Growth_of_Pseudomonas_fluorescence_SBW25_in_48-well_Plate_Format
Rosemarie Wilton
Abstract
This protocol provides a method for culturing Pseudomonas fluorescens SBW25 in 48-well microplates. The protocol is specific for use with a BioTek Epoch 2 plate reader, but other plate readers can be used with appropriate modifications. One advantage of the Epoch 2 instrument is the ability to run with the plate lid at a higher temperature than the well contents. This prevents condensation on the lid and resulting noise in the OD readings.
This protocol is suitable for many Pseudomonas strains.
I thank members of the ORNL team, Joshua Elmore and Quint Peabody V, for assistance during development of this protocol and tips on use of the Epoch2 plate reader. I thank the NREL team for the M9 Minimal Media recipe.
Steps
Culturing Pseudomonas fluorescens SBW25
DAY1: Overnight culture
Inoculate 5mL LB, in a vented or loosely capped culture tube (e.g. CELLTREAT 50 mL Bio-Reaction tube), from a glycerol stock or with a single colony from a plate.
Grow overnight at 30°Cwith shaking at 250rpm.
DAY2: Four hour culture
NOTE: Pseudomonas cells typically have a long lag time if transferred from a stationary-phase overnight culture directly to minimal medium. The additional four hour culture period in fresh LB allows the cells to return to exponential growth phase, and minimizes the subsequent lag upon transfer to minimal medium. NOTE: Pseudomonas cells typically have a long lag time if transferred from a stationary-phase overnight culture directly to minimal medium. The additional four hour culture period in fresh LB allows the cells to return to exponential growth phase, and minimizes the subsequent lag upon transfer to minimal medium.
Dispense 10mL LB into vented or loosely capped 50 mL culture tube.
Inoculate with 250µLovernight culture.
Incubate for four hours at at 30°Cwith shaking at 250rpm
Pellet cells by centrifugation, 4000rpm for 0h 5m 0s.
Pour off supernatant and resuspend in 5mL M9 salts.
Collect cells by centrifugation, and repeat M9 salts wash step.
Resuspend pellet in5mL sterile M9 salts.
Remove a small aliquot to measure OD600 on a spectrophotometer.
Plate set-up and data collection
Prepare M9 minimal medium containing desired carbon source (M9-C) and filter sterilize.
Dilute washed cells to a final OD600 of 0.05 to 0.1 OD with M9-C.
Dispense 600 ul/well to 48-well plate and cover with the supplied lid.
Each unique well condition or sample type should be performed in triplicate.
NOTE: NOTE: If long incubation periods are anticipated (>24 hours), fill edge wells with M9-C and only use the 24 inner wells for culturing. This will minimize evaporation during long culture times.
Start up the Gen5 software on the Epoch2 system and place the plate in the drawer.
Enter the protocol shown below; run times and sampling intervals can be adjusted as needed.
48-well plate notes:
Notice that we have left the "Use Lid" box unchecked (even though we use the lid). This is because the Default 48 well plate plus lid is higher than the instrument max of 23.5 mm. To prevent error message, leave lid box unchecked or generate a custom plate description for the Greiner plate (System>Plate Types>View/Modify).
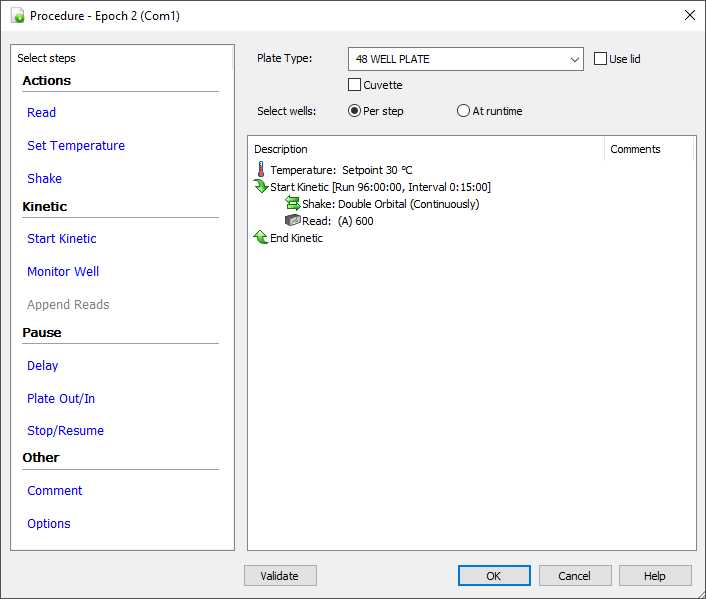
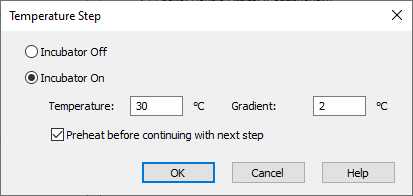
Adjusting the Well and Lid temperature:
The Epoch 2 allows the lid to be run at a higher temperature to avoid condensation. This is set in the Gradient box below. This should be set to 1 or 2ºC.
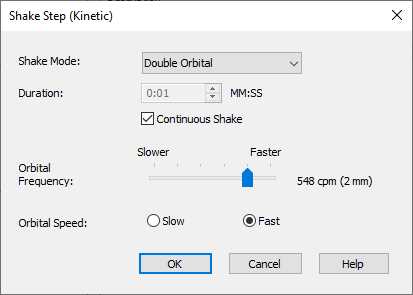
Adjusting the Shaking mode and speed:
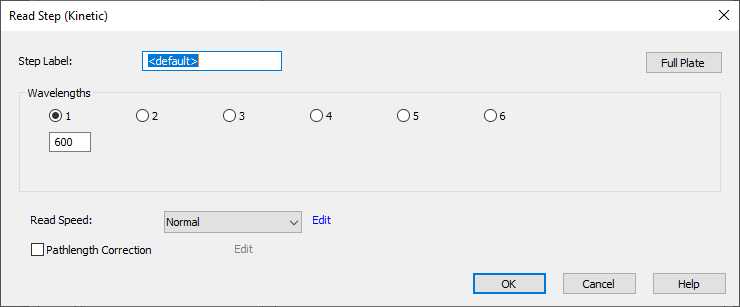
Adjusting the Read step:
Edit the Read Speed to bring up the second window. Increase the delay time to 250 msec to allow wells to stabilize before reading.

Once protocol has been set up, start the run and monitor data collection over time. The run can be stopped at earlier timepoints without losing the data.

