SARS-CoV-2 nsp3 macrodomain Time-Resolved FRET peptide displacement assay
Haim Barr, Noa Lahav
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abstract
This protocol details the Time-Resolved FRET (TR-FRET) assay for SARS-CoV-2 nsp3 macrodomain (Mac1) binding of adenosine diphosphate (ADP)–ribosylated (ADPr) peptide. This method is intended to measure the activity of Mac1 by using a specific ADPr-modified peptide that allows the detection of binding. When bound, the biotinylated-peptide and the HIS-tagged Mac1 form a proximity complex that is detected by TR-FRET using Streptavidin-Eu Cryptate and anti-HIS-XL665 as a donor/acceptor pair. Excitation of the Eu Cryptate complex at 325 nm emits a resonant energy of 625 nm which in turn excites the XL665 to emit fluorescence at 665 nm. This energy transfer occurs only when ADPr modified peptide is in sufficient proximity to Mac1 and inhibitors which displace the peptide will prevent energy transfer. Binding activity is reported as the ratio of Acceptor/Donor (Em/Em) X 10,000.
Experiment Concentrations (From Stock to Assay)
| A | B | C | D | E |
|---|---|---|---|---|
| Reagent | Stock | Loaded into Combi | Final in assay plate | Units |
| His-SARS CoV-2 MAC1 | 183000 | 50 | 12.5 | nM |
| Substrate (Biotin-ADPr) | 10000000 | 1600 | 400 | nM |
| Detection solution | ||||
| Streptavidin-XL665 (SA-XL) | 1 | 0.25 | 0.125 | % |
| MAb Anti-6HIS-Eu cryptate Gold | 100 | 0.25 | 0.125 | % |
| Assay buffer | ||||
| HEPES pH=7.0 | 250 | 25 | 25 | mM |
| NaCl | 200 | 20 | 20 | mM |
| BSA | 0.5 | 0.05 | 0.05 | mg/ml |
| Tween 20 | 0.5 | 0.05 | 0.05 | % |
| HTRF PPI Europium Detection Buffer | 100 | 10 | 10 | % |
For more information, please check out the "Materials" Section
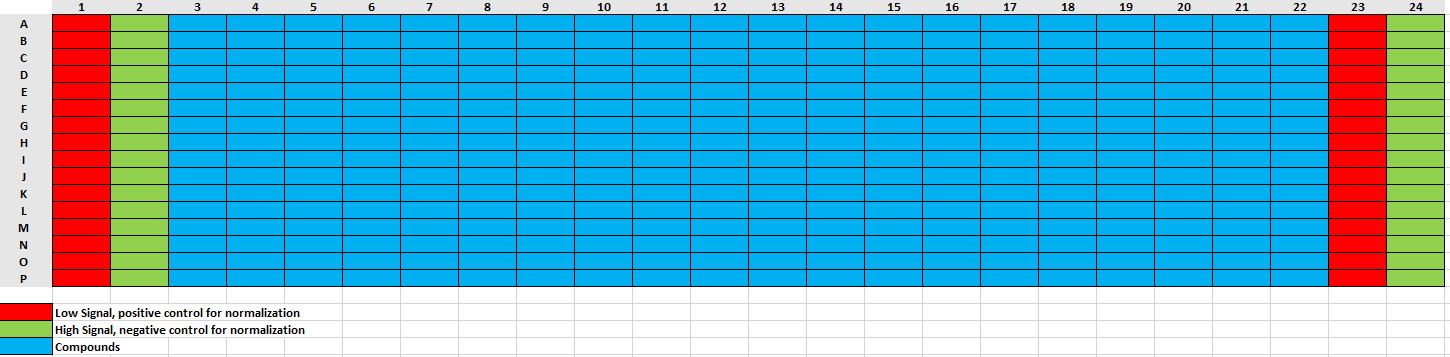
Compound Plate Design for Dose Response:
Total Assay Volume: 16 µL
**Compounds Top Assay Concentration:** 100 µM
**Dilution Factor:** 3
**Dose Response Points:** 10
**Number of Replicates:** 2
Backfill with DMSO: Yes
Compounds Plate Design for 2-Point Assay:
Total Assay Volume: 16 µL
**Compounds Assay Concentration:** 100 µM and 50 µM
**Dilution Factor:** 2
**Dose Response Points:** 2
**Number of Replicates:** 2
Backfill with DMSO: Yes
Before start
Note: Inhibitor compounds stock concentration is 20millimolar (mM). Compounds are pre-dispensed into 384 plates and stored at -20˚C until use.
Steps
Prepare Reagents
PREPARE all of the reagents/buffers required for this experiment.
