Protocol for detection of Salmonella Typhi and Salmonella Paratyphi A in Produce
Renuka Kapoor, Ashutosh Wadhwa, Christine Moe
Abstract
The protocol describes method for qualitative detection (presence/ absence) of Salmonella Typhi and Salmonella Paratyphi A on produce by enrichment culture followed by real-time PCR.
Steps
1. Processing of produce sample
The following steps describe processing of produce samples up to enrichment stage.
Put on gloves and spray hands with 70% ethanol and rub hands together to sanitize all surfaces of the gloves.
Shake the bag again for 0h 0m 30s.
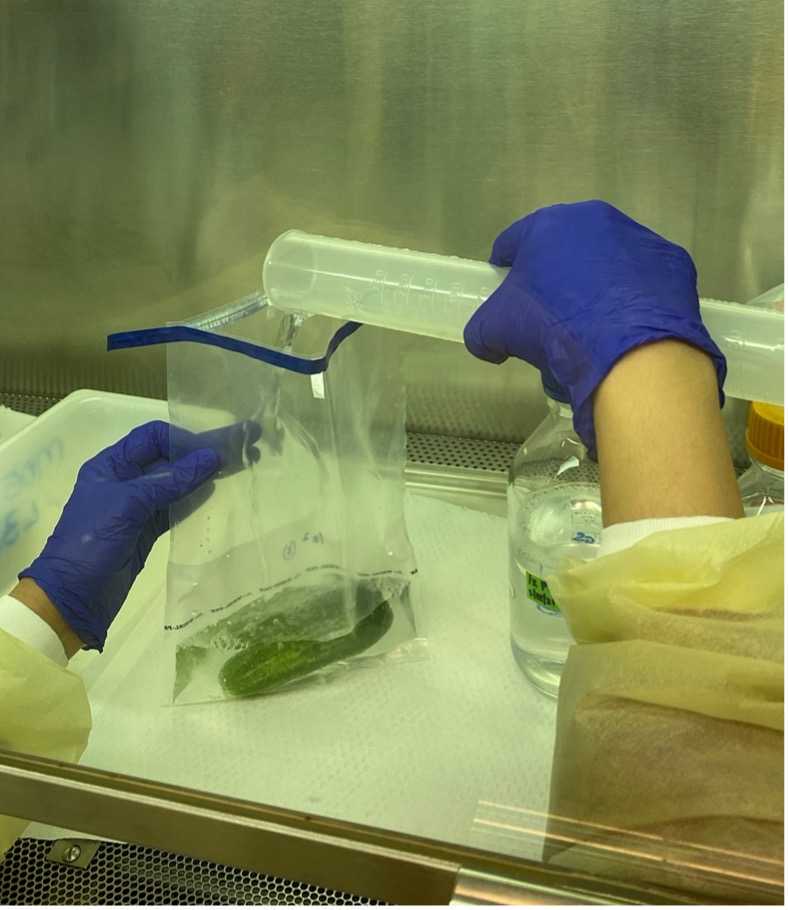
Open the Whirl-Pak bag.
Close the Whirl-Pak bag containing produce wash.
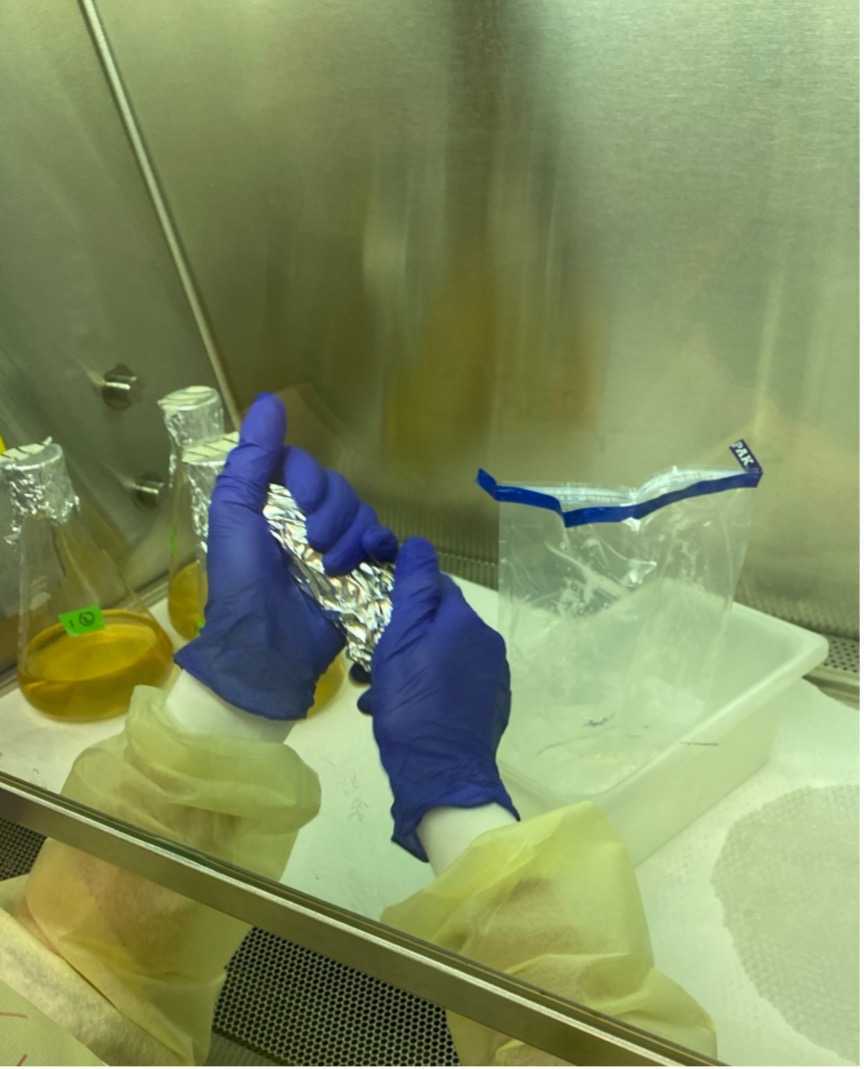
Weigh the produce (using aluminum foil) and record the weight.
Clean your work surface with 70% ethanol. Steps 3-6 should be done inside a biosafety hood.
Spray the outside of the Whirl-Pak bag containing the sample with 70% ethanol and rub it well.
Incubate the bag for 0h 10m 0s at 37°C.
2. Enrichment culture
The following steps are for optional enrichment of the sample. If enrichment is not performed, the produce wash can be used directly for membrane filtration and DNA extraction (Section 3).
Fill a 1 liter flask with 450mL of Universal Pre-enrichment (UP) Broth (USEPA Standard Analytical Protocol for Salmonella Typhi in Drinking Water).
3. DNA Extraction
The following steps are for membrane filtration and DNA extraction. They can be performed following step 1.15 or step 2.3.
Clean your workspace and set up your filter units.
Proceed with the DNA Extraction according to manufacturer’s protocol (Qiagen DNeasy PowerWater kit).
Allow the sample to filter until liquid is no longer visible on the filter.
4. Real-time PCR
Test DNA extracts for S . Typhi and S . Paratyphi A using Taqman-based quantitative real-time PCR (qPCR) platform.
Detection of S . Typhi
S . Typhi is detected using duplex PCR protocol developed by researchers at the University of Washington (Scott Meschke and team) usIng primers and probes targeting the tviB and staG genes (Nair et al., 2019).
tviB _F 5’TGTGGTAAAGGAACTCGGTAAA-3’;
tvi B_R 5’-GACTTCCGATACCGGGATAATG-3’;
