Fast Agarose Gel Electrophoresis-Chem 384/584
Addgene The Nonprofit Plasmid Repository, Ken Christensen
Abstract
This protocol is for agarose gel electrophoresis. To see the full abstract and additional resources, visit the Addgene protocol page.
Steps
Pouring a Standard 1% Agarose Gel
Measure .6g of agarose.
Mix agarose powder with 60mL 1xTAE in a microwavable flask. See TAE Recipe.
Microwave for 0h 1m 0s to 0h 3m 0s until the agarose is completely dissolved (but do not overboil the solution, as some of the buffer will evaporate and thus alter the final percentage of agarose in the gel. Many people prefer to microwave in pulses, swirling the flask occasionally as the solution heats up.)
Let agarose solution cool down to about 50°C (about when you can comfortably keep your hand on the flask), about 0h 5m 0s.
Add Midori Green (25,000x) to a final concentration of 1x (about 2µL -3µL of lab stock solution per 60mL gel). EtBr binds to the DNA and allows you to visualize the DNA under ultraviolet (UV) light.
Pour the agarose into a gel tray with the well comb in place.
Place newly poured gel at 4°C for 0h 10m 0s-0h 15m 0s OR let sit at room temperature for 0h 20m 0s-0h 30m 0s, until it has completely solidified.
Loading Samples and Running an Agarose Gel
Add loading buffer (e.g., to6x Purple Loading Dye from NEB) to each of your DNA samples.

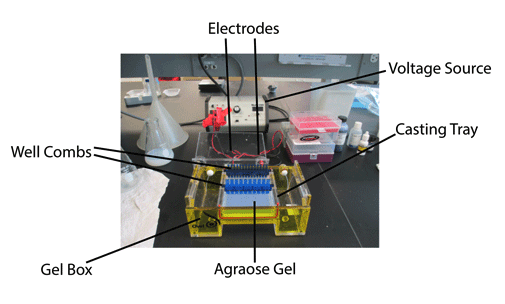
Once solidified, place the agarose gel into the gel box (electrophoresis unit).
Fill the gel box with 0.25xTAE (or TBE) until the gel is covered.
Carefully load a molecular weight ladder into one lane of the gel.
Run the gel at 250-300 V until the dye line is approximately 75-80% of the way down the gel. A typical run time is about 0h 15m 0s-0h 25m 0s, depending on the gel concentration and voltage.
Turn OFF power, disconnect the electrodes from the power source, and then carefully remove the gel from the gel box.
Using any device that has UV light, visualize your DNA fragments. The fragments of DNA are usually referred to as ‘bands’ due to their appearance on the gel.