Protocol for Transfection of Bodo saltans with SaCas9 RNP complex in conjunction with eGFP-NEO plasmid by electroporation
Fatma Gomaa, Zhu-Hong Li, Roberto Docampo, Virginia Edgcomb
Abstract
Developing transfection protocol for Bodo saltans , using SaCas9/sgRNA ribonucleoprotein (RNP) complex in conjunction with DNA repair template to disrupt the Paraflagellar rod 2 gene ( BsPFR2 ) and increase the efficiency of targeted homologous recombination when a repair template DNA is provided. The exogenous repair template is double stranded DNA and it consists of eGFP fused with the drug selection gene nptII/neo and flanked by 500 bp of the untranslated regions (UTRs) upstream and downstream of the targeted BsPFR2 as homologous repair arms.
Steps
Step 1: Plasmid construction to target the PFR-2 gene
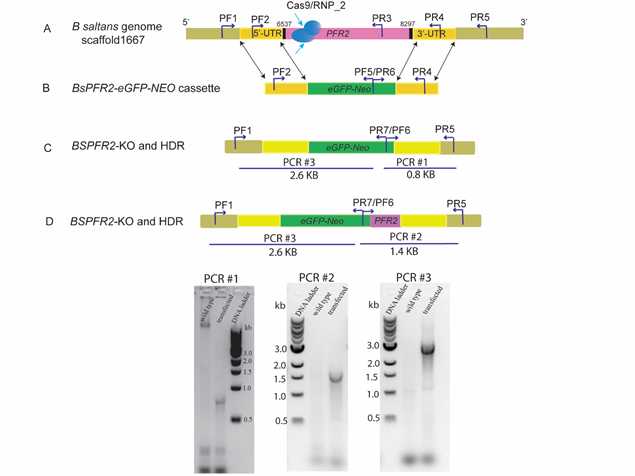
-A 2512 bp promoter-less cassette is designed to target and knock out the B. saltans 69 KDa paraflagellar rod protein 2C (PFR-2), (GenBank accession #CYKH01000743: scaffold1667, positions 3455 to 6406).
-This cassette is designed to replace the PFR-2 gene with a fusion of the eGFP (enhanced green fluorescent protein) and Neomycin genes. It contains 500 bp homologous arms at the 5' and 3' ends.
-The construct was linearized with the restriction enzyme Xbal (New England BioLabs) prior to electroporation. The plasmid sequence was deposited in GenBank under accession number (MZ522125).
Step 2: Co-delivery of SaCas9 RNP complex and the DNA repair template for BsPFR2 disruption
-10 ml of B. saltans cells from cultures at log phase (2-3 days old cultures) were filtered using a 7 micron nylon filter and washed 3 times in ddH2O by centrifugation at 950xg for 4 minutes.
-Total cell count used for electroporation was between 1x106 to 2x106
-2 µg of sgRNA were annealed with 4 µg of SaCas9 for 5 minutes at 37°C, then this mixture was combined with 50 µg of PFR-GFP-Neo plasmid and incubated 2 minutes at room temperature.
- B. saltans cells were electroporated using a square wave electroporator (NEPA21, Bulldog Bio, Inc.), with the electroporation parameters presented in Table 1.
-The cells were recovered immediately after electroporation and incubated in B. saltans growth media.
-G418 was added to transfected cultures 48 hours post-electroporation at concentration 2 µg/ml and increased gradually to 3 µg/ml over few weeks.
-Genotyping analysis using PCR primers sets targets different regions on the plasmid as well as on the genome of B. saltans to confirm the on-target plasmid integration are presented in Figure 1
-Table 1: NEPA 21 electroporation parameters used in our study
| A | B | C | D | E | F | G | H | I | J | K | L | M |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Poring pulse | **** | **** | Transfer pulse | **** | **** | |||||||
| **** | V | PD (ms) | PI (ms) | N | decay rate | polarity | V | PD (ms) | PI (ms) | N | decay rate | polarity |
| 1 | 200 | 25 | 0 | 1 | 10% | + | 60 | 99 | 999 | 5 | 40% | +/- |
| 2 | 99 | 5 | 50 | 7 | 10% | + | 99 | 50 | 50 | 5 | 40% | +/- |
| 3 | 150 | 5 | 50 | 3 | 10% | + | 50 | 10 | 999 | 5 | 40% | +/- |
-V: voltage strength; PD: pulse duration; PI: pulse interval; N: number of pulses