2,4-dinitrophenylhydrazine alpha-ketoglutarate detection assay for Prolyl Hydroxylase Domain (PHD) proteins
sjwong
Abstract
The 2,4-dinitrophenylhydrazine (2,4-DNPH) alpha-ketoglutarate detection assay was developed to support the study of prolyl hydroxylase domain (PHD) proteins in a substrate-independent manner. This protocol was extensively optimized for the PHD protein reaction, and is applicable to the study of enzyme kinetics or to high-throughput screening.
Attachments
Steps
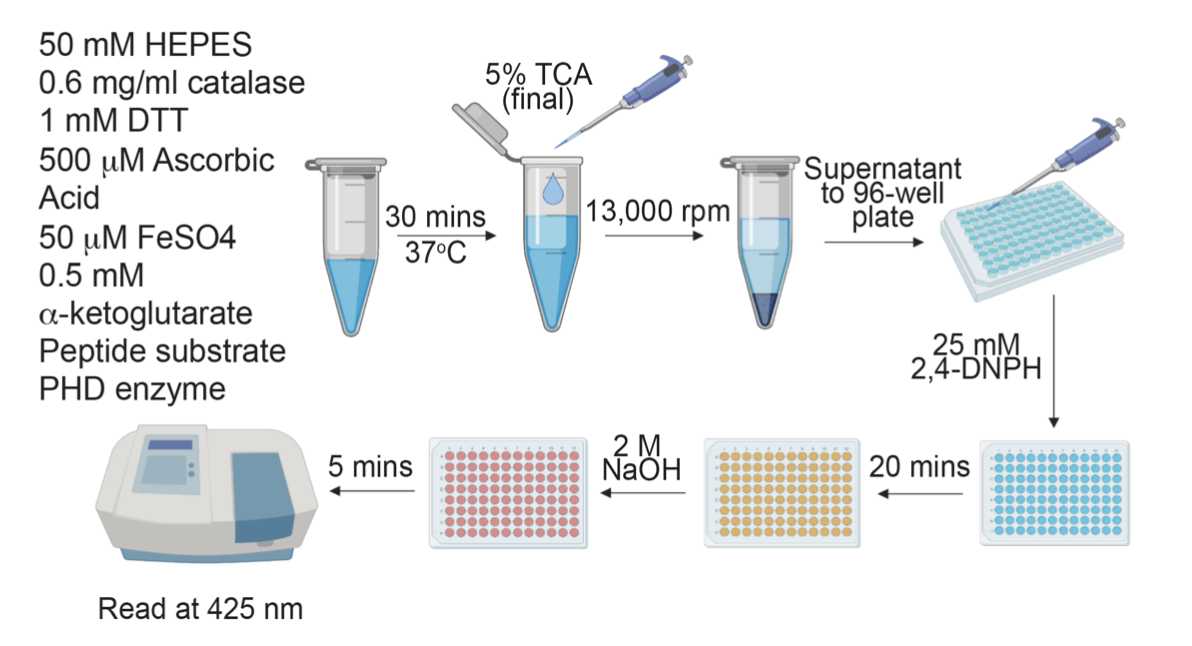
Overview of assay schematic
In vitro hydroxylation assay
Prepare 5 Eppendorf tubes containing 50 µl of 10% TCA.
- Label tubes: 0 min, 1 min, 2 min, 5 min, 15 min.
Prepare cofactor solution containing HEPES/MES, catalase, DTT, ascorbic acid, FeSO4, a-ketoglutarate, and peptide in a 150 µl volume in an Eppendorf tube (using the working concentrations).
Add 150 µl of 20 µM PHD enzyme into the cofactor solution.
Vortex briefly.
Place into a 37 oC tabletop shaking incubator and start the timer (counting up).
- This step equilibrates the temperature of the reaction to 37 oC
At T = 1 min on the timer, withdraw 50 µl of the reaction solution and quench in the “0 min” tube containing 10% TCA, and replace the reaction tube in the incubator.
Repeat this for the other time points.
- At T = 2 min, withdraw 50 µl of the reaction solution and quench in the “1 min” tube
- At T = 3 min, withdraw 50 µl of the reaction solution and quench in the “2 min” tube
- At T = 6 min, withdraw 50 µl of the reaction solution and quench in the “5 min” tube
- At T = 16 min, withdraw 50 µl of the reaction solution and quench in the “15 min” tube
Briefly vortex the quenched reactions.
Keep the quenched reactions at 4 oC until ready for downstream processing.
- Reactions have been stored up to 3 days with no loss of signal.
Color development with 2,4-DNPH
Centrifuge the quenched reactions at 13,000 rpm for 15 minutes.
Meanwhile, add 10 µl of 0.5 M sodium phosphate to 5 wells of a 96-well plate.
Transfer 90 µl of the supernatant of the quenched reaction to a well containing 10 µl of 0.5 M sodium phosphate (VT = 100 µl).
- Do the same for the other 4 quenched supernatants.
Using a multi-channel pipette, add 100 µl of 50 mM 2,4-DNPH to the wells (VT = 200 µl). Pipette up and down gently to mix.
Leave at room temperature for 20 minutes.
Using a multi-channel pipette, add 50 µl of 6 M NaOH to the wells (VT = 250 µl). Pipette up and down gently to mix.
Leave at room temperature for 5 minutes.
Read at 425 nm on a spectrophotometer.
Data handling
Calculate the amount of a-ketoglutarate consumed from a standard curve processed in the same way as the samples.
The initial rate should be taken as the linear portion of the curve. In this case, from T = 0 to 2 mins.