CODEX® Multiplexed Imaging | Tissue Staining and Reporter Plate Preparation
Diane Saunders, Marcela Brissova, Alvin C. Powers, Conrad Reihsmann
Disclaimer
This protocol is adapted from the CODEX User Manual, revision C (Akoya Biosciences, Dec. 2020). For a quick reference and overview, see FF Tissue Staining and Reporter Plate Preparation. Although the protocol is designed for flash frozen (unfixed) tissue, we have validated that it also works for lightly PFA-fixed tissue.
Abstract
This protocol describes the staining and pre-imaging preparation currently in use by the Vanderbilt Diabetes Research Center Islet & Pancreas Analysis (IPA) Core and Powers/Brissova Research Group prior to performing multiplexed imaging of the human pancreas using the CO-Detection by indEXing (CODEX®) platform (now PhenoCycler™; Akoya Biosciences). See also CODEX® Multiplexed Imaging | Modality overview.
Steps
Tissue Preparation
Prepare pre-staining reagents (per individual coverslip):
-
15-mL beaker with
10mLacetoneNoteAfter acetone fixation, all incubations and washes in this portion of the protocol are done in 6-well plates using approximately 3mL of reagent per well (one well per coverslip). If using an alternative container, this volume must be sufficient to submerge the coverslip. -
2 wells Hydration Buffer
-
1 well Pre-staining Fixation Solution (1.6% PFA in Hydration Buffer)
-
1 well Staining Buffer
Remove coverslip from freezer and place face up in container with layer of0h 2m 0s to dry.
Place coverslip into an individual 10-mL beaker containing acetone , making sure entire tissue area is submerged. Incubate for . 0h 10m 0s.
Carefully remove coverslip from beaker and place back into Drierite container for another . 0h 2m 0s.
Transfer coverslip to 6-well plate and perform two washes in 3 mL Hydration Buffer , incubating for 0h 2m 0s each time. If staining more than one sample at a time, make sure coverslip order/orientation in 6-well plates is kept consistent so you don't lose track of samples.
From second well of Hydration Buffer, transfer coverslip to well containing 3 mL Pre-Staining Fixation Solution (1.6% (v/v) PFA in Hydration Buffer) and incubate for . 0h 10m 0s.
Wash coverslip twice in 3 mL Hydration Buffer to remove fixative. No incubation is required, and aliquots from step 5 can be reused.
Incubate coverslip in 3 mL Staining Buffer for 0h 20m 0s. Place 6-well plate onto a fixed-angle rocker for the incubation period.
Primary Antibody Stain
Prepare CODEX Blocking Buffer for primary antibody cocktail:
- Staining Buffer -
91% (v/v) - N/G/J/S blockers -
2.4% (v/v)each
| A | B |
|---|---|
| Total number of samples | 1 |
| Total volume (μl) | =210*B1 |
| Staining Buffer (μl) | =B2-(B4+B5+B6+B7) |
| N blocker (μl) | =B1*5 |
| G blocker (μl) | =B1*5 |
| J blocker (μl) | =B1*5 |
| S blocker (μl) | =B1*5 |
Table 1: Blocking Buffer. Copy and paste all cells above into an Excel sheet, then enter value into cell B1. The volumes for each reagent will automatically be returned in cells B2-B7.
Calculate required amounts for each primary antibody (based on optimized dilution) as well as the total antibody volume. Remove the total volume from the Blocking Buffer , then add primary antibodies. Vortex gently.
ⓘ Preconjugated antibody dilutions: PhenoCycler Antibody Dilutions
Place coverslip face up in humidity chamber (can use an empty pipet tip box with damp paper towel in the bottom; coverslips rest on the removable insert). Carefully pipette 200µL of antibody cocktail solution on top, making sure solution pools to cover the entire tissue area.
