrusion on the rack to ensure the right orientation. Both the plate and the elution strip should be on the same side of the turntable.
-
Select the MVRI_DUO_SV_300ul Protocol and press start.
-
Close the front lid while the KingFisher is running.
Un-loading the device:
-
After completion of the run, a final prompt will appear. “Unload RNA plate and RNA Elution Strip”.
-
Unload the elution strip containing the RNA, cap and place the elution strip containing the RNA on ice.
-
Remove the sample plate from the device then press the “Check Mark”. Discard the plate and content following the appropriate laboratory procedure. Wipe clean equipment, then switch on UV light for disinfection.
-
Transfer eluted viral RNA to labelled containers and keep on ice for immediate use or store frozen until needed.
Automated extraction using King Fisher Flex – Workflow C MVRI_Flex_SV_300ul.bdz
Read the King Fisher Flex instrument manual for installation and operating instructions in its entirety before operating the magnetic particle processor.
Please find attached the associated King Fisher Flex protocol for importing into your equipment.
Plasticware for small volume sample
extractions on King Fisher Flex equipped with 96-tip magnetic head.
| A | B |
|---|---|
| Item | ThermoFisher product code |
| KingFisher deep-well 96 plate (50) | 95040450 |
| KingFisher 96 tip comb for deep-well magnets (100) | 97002534 |
| KingFisher 96 KF plate For tip comb placement and eluate storage (48) | 97002540 |
Preparing sample plate
-
Aliquot 600 μl of the Lysis/Binding solution supplemented with carrier RNA and 100% Isopropanol (see step 3 in Reagent preparation) to a 96 deep-well plate marked as Sample plate.
-
Add 300 μl of samples to the 96 deep-well plate containing the Lysis/Binding Solution. Mix by gently pipetting up and down a few times. (Discard tube containing the punches).
Setting up the processing plates and running the extraction program
-
Add 20 μl of prepared bead mix to the wells of the Sample plate containing the Lysed sample solution using a new tip for every addition and rinsing it with the sample solution to prevent loss of beads.
-
Label and prepare the processing plates according to the table below:
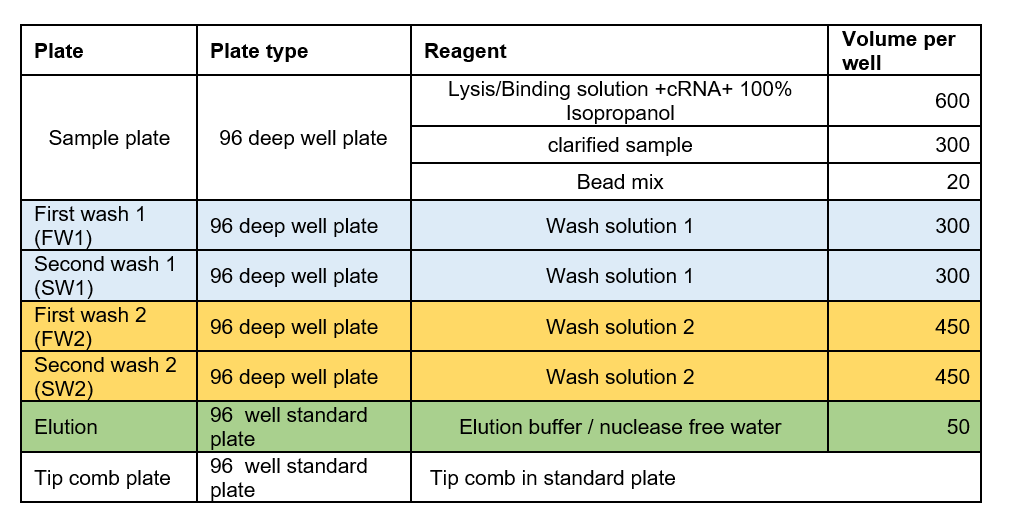
-
Add 300 μl of prepared wash solution 1 to the 96 deep well plates marked FW1 and SW1.
-
Add 450 μl of prepared wash solution 2 of the 96 deep well plates marked FW2 and SW2.
-
Add 50 μl of nuclease free water to wells of the 96-standard plate marked Elution.
-
Place a 96 tip comb in a 96-standard plate.
-
Check to confirm that the instrument is set up with 96 deep-well magnetic head and 96 deep-well heat block. Select the MVRI_Flex_SV_300ul protocol on the equipment and load the plates onto the King Fisher Flex as directed, then start the protocol.
-
Close the front lid of the device.
Un-loading the device:
-
After completion of the run, a final prompt will appear. “Unload plate containing the RNA”.
-
Transfer eluted viral RNA to labelled containers and keep on ice for immediate use or store frozen until needed.
-
Empty and wipe clean equipment. Dispose of processing plates and their contents by following standard laboratory processes.

