Molecular Cloning
Lizhen Zhu, Ruixin Lin, Xiaoqi Ye, Ruiyan zeng, 18814306949 Minkun Huang, 624787660
Abstract
SZPT-CHINA Experimental methods of molecular cloning
Steps
Vector linearization and target fragment preparation
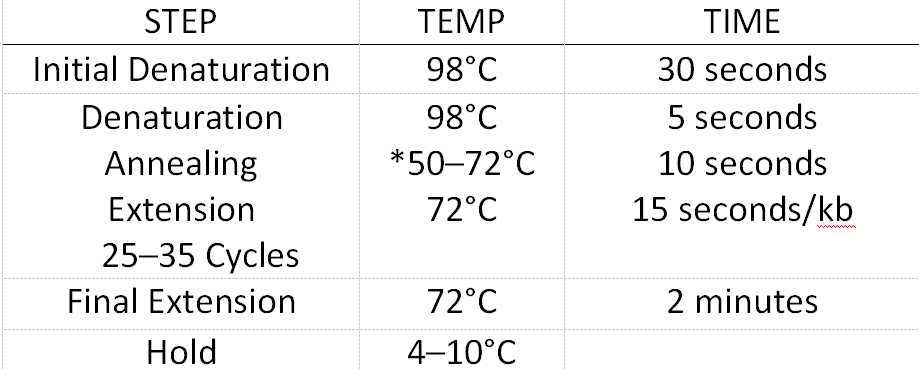
Enzyme digestion Connection
Enzyme digestion Connection
Enzyme digestion and connection system(10µL )
3µL
1µL
1µL
0.5µL
4.5µL
Transformation
Transformation
Take 50 µL of competent cells in a sterile Eppendorf tube.
Add 2~5 µL of the plasmid to be transformed into each tube, gently mix the plasmid and competent cells with a pipette tip, and ice bath for 25 minutes.
Heat shock the ice-bath mixture in a 42℃ water bath for 45s, do not shake the centrifuge tube.
Take out the ice bath and cool for 2 minutes.
Add 800 µL of non-resistant LB medium preheated to 37°C to each tube, shake gently at 37°C, 150r/min for 1h.
Centrifugally concentrate to 100μL, spread it on an LB plate containing antibiotics, and incubate inverted at 37°C for 12-16h.
Make a negative control at the same time (use distilled water instead of plasmid).
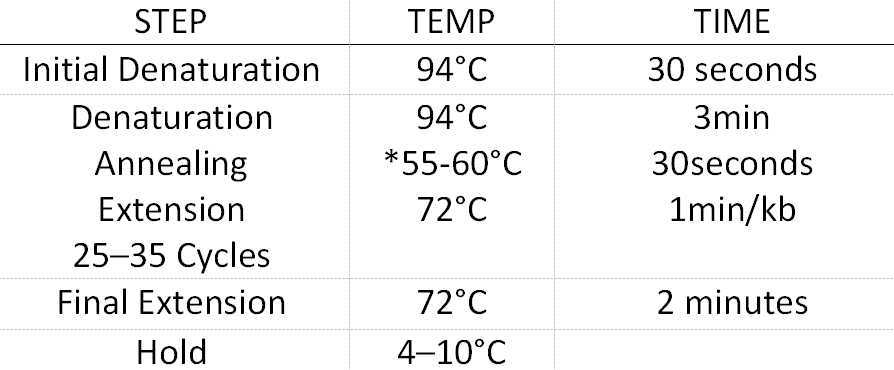
Colony PCR screening of positive clones
Colony PCR screening of positive clones
Add 25ul of sterile water to the 96-well plate, pick about 15-20 colonies from the successfully transformed DH5α bacterial plate with a pipette tip, and mix them evenly. (Make a corresponding mark on the picking plate).
Put the mixed bacteria solution into 10ul to 200ul PCR tube and mark it.
Take 0.1ul of bacterial solution in a 96-well plate and add it to the antibiotic-containing plate with a pipette tip and mark it.
Verification result of 1% agarose gel electrophoresis.