Cell culture of THP-1 monocytes and differentiation into macrophages with PMA
Sylvia Oghogho Omage, Maria Wallert, Stefan Lorkowski
Abstract
THP-1 is a human monocytic cell line originally isolated from a 1-year old male subject with acute monocytic leukemia (Tsuchiya et al., 1980). The cells are easy to culture and are safe, as no viruses have been reported to be present in the cells (Chanput et al., 2014). THP-1 monocytes are round, floating cells with a doubling time of 35-50 h (Chanput et al., 2014). They have been used as a model for studying immune response, monocyte and macrophage function, lipid metabolism and functions of drugs (Chanput et al., 2014). THP-1 cells can be differentiated into macrophages for use in experiments. As macrophages, the cells become adherent to the cell culture vessel. Agents used for differentiation of THP-1 cells include phorbol 12-myristate 13-acetate (PMA), vitamin D, and macrophage colony-stimulation factor M-CSF (Chanput et al., 2014). This cell line is sensitive to culture conditions; e.g. the cells react differently to differentiation substances depending on the cell density (Liu et al., 2023) . The protocol presented herein is routinely used for culturing and differentiating THP-1 cells with PMA.
Before start
Note: All steps with cells should be carried out using a safety cabinet to avoid contamination of cells.
Steps
Thawing of THP-1 cells
Transfer the cryotube containing THP-1 monocytes from the liquid nitrogen tank to the cell culture lab on ice. Note : Wear protective gloves, goggles and a lab coat when handling liquid nitrogen to prevent burns.
Thaw the cells immediately in a 37 °C water bath until only a little chunk of ice is visible. Note: This procedure and the next steps must be performed quickly because the cryoprotectant dimethyl sulfoxide (DMSO) is cytotoxic above 4 °C.
Prepare RPMI-1640 cell culture medium supplemented with FBS and PSG with final concentrations of 10 % (v/v) and 1 % (v/v), respectively.
Mix the thawed cells (1 ml) with 9 ml of pre-warmed supplemented RPMI-1640 medium in a 15 ml tube and resuspend thoroughly.
Centrifuge at 300 x g for 5 minutes at room temperature and carefully aspirate the supernatant with a sterile Pasteur pipette.
Resuspend the cell pellet in 5 ml of supplemented RPMI-1640 medium and transfer the cell suspension to a 25 cm2cell culture flask.
Place the flask in a 37 °C incubator with 5 % CO2 atmosphere.
Leave the cells in the incubator and observe their growth over a period of days. When the cells have grown and are about 80 % dense, transfer them to a bigger flask and add fresh supplemented RPMI-1640 medium; e.g. cells can be transferred to a 75 cm2 cell culture flask with 25 ml of fresh supplemented 1640 medium added .
The cells should be split twice a week, at a density of around 80 %.
Passaging of THP-1 cells
Prepare the supplemented RPMI-1640 medium by mixing RPMI-1640 with FBS and PSG to final concentrations of 10 % (v/v) and 1 % (v/v), respectively.
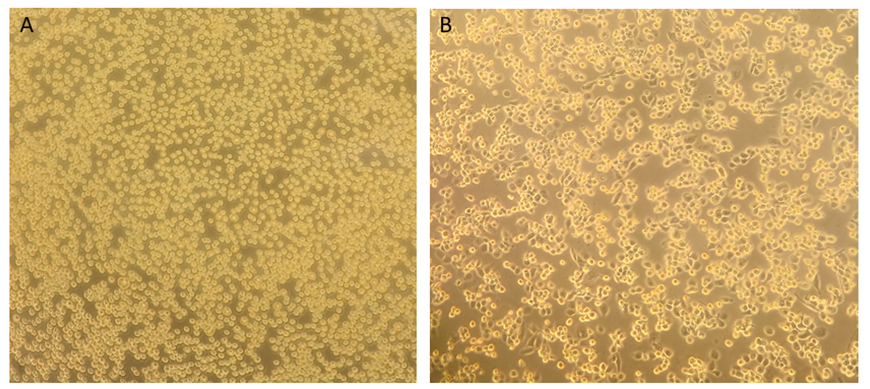
Evaluate cells using an inverted microscope. Cells should be round, bright and floating; there should be no bacterial or fungal contamination or irregular-shaped floating debris ( see Figure 1.A ). The cells should typically not clump together.
Collect cells in a 50 ml falcon tube.
Centrifuge at 300 x g for 5 minutes at room temperature.
Aspirate the medium and resuspend the cells with 5 ml of fresh supplemented RPMI-1640 medium.
Prepare a dilution of the cells for counting (1:30 to 1:50 dilution is recommended).
Count the cells under the microscope using a Neubauer counting chamber or a cell counter; typically, approximately 55 x 106 cells ± 20 % should be in 50 ml of medium.
