Schistosoma mansoni cercariae sexing
Sarah K Buddenborg, Matt Berriman
Abstract
This DNA extraction method for Schistosoma mansoni cercariae is based on the HOTSHOT method https://health.uconn.edu/mouse-genome-modification/protocols/hotshot-method-of-dna-preparation/. DNA isolation is followed by PCR amplification of the "W1" female W chromosome repetitive region. The 476 bp W1 repeat was identified by Webster, Mansour, and Bieber (1989). PCR primers for the W1 repeat were designed by Gasser, Morahan, and Mitchell (1991). Existing primers for the S. mansoni actin gene are used as a positive control (Delcroix et al, 2006).
W1a - 5' CAA CAC AGT GAA ATT CTT CC 3' (positions 10-29)
W1b- 5' GAA TTC ACC ACT CGA CAT TC 3' (positions 463-482)
Before start
-
Pre-heat heat block to
95°C -
Pre-cool centrifuge to
4°C -
Fill an ice bucket with ice
Steps
Cercariae Collection
While holding with wide-tip featherweight forceps, rinse patent Biomphalaria glabrata snails, individually, with MilliQ water
Place rinsed snails into individual wells of 6- or 12-well plates
Add 3-4ml of MilliQ water to each well
Induce shedding of cercariae by place snails under a direct light for up to 2 hours or until cercariae are visible in well with a naked eye
After 2 hrs, remove snails that are not shedding cercariae and place into a new tank with food. These snails can be re-shed in one week. Some monomiracidium-infected snails will not begin shedding until up to 8 weeks after exposure. At this time, if they are still not shedding cercariae, it is safe to assume they were not infected and can be disposed of properly.
For snails that are shedding low amounts of cercariae (<100), place into a new tank with food and re-shed in one week
Put an "X" on the wells of the snails that are shedding and uniquely number each. This numbering must be preserved throughout.
Prepare numbered 5ml Eppendorf tubes each fitted with a 100µm pluriStrainer Mini
Using a new sterile transfer pipet for each snail, pipette water containing the cercariae through the pluriStrainer
Rinse the well and snails with fresh MilliQ water and collect this water as above to fill each 5ml tube to the top
Discard pluriStrainers, close, and submerge 5ml tubes containing cercariae into ice for 0h 30m 0s
Centrifuge tubes at 2000rpm,4°C
After centrifugation, place tubes immediately back on ice and aspirate/pipette off liquid, taking care not to disrupt the cercariae pellet. Try to leave no more than 50µl of water on the cercariae pellet as the more dilute the lysate is, the more difficult amplification will be.
Cercariae and Cell Lysis
Add 1x volume of 2x Alkaline Lysis Reagent to each cercariae pellet and mix well (i.e. add 25µl 2x Alkaline Lysis Reagent to 25µl of cercariae in MilliQ)
Incubate at 95°C for ~1 hr or until cercariae dissolve, mixing at least every 15 min. Centrifuge using a mini-centrifuge if needed
After tissue is dissolved, place on ice to cool
Lysate Neutralization
Add 1x volume of 2x Neutralizing Reagent and briefly vortex to mix (i.e. add 50µl 2x Neutralizing Reagent to 50µl of lysate)
PCR amplification of sex-specific region
Prepare two PCR master mixes on ice (for actin control and W1 primer sets) each with enough for your samples, 1 female positive control, 1 male negative control, and 1 water blank control (i.e. if you have 8 samples, you will need 11 reactions for actin primers and 11 reactions for W primers)
| A | B | C |
|---|---|---|
| Reagent | Final concentration | Volume per reaction |
| Nuclease-free water | - | 6.32µl |
| Platinum II Taq | 0.04U/µl | 0.08µl |
| 5x Platinum II Buffer | 1x | 2µl |
| 10mM dNTP | 0.2mM each | 0.2µl |
| 10uM F | 0.2uM | 0.2µl |
| 10uM R | 0.2uM | 0.2µl |
| DNA* | - | 1µl |
| 10µl reaction |
*If you think your DNA concentration is really low/high, you can measure first on Nanodrop (blank with Neutralization Buffer) and change input volume as necessary. Keep in mind that using more than 10% of the overall volume may inhibit the PCR reaction.
Run the PCR program as follows for both primer sets:
| A | B | C | D |
|---|---|---|---|
| Initial denaturation | 94°C | 2 min | |
| Denature | 98°C | 5 sec | |
| Anneal and extend | 60°C | 15 sec | To step 2 x25 |
| Hold | 4°C | ~ |
Analyze PCR Amplicons
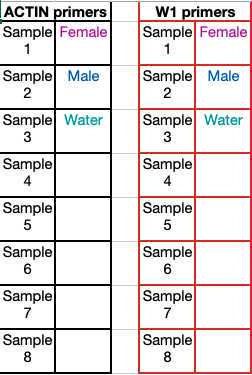
Run a 1% agarose gel to analyze 5-10µl of your PCR product. Males will have PCR product band for actin only; Females will have PCR product band for actin and W1.

