NEBNext® Ultra™ II DNA PCR-free Libray Prep Kit for Illumina® (NEB #E7410S/L, #E7415S/L)
New England Biolabs
Abstract
The NEBNext Ultra II DNA PCR-free Library Prep Kit for Illumina contains the enzymes and buffers required to convert a broad range of input amounts of DNA into high quality libraries for next-generation sequencing on the Illumina platform without PCR amplification. The fast, user-friendly workflow also has minimal hands-on time.
Each kit component must pass rigorous quality control strandards, and for each new lot the entire set of reagents is funtionally validated together by construction and sequencing of indexed libraries on Illumina sequencing platform.
For larger volume requiremnts, customized and bulk packaging is abailable by pruchasing through the OEM/Bulks department at NEB. Please contact OEM@neb.com for further information.
Before start
Starting Material: 250 ng-1 μg purified, genomic DNA sheared to 400 bp range. We recomment that DNA be sheared in 1X TE. If the DNA volume post shearing is less than 50 μl, add 1X TE to a final volume of 50 μl. Alternatively, sample can be diluted with 10 mM Tris-HCl, pH 8.0 or 0.1X TE.
Steps
NEBNext End Prep
Ensure that the Ultra II End Prep Reaction Buffer is completely thawed. If a precipitate is seen in the buffer, pipette up and down several times to break it up, and quickly vortex to mix. Place on ice until use.
Add the following components to a 0.2 ml thin wall PCR tube on ice:
| A | B |
|---|---|
| COMPONENT | VOLUME PER ONE LIBRARY |
| Fragmented DNA | 50 μl |
| (green) NEBNext Ultra II End Prep Reaction Buffer | 7 μl |
| (green) NEBNext Ultra II End Prep Enzyme Mix | 3 μl |
| Total Volume | 60 μl |
Set a 100 μl or 200 μl pipette to 50 μl and then pipette the entire volume up and down at least 10 times to mix thoroughly. Perform a quick spin to collect all liquid for the sides of the tube.
Place in a thermal cycler, with the heated lid set to 75°C, and run the following program:
0h 30m 0s at 20°C
0h 30m 0s at 65°C
Hold at 4°C
Proceed immediately to the next section once the reaction temperature reaches 4°C.
Adaptor Ligation
Add the following components directly to the End Prep Reaction Mixture:
| A | B |
|---|---|
| COMPONENT | VOLUME |
| End Prep Reaction Mixture (Step 4) | 60 μl |
| NEBNext UMI Adaptors for Illumina* | 2.5 μl |
| (red) NEBNext Ultra II Ligation Master Mix** | 30 μl |
| (red) NEBNext Ligation Enhancer | 1 μl |
| Total Volume | 93.5 μl |
- The NEBNext UMI adaptors are provided in NEBNext Multiplex Oligos for Illumina (Unique Dual Index UMI Adaptors DNA Set 1, NEB #E7395). Please refer to the NEB #E7395 manual for valid barcode combinations.
** Mix the Ultra II Ligation Master Mix by pipetting up and down several times times prior to adding to the reaction.
Set a 100 μl or 200 μl pipette to 80 μl and then pipette the entire volume up and down at least 10 times to mix thoroughly. Perform a quick spin to collect all liquid from the sides of the tube
Caution: The NEBNext Ultra II Ligation Master Mix is viscous. Care should be taken to ensure adequate mixing of the ligation reaction, as incomplete mixing will result in reduced ligation efficiency. The presence of a small amount of bubbles will not interfere with performance.
Incubate at 20°C for 0h 15m 0s in a thermal cycler with the heated lid off. Move immediately to the next step or place your sample at -20°C.
Safe Stopping Point: Samples can be stored overnight at . -20°C.
Size Selection of Adaptor-ligated DNA
Caution: The following cleanup protocol is for libraries with ~350 bp or ~450 bp inserts only (Step 20). Size selection conditions were optimized with NEBNext Sample Purification Beads and SPRIselect beads. However, AMPure XP beads can be used following the same conditions. If using AMPure XP beads, please allow the beads to warm to room temperature for at least before use. 0h 30m 0s before use.
Bring the volume of the reaction to ~100µL by adding 7µL 0.1X TE (dilute 1X TE Buffer 1:10 with water).
Vortex NEBNext Sample Purification Beads or SPRIselect Beads to resuspend.
Add 50µL of resuspended NEBNext Sample Purification Beads or SPRIselect beads to the 100 μl sample from Step 9. Mix well by pipetting up and down at least 10 times. Be careful to expel all of the liquid out of the tip during the last mix. Vortexing for 3-5 seconds on high can also be used. If centrifuging samples after mixing, be sure to stop the centrifugation before the beads start to settle out.
Incubate samples for at least 0h 5m 0s at room temperature.
Place the tube/ plate on an appropriate magnetic stand to separate the beads from the supernatant. If necessary, quickly spin the sample to collect the liquid from the sides of the tube or plate wells before placing on the magnetic stand.
After 0h 5m 0s (or when the solution is clear), carefully remove and discard the supernatant that contains unwanted DNA. Be careful not to disturb the beads that contain the desired DNA targets.
Caution: do not discard beads.
Add 200µL of 80% freshly prepared ethanol to the tube/ plate while in the magnetic stand. Incubate at room temperature for 0h 0m 30s, and then carefully remove and discard the supernatant. Be careful not to disturb the beads that contain DNA targets.
Repeat the previous step once. Be sure to remove all visible liquid after the second wash. If necessary, briefly spin the tube/ plate, place back on the magnet and remove traces of ethanol with a p10 pipette tip.
Air dry the beads for up to 0h 5m 0s while the tube/ plate is on the magnetic stand with the lid open.
Caution: Do not overdry the beads. This may result in lower recovery of DNA target. Elute the samples when the beads are still dark brown and glossy looking, but when all visible liquid has evaporated. When the beads turn lighter brown and start to crack, they are too dry.
Remove the tube/ plate from the magnetic stand. Elute the DNA target from the beads into 102µL of 0.1X TE. Mix well on a vortex mixer or by pipetting up and down 10 times. Incubate for 0h 2m 0s at room temperature. If necessary, quickly spin the sample to collect the liquid from the sides of the tube or plate wells before placing back on the magnetic stand.
Place the tube/ plate on a magnetic stand. After 0h 5m 0s (or when the solution is clear), transfer 100µL to a new tube.
Add appropriate amounts of resuspended Sample Purification or SPRIselect Beads to the sample for the desired insert sizes. Mix well by pipetting up and down at least 10 times. Be careful to expel all of the liquid out of the tip during the last mix. Vortexing for 3-5 seconds on high can also be used. If centrifuging samples after mixing, be sure to stop the centrifugation before the beads start to settle out.
| A | B |
|---|---|
| INSERT SIZE | BEADS VOLUME |
| 350 bp | 65 μl |
| 450 bp | 58 μl |
Incubate samples for at least 0h 5m 0s at room temperature.
Place the tube/ plate on an appropriate magnetic stand to separate the beads from the supernatant. If necessary, quickly spin the sample to collect the liquid from the sides of the tube or plate wells before placing on the magnetic stand.
After 0h 5m 0s (or when the solution is clear), carefully remove and discard the supernatant that contains unwanted DNA. Be careful not to disturb the beads that contain the desired DNA targets.
Caution: do not discard beads.
Add 200µL of 80% freshly prepared ethanol to the tube/ plate while in the magnetic stand. Incubate at room temperature for 0h 0m 30s, and then carefully remove and discard the supernatant. Be careful not to disturb the beads that contain DNA targets.
Repeat the previous step once. Be sure to remove all visible liquid after the second wash. If necessary, briefly spin the tube/ plate, place back on the magnet and remove traces of ethanol with a p10 pipette tip.
Air dry the beads for up to 0h 5m 0s while the tube/ plate is on the magnetic stand with the lid open.
Caution: Do not overdry the beads. This may result in lower recovery of DNA target. Elute the samples when the beads are still dark brown and glossy looking, but when all visible liquid has evaporated. When the beads turn lighter brown and start to crack, they are too dry.
Remove the tube/ plate from the magnetic stand. Elute the DNA target from the beads into 22µL of 0.1X TE. Mix well on a vortex mixer by pipetting up and down 10 times. Incubate for 0h 2m 0s at room temperature. If necessary, quickly spin the sample to collect the liquid from the sides of the tube or plate wells before placing back on the magnetic stand.
Place the tube/ plate on a magnetic stand. After 0h 5m 0s (or when the solution is clear), transfer 20µL to a new tube.
Quantitate the library using qPCR (NEBNext Library Quant Kit for Illumina, NEB #E7630S/L).
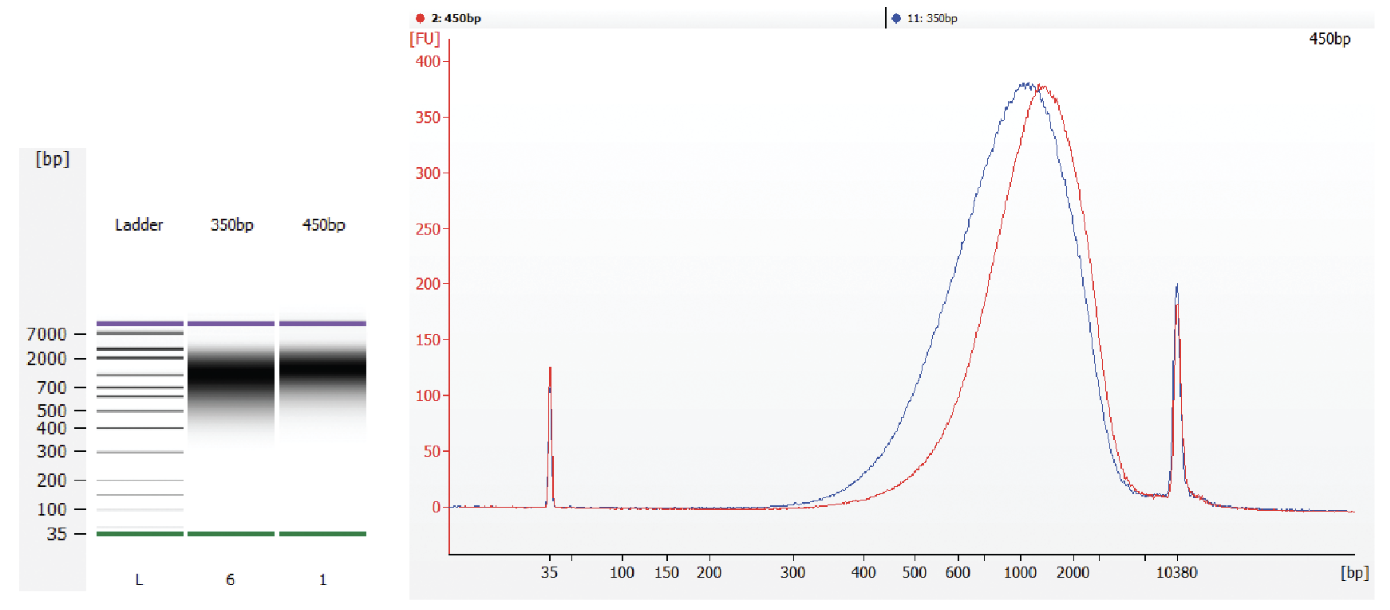
Verify fragment size by checking the library size distribution on an Agilent Bioanalyzer or TapeStation. Run 1µL library on a DNA High Sensitivity Chip (Bioanalyzer) or High Sensitivity D5000 ScreenTape® (TapeStation). See Figure 1 for an example.
Safe Stopping Point: It is safe to store the library at . -20°C.
Figure 1: Examples of Ultra II DNA PCR-free libraries on a Bioanalyzer. The PCR-free libraries migrate slower due to the single strand regions of the adaptors, thus appearing significantly larger than the actual fragment sizes.