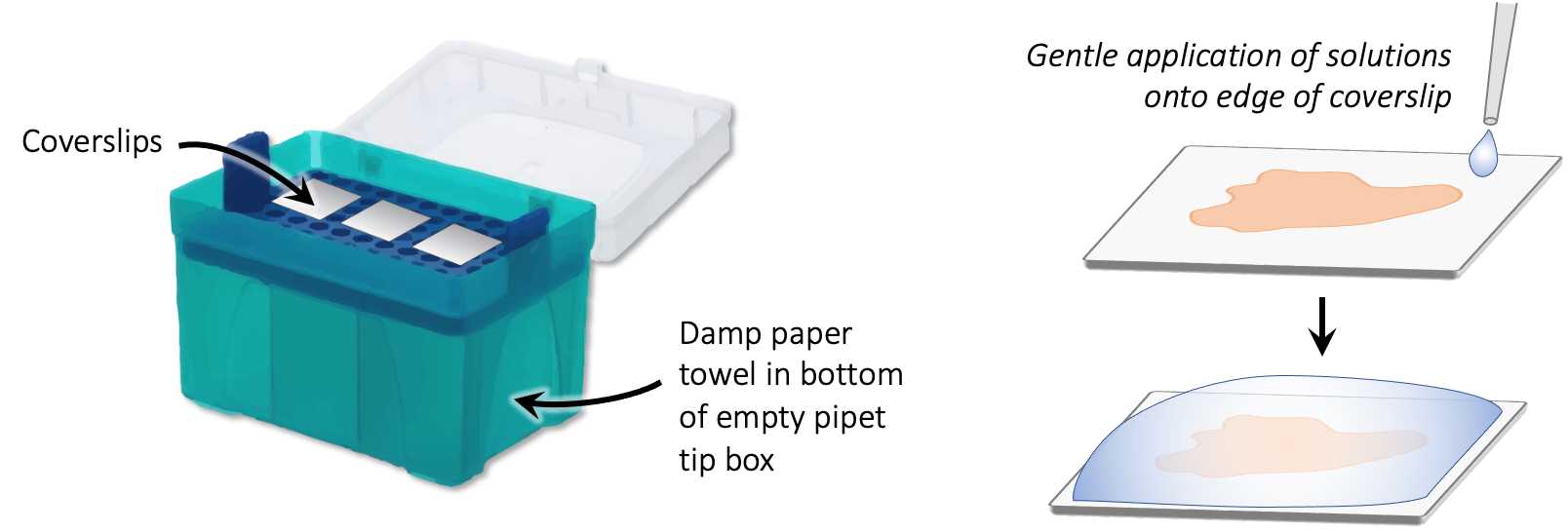
Incubate at Room temperature for . 3h 0m 0s.
Post-Stain Procedure
Prepare post-staining reagents (per individual coverslip):
- 1 well Post-staining Fixation Solution (1.6% PFA in Staining Buffer)
- 2 wells Staining Buffer
- 1 well methanol
On ice - 3 wells 1X DPBS
Transfer coverslip from humidity chamber to 6-well plate and perform two washes in 3 mL Staining Buffer , incubating for 0h 2m 0s each time.
From second well of Staining Buffer, transfer coverslip to well containing 3 mL Post-Staining Fixation Solution (1.6% (v/v) PFA in Staining Buffer) and incubate for . 0h 10m 0s.
Wash coverslip three times in 3 mL DPBS to remove fixative. No incubation is required.
Incubate coverslip in 3 mL cold methanol On ice for 0h 5m 0s.
Wash coverslip three times in 3 mL DPBS to remove methanol. No incubation is required, and aliquots from step 16 can be reused.
Prepare Final Fixative (2% (v/v) Fixative Reagent in DPBS).
⚠ Thaw Fixative Reagent right before use and discard any unused volume. One tube provides adequate volume to fix up to 5 samples.
Place coverslip face-up in humidity chamber and carefully pipette 200µL of Final Fixative onto coverslip, making sure it covers the entire tissue area. Incubate at Room temperature for 0h 20m 0s.
Wash coverslip three times in 3 mL DPBS to remove fixative. No incubation is required, and aliquots from steps 16/18 can be reused.
Transfer coverslip into 3 mL of Storage Buffer and label 6-well plate. Add a piece of parafilm before placing lid to ensure solution does not evaporate. Store at 4°C for up to 2 weeks. Label the top of the plate with Sharpie or tape to keep track of sample(s).
Preparation of Reporter Plate
Gather supplies and reagents:
Prepare Reporter Stock Solution:
- Nuclease-free water -
81.33% (v/v) - 10X CODEX Buffer -
10.00% (v/v) - Assay Reagent -
8.33% (v/v) - CODEX Nuclear Stain -
0.33% (v/v)
| A | B |
|---|---|
| Total number of samples | 3 |
| Reporter cycles per sample | 10 |
| Total volume (mL) | =(B1*(B2+2))*.250 |
| Nuclease-free water (mL) | =B3-((B5+B6+B7)/1000) |
| 10X CODEX buffer (μl) | =ROUNDDOWN((B3*1000)/10,0) |
| Assay Reagent (μl) | =ROUNDDOWN((B3*1000)/12,0) |
| CODEX Nuclear Stain (μl) | =ROUNDDOWN((B3*1000)/300,0) |
Table 2: Reporter Stock Solution. Copy and paste all cells above into an Excel sheet, then enter values into cells B1-B2. The volumes for each reagent will automatically be returned in cells B3-B7.
⚠ Ensure you prepare adequate Reporter Stock Solution to account for Blank cycles (no reporters); formulas in Table 2 reflect this.
Label one 1.5 mL light-blocking microcentrifuge tube for each reporter cycle and aliquot Reporter Stock Solution (250µL per sample). You should have Reporter Stock Solution remaining (for Blank cycles) after aliquoting.
Prepare reporter mixes by adding appropriate reporters to microcentrifuge tubes (5µL per sample for each reporter):
| A | B |
|---|---|
| Total number of samples | 3 |
| Total volume Reporter Stock Solution (μl) | =250*B1 |
| Reporter for channel 1 (μl) | =5*B1 |
| Reporter for channel 2 (μl) | =5*B1 |
| Reporter for channel 3 (μl) | =5*B1 |
Table 3: Reporter mixes (per cycle). Copy and paste all cells above into an Excel sheet, then enter value into cell B1. The volumes for each reagent will automatically be returned in cells B2-B5.
⚠ Make sure to vortex reporter tubes briefly before adding to reporter mix.
Mix contents of tubes by gently pipetting up and down with pipette or by brief vortex. Spin tubes using benchtop microcentrifuge to collect solution at bottom of tubes.
Add 250µL of reporter mix to each corresponding well in a light-protected 96-well plate, making sure to leave blank wells before and after reporter mixes. Fill these wells (corresponding to Blank cycles) with 250µL Reporter Stock Solution (no reporters in solution).
<img src="https://static.yanyin.tech/literature_test/protocol_io_true/protocols.io.n92ldzro9v5b/hjrqbh5qp0_update.jpg" alt="Figure 2. Schematic of 96-well Reporter Plate. In this example a total of 24 primary antibodies will be visualized in eight imaging cycles, with additional cycles before (#1) and after (#10) serving as "Blank" cycles with DAPI only. After reporter mixes are made up in tubes, each mix (labeled by its cycle number, #2-9) is transferred to its respective well (B2-B9). Reporter Stock Solution is added to wells B1 and B10 for Blank cycles." loading="lazy" title="Figure 2. Schematic of 96-well Reporter Plate. In this example a total of 24 primary antibodies will be visualized in eight imaging cycles, with additional cycles before (#1) and after (#10) serving as "Blank" cycles with DAPI only. After reporter mixes are made up in tubes, each mix (labeled by its cycle number, #2-9) is transferred to its respective well (B2-B9). Reporter Stock Solution is added to wells B1 and B10 for Blank cycles."/>
Seal plate with foil plate seal and store at 4°C until imaging.