Prepare a new flask of cells by splitting the cells 1:5 in 50 ml of fresh supplemented RPMI-1640 medium.
Differentiation of THP-1 monocytes to macrophages
Prepare PMA stock solution: PMA is dissolved in DMSO to a final concentration of 1 mg/ml.
Note: PMA should not be exposed to light. It should be aliquoted and stored at -20° C; it should not be subjected to several freeze-thaw cycles to preserve its efficacy.
Prepare β-mercaptoethanol stock solution: β-mercaptoethanol is diluted with water to a final concentration of 2.5 M, and stock solution can be stored at 4° C.
Note : β-mercaptoethanol should be handled under the biological safety cabinet because its vapors are toxic.
Collect cells in a 50 ml falcon tube.
Centrifuge at 300 x g for 5 minutes at room temperature.
Aspirate the medium and resuspend the cells with 5 ml of fresh supplemented RPMI-1640 medium.
Count the cells with a microscope using a Neubauer counting chamber or a cell counter.
Seed cells by mixing the required number of cells for seeding with the required volume of differentiation medium in a 50 ml falcon tube.
Dispense the appropriate volume of the cell-medium mixture into appropriate vessels.
The following steps are for seeding two 25 cm2 flasks with 3.3 x 106 cells each as an example. Since two flasks require 6 x 106cells, prepare the cell suspension with an additional 10 % , i.e 7.26 x 106 cells.
Calculations
Required volume of cell suspension (number of cells needed ÷ number of cells counted): For example, if the number of cells counted cis 12 x 106 cells, the volume of the cell suspension needed is: 7.26 x 106÷ 12 x 106 = 0.605 ml of cells.
Required volume of RPMI-1640 medium: 5 ml of medium is used to seed 3.3 x 106 cells in a 25 cm2 flask. For this, 5 ml x 2 flasks (+ 10 % extra) = 11 ml of medium. Next, subtract the volume of cells needed to get the final volume of medium needed, i.e 11 ml of medium-0.605 ml of cells = 10.4 ml of medium.
Required volume of PMA stock solution: A final concentration of 100 ng/ml PMA is used for differentiation. In the example, 1.1 µl of the 1 mg/ml PMA stock solution is needed.
Required volume of β-mercaptoethanol stock solution: A final concentration of 50 µM of β-mercaptoethanol is used for differentiation. In this example, 0.22 µl of the 2.5 M β-mercaptoethanol stock solution are needed.
Mix the required volume of the cell suspension with supplemented RPMI-1640 medium containing 100 ng/ml PMA and 50 mM β-mercaptoethanol In a labeled falcon tube.
Resuspend properly and dispense 5 ml into each 25 cm2 flask for subsequent experiments.
Gently swirl flasks and place flasks in an incubator at 37 °C with 5 % (v/v) CO2 atmosphere for 96 h.
After 96 h, THP-1 cells are fully matured into macrophages and firmly attached to the bottom of the culture vessel ( see Figure 1.B ). Cells can be now used for subsequent experiments.
Table 1 provides an overview of the recommended number of cells to seed in vessels of different sizes and the required volumes of supplemented RPMI-1640 medium.
Note: The cell density can be adjusted to suit the requirements of an experiment.
Table 1: Recommended number of cells to seed in vessels.
| A | B | C |
|---|---|---|
| Multiwell plates | Cell number [*10^6] | Volume of RPMI-1640 medium [ml] |
| 6 well | 1.4 | 3 |
| 12 well | 1.0 | 2 |
| 24 well | 0.38 | 2 |
| 96 well | 0.057 | 0.350 |
| Flasks | Cell number [*10^6] | Volume of RPMI-1640 medium [ml] |
| 25 cm^2 | 3.3 | 5 |
| 75 cm^2 | 10 | 15 |
| 150 cm^2 | 20 | 30 |
Freezing of THP-1 cells
Prepare freeze container (e.g. Mr. Frosty, Nalgene) with 2-propanol and cool it to 4 °C.
Note : Cooling in a freeze container with 2-propanol ensures a slow and gentle temperature decline. A cooling rate of -1 °C/min is the optimal rate for cell preservation.
Prepare freezing medium: RPMI-1640 containing 20 % (v/v) FBS, 0.1 mg/ml PSG and 10 % (v/v) sterile DMSO.
Collect cells in a 50 ml falcon tube.
Centrifuge at 300 x g for 5 minutes at room temperature.
Aspirate the medium and resuspend the cells with 5 ml of fresh suplemented RPMI-1640 medium.
Count the cells and calculate the number of needed cryotubes and volume of freezing medium. Label cryotubes.
Adjust cell concentration to 10 x 106 cells/ml and dispense 1 ml per cryotube.
Place labeled cryotubes in freeze container and store container at -80 °C overnight before transferring the cryotubes to a liquid nitrogen tank.