Sidoli Phosphoenrichment From Clean Dry Peptides
Edwin Yoo
Published: 2023-02-20 DOI: 10.17504/protocols.io.n2bvj8jxngk5/v1
Abstract
Sidoli Phosphoenrichment From Clean Dry Peptides
Steps
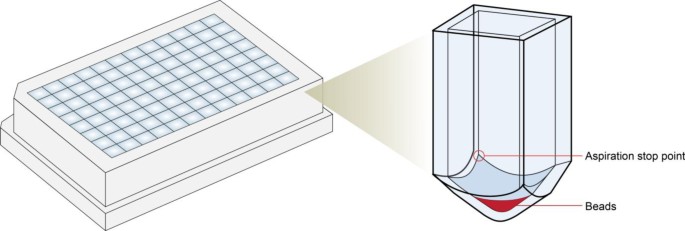
1. Can do this protocol carefully on deep well 96 well plate to multiplex. Need to aspirate supernatant from bottom corner of the deep well plate as to avoid aspirating titanium.
Obtain trypsinized peptides that are clean and dried (ie. completed desalting or S-trap).
Note
2.
- Dilute your peptide solution at least 10 times with loading buffer (alternatively if you have 100 μL sample you can add 50 μl water, 50 μL 100% TFA, 800 μL Acetonitrile and 76 mg Glycolic acid to make the sample up to the proper loading buffer). If completely dry, can just resuspend with 100 ul loading buffer.
3.
4.
- Place the tubes on the shaker (highest shaking) at room temperature for 5-10 min - Centrifuge to pellet the beads (table centrifuge <15 sec)
5.
- Remove the supernatant
6.
- Wash the beads with 70-100 μL loading buffer - mix for 15 sec, transfer to another eppendorff tube and then centrifuge to pellet the beads (the transfer is performed due to the fact that peptides and phosphopeptides stick to surfaces and can be eluted in the last elution step)
7.
- Wash with 70-100 μL washing buffer 1 - mix for 15 sec and then centrifuge to pellet the beads. Remove supernatant.
8.
- Wash with 50-100 μL washing buffer 2 - mix for 15 sec and then centrifuge to pellet the beads. This step is important to remove peptides that bind in a HILIC mode to TiO2. Remove supernatant.
9.
- Dry the beads for 5-10 min in the vacuum centrifuge or on the table.
10.
- Elute the phosphopeptides with 50 μL Elution buffer – mix well and leave the solution and the beads for 5-10 min to allow an efficient elution
11.
- Centrifuge the solution for 1 min, recover the supernatant (as it contains the phosphopeptides) and dry it in a SpeedVac centrifuge. Ready for injection.