Assay Buffer
| A | B | C | D | E |
|---|---|---|---|---|
| Reagent | Stock | Loaded into Combi | Final in assay plate | Units |
| HEPES pH=7.0 | 250 | 25 | 25 | mM |
| NaCl | 200 | 20 | 20 | mM |
| BSA | 0.5 | 0.05 | 0.05 | mg/ml |
| Tween 20 | 0.5 | 0.05 | 0.05 | % |
| HTRF PPI Europium detection buffer | 100 | 10 | 10 | % |
Reagents (dilute reagents in assay buffer for required volume)
| A | B | C | D | E |
|---|---|---|---|---|
| Reagent | Stock | Loaded into Combi | Final in assay plate | Units |
| His-SARS-CoV-2 MAC1 | 183000 | 50 | 12.5 | nM |
| Substrate (Biotin-ADPr) | 10000000 | 1600 | 400 | nM |
Detection Solution (dilute reagents in assay buffer for required volume)
| A | B | C | D | E |
|---|---|---|---|---|
| Reagent | Stock | Loaded into Combi | Final in assay plate | Units |
| Streptavidin-XL665 (SA-XL) | 1 | 0.25 | 0.125 | % |
| MAb Anti-6HIS-Eu cryptate Gold | 100 | 0.25 | 0.125 | % |
Prepare 384-well Plate
PRIME Multi-Drop Combi Tube Dispensing Cassette with Assay Buffer by selecting the PRIME button on the Combi Dispenser until the tubes are filled completely.
- Note: Be sure to cycle dispensing several times on a a clean plate lid (This confirms there are no bubbles in the Dispensing Cassette).
DISPENSE 4µL to Columns 1 and 23 of assay plate
- Note: These will represent the inhibitor control columns
EMPTY Multi-Drop Combi Tube Dispensing Cassette (by selecting the EMPTY button on the Combi Dispenser until the tubes of the cassette are emptied). Discard the Assay Buffer discharged from the cassette.
PRIME Multi-Drop Combi Tube Dispensing Cassette with His-SARS COV2 MAC1 Enzyme by selecting the PRIME button on the Combi Dispenser until the tubes are filled completely.
- Note: Be sure to cycle dispensing several times on a a clean plate lid (This confirms there are no bubbles in the Dispensing Cassette).
DISPENSE 4µL 50nanomolar (nM) to Columns 2-22 and 24 of assay plate
Note:
50nanomolar (nM)is four times the final concentration for the assay. It will be diluted to be a final concentration of12.5nanomolar (nM)- Column 2 and Column 24 are neutral control columns (Contain: Enzyme, Substrate, DMSO; no experimental compounds )
EMPTY Multi-Drop Combi Tube Dispensing Cassette (by selecting the EMPTY button on the Combi Dispenser until the tubes of the cassette are emptied). Discard the 50nanomolar (nM) discharged from the cassette.
PRIME Multi-Drop Combi Tube Dispensing Cassette with Assay Buffer by selecting the PRIME button on the Combi Dispenser until the tubes are filled completely. Then, EMPTY the Multi-Drop Combi Tube Dispensing Cassette (by selecting the EMPTY button on the Combi Dispenser until the tubes of the cassette are emptied). Discard the Assay Buffer discharged from the cassette.
CENTRIFUGE plate 1500rpm to remove bubbles
INCUBATE plate for 0h 15m 0s atRoom temperature
PRIME Multi-Drop Combi Tube Dispensing Cassette with1600nanomolar (nM) by selecting the PRIME button on the Combi Dispenser until the tubes are filled completely.
- Note: Be sure to cycle dispensing several times on a a clean plate lid (This confirms there are no bubbles in the Dispensing Cassette).
DISPENSE 4µL 1600nanomolar (nM) into Columns 1-24 (full plate)
Note:
1600nanomolar (nM)is four times the final concentration for the assay. It will be diluted to be a final concentration of400nanomolar (nM)
EMPTY Multi-Drop Combi Tube Dispensing Cassette (by selecting the EMPTY button on the Combi Dispenser until the tubes of the cassette are emptied). Discard the 1600nanomolar (nM) discharged from the cassette.
CENTRIFUGE plate 1500rpm to remove bubbles
PRIME Multi-Drop Combi Tube Dispensing Cassette with Assay Buffer by selecting the PRIME button on the Combi Dispenser until the tubes are filled completely. Then, EMPTY the Multi-Drop Combi Tube Dispensing Cassette (by selecting the EMPTY button on the Combi Dispenser until the tubes of the cassette are emptied). Discard the Assay Buffer discharged from the cassette.
PRIME Multi-Drop Combi Tube Dispensing Cassette with 0.25% volume by selecting the PRIME button on the Combi Dispenser until the tubes are filled completely.
- Note: Be sure to cycle dispensing several times on a a clean plate lid (This confirms there are no bubbles in the Dispensing Cassette).
DISPENSE 8µL 0.25% volume into full plate
Note:
0.25% volumeis two times the final concentration for the assay. It will be diluted to be a final concentration of0.125% volume
EMPTY Multi-Drop Combi Tube Dispensing Cassette (by selecting the EMPTY button on the Combi Dispenser until the tubes of the cassette are emptied). Discard the 1600nanomolar (nM) discharged from the cassette.
