Mouse Pancreatic Islet Isolation
Islet and Pancreas Analysis Core
Abstract
This SOP defines the methods used by the Vanderbilt Diabetes Center Islet and Pancreas Analysis (IPA) Core for isolation of pancreatic islets from wild type and transgenic mice.
Steps
Reagent preparation
0.6 mg/mL collagenase P solution – Weigh out between 20-30 mg of collagenase P into a 50 mL conical tube. Divide the precise mg mass by 0.6 to determine volume (mL) of HBSS to add to the tube. Once HBSS is added, vortex for about 30 seconds until collagenase goes into solution. Once prepared, keep collagenase P solution on ice.
Pipet 6.5 mL aliquots into 15 mL conical tubes (each tube can hold up to 2 pancreata).
HBSS with 10% FBS – Remove and discard 50 mL from a 500 mL bottle of HBSS, then add 50 mL FBS. Store at 4°C for up to 1 month.
HBSS with 1% FBS – Remove and discard 5 mL from a 500 mL bottle of HBSS, then add 5 mL FBS. Store at 4°C for up to 1 month.
Ketamine (15 mg/mL)/xylazine (3 mg/mL) working solution – Use 5 mL syringes with 18G needles. Label all bottles with additive, mix date, and expiration date of earliest-expiring ingredient.
Pull 2 mL of xylazine and inject into a 10 mL bottle of ketamine.
Pull 2 mL of ketamine/xylazine and inject into a 10 mL bottle of saline.
Surgery preparation
Set water bath to 37°C.
Remove Histopaque from the refrigerator to warm up to room temperature.