CENTRIFUGE 1500rpm plate to remove bubbles
INCUBATE plate for 1h 0m 0s at Room temperature
Recommended: Clean/Empty the Multi-Drop Combi Reagent Dispenser and Dispensing Cassette during this incubation step
Read Plate Fluorescence
READ and RECORD the plate Relative fluorescence units (RFU) via the " Mac1 Protocol " on the PHERAstar FS Control Software.
Equipment
| Value | Label |
|---|---|
| PHERAstar FS | NAME |
| Microplate reader | TYPE |
| BMG LABTECH | BRAND |
| 0471B0001A | SKU |
Diagram of assay
Figure 1 graphical depiction of assay principal and its use in screening campaign
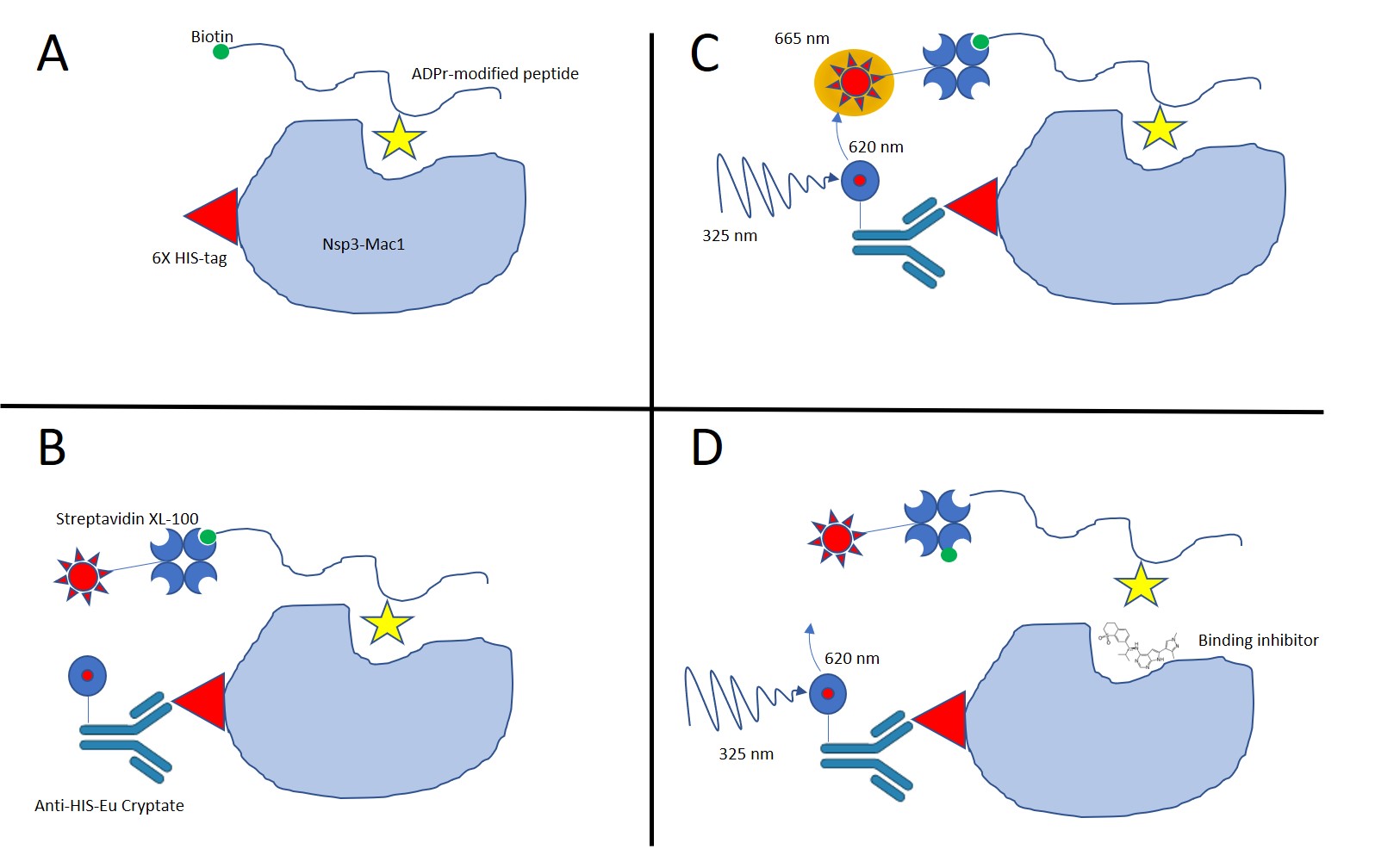
Experimental Design
Keywords
Mac1, Nsp3, TR-FRET, HTRF, ADPr, Displacement, Screening, Assay, Inhibitor, Fragment, Binding, Macrodomain