Using an 18G needle, pull up 4 mL of collagenase solution into a 5 mL syringe. Place syringe on ice.
Surgery
Inject mice intraperitoneally with ketamine/xylazine solution (1 μl/mg body weight) using a 1 mL syringe with a 27G needle. Place mice into a plastic bin with air holes.
After 5-10 minutes, check to see if mice are fully anesthetized by gently pinching feet and tail. If there is a jerking or squeaking response, inject about 100 μL more of ketamine/xylazine solution, and wait a few more minutes. Continue checking for full anesthetization before beginning surgery.
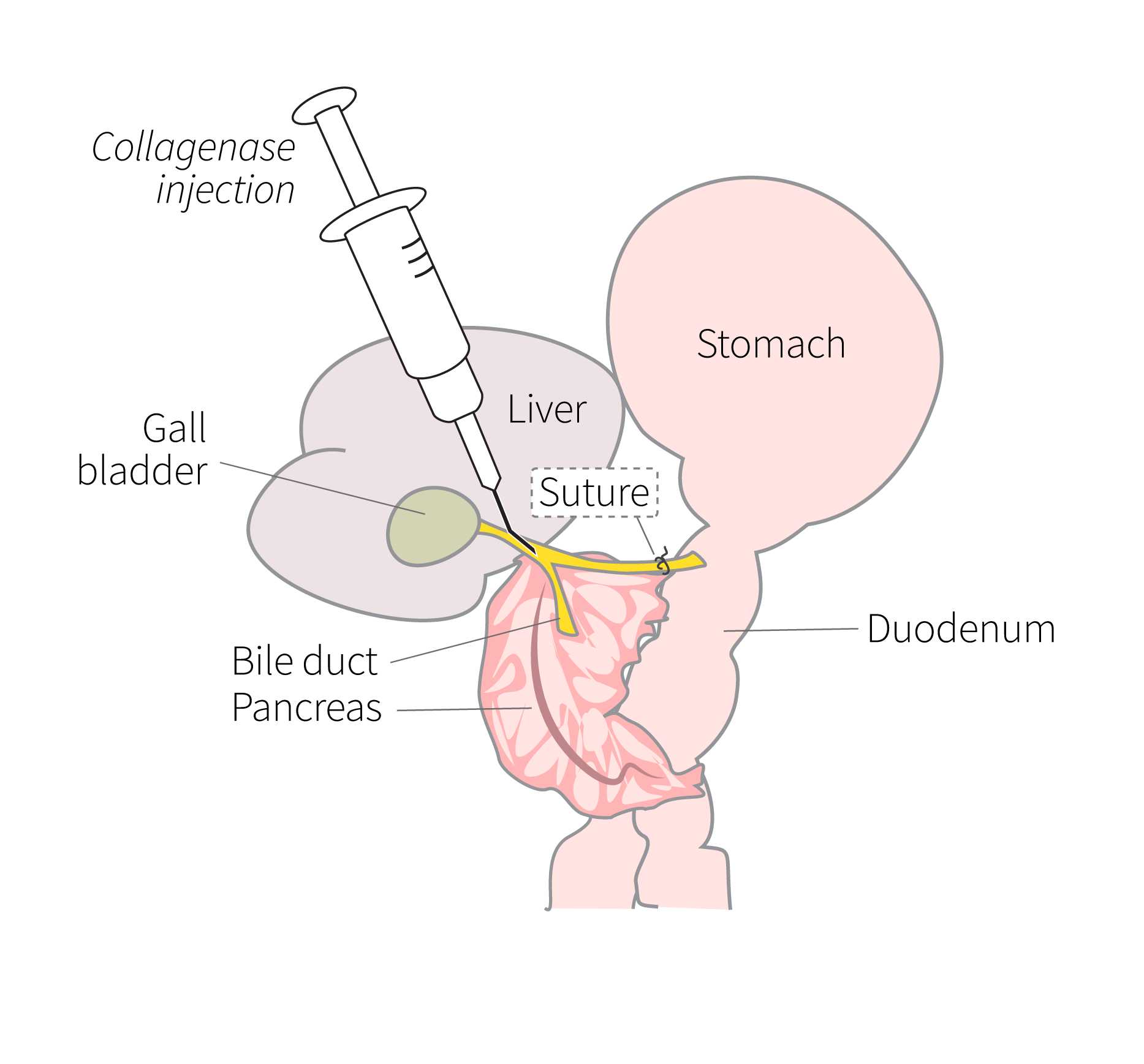
Place mouse face up on the surgical stage, tape limbs down, and wipe with 70% ethanol. Open the body cavity using forceps and scissors, and move organs to the left side of the body to locate the bile duct.
Ligate the bile duct with suture at the duodenum.
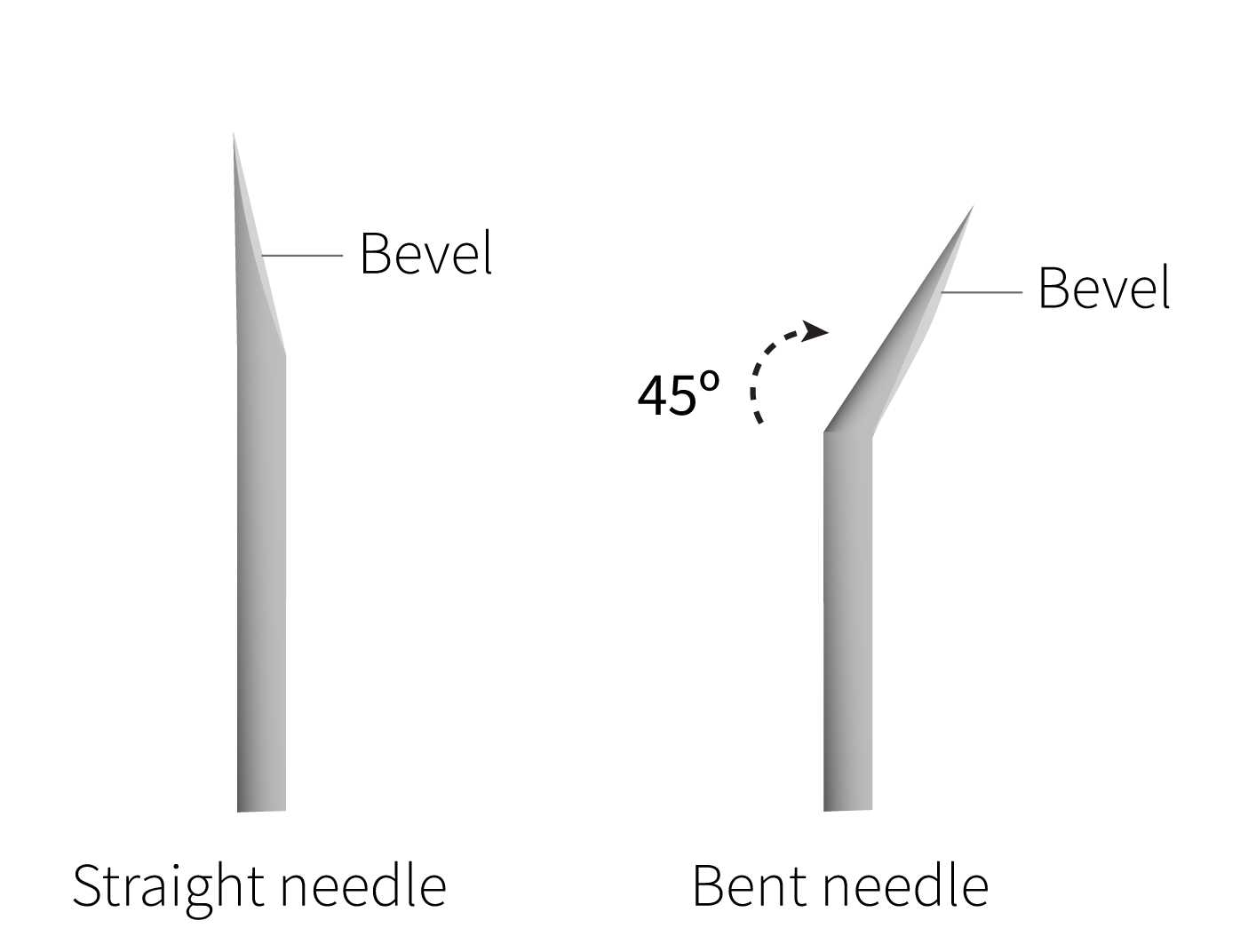
Remove the 18G needle on the collagenase syringe and replace with the bent 30G needle.
Remove suture so that only the knot on the bile duct remains to prevent leakage.
Separate the pancreas from the duodenum and stomach, using two pairs of forceps. Move the intestines over to the side and separate the pancreas, starting from the head. Remove the pancreas with the spleen still attached.
Detach the spleen, blot pancreas on the surgical stage to remove some of the blood, and place into a conical tube containing the collagenase solution. Place the tube back on ice.
Perform cervical dislocation on the mouse and transfer carcass to a biohazard bag for appropriate disposal.
Pancreas digestion
Using tri-grip clamps, secure each tube in the wrist action shaker so that all tubes are fully immersed in the 37°C water bath. Start the shaker.
Shake tubes with the wrist action shaker for total of 0h 8m 0s. After 4 minutes, remove tubes from the bath and shake vigorously by hand 3-5 times to begin to break up tissue. Then, place the tubes back in the water bath to continue shaking for the remaining 4 minutes.
Remove tubes from the bath and shake by hand for up to 0h 2m 0s. Shake until tissue has dissolved into a homogeneous slurry.
Return tubes to ice and add cold HBSS with 10% FBS to bring volume to 15mL.
Centrifuge tubes at200rcf,0h 0m 0s for 0h 1m 0s at 25°C.
Decant the tubes and wash and centrifuge pellets 3X with 10 mL HBSS with 10% FBS . Vortex each time after adding HBSS to mix before centrifugation.
After the final wash, discard supernatant and add 10 mL HBSS with 10% FBS to the tube, pipet up and down to mix, and transfer to a 50 mL conical tube. Wash the original tube with another 5 mL HBSS with 10% FBS and add to 50 mL tube, and transfer that as well, for a total of 15mL of digest.
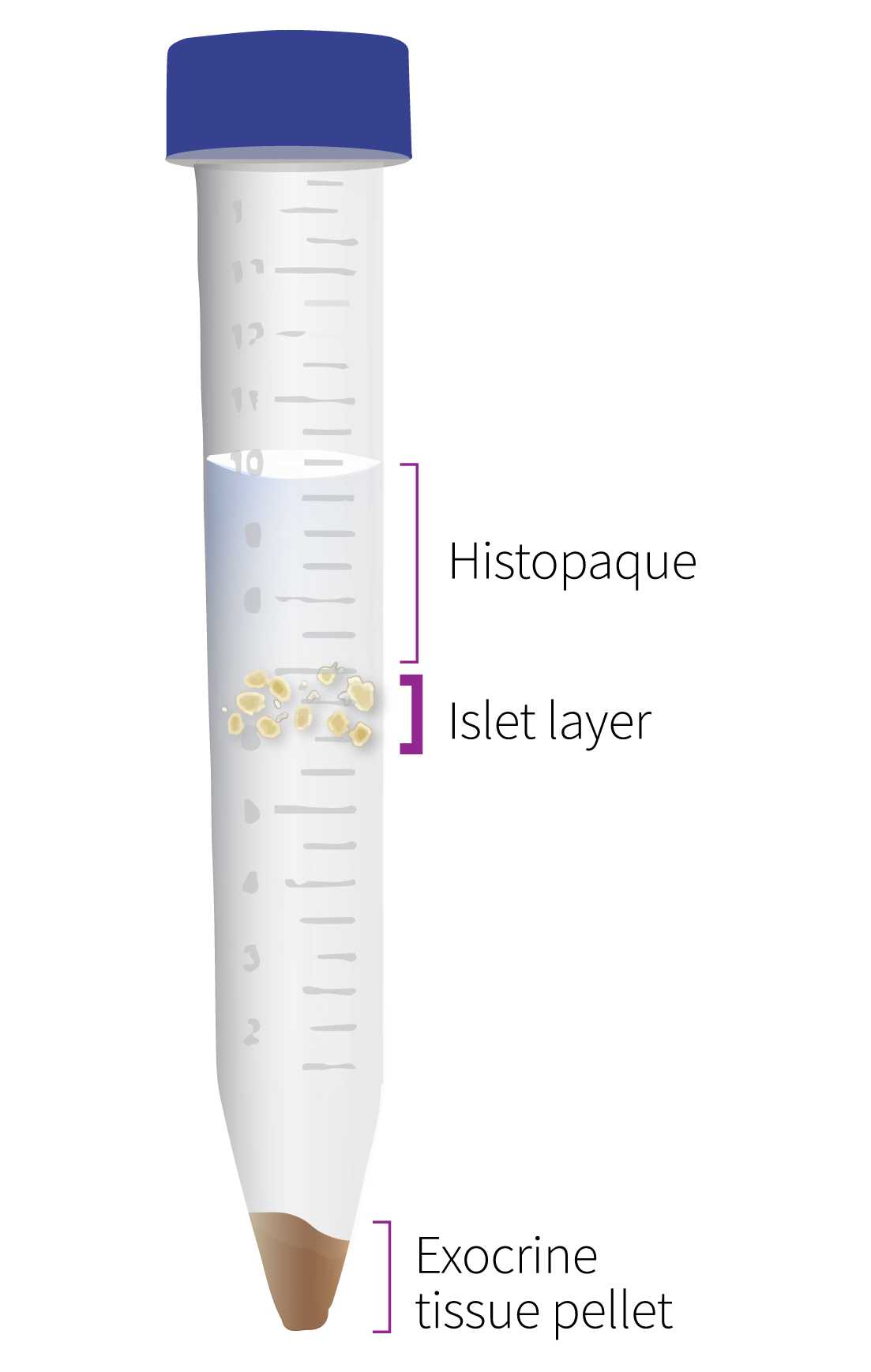
Vortex the tubes. Using a manual bulb pipet filler, very gently underlay the digest with 15 mL of Histopaque gradient .
Centrifuge tubes at 500rcf,0h 0m 0s for 0h 10m 0s at 25°C.
Centrifuge tubes at 500rcf,0h 0m 0s for 0h 10m 0s at 25°C.
Decant the tubes and wash and centrifuge pellets 3X with 25 mL HBSS with 1% FBS . Vortex each time after adding HBSS to mix before centrifugation.
After the final decanting, add 10 mL HBSS with 10% FBS to the tube, pipet up and down to mix, and transfer to a 10 cm untreated culture dish. Wash the tube with another 5 mL HBSS with 10% FBS , and transfer that as well, for a total of 15mL of digest.
Place the cell culture plate containing the digest on the stage of an inverted microscope. View and pick islets at 4x magnification, using a P-200 pipette with ART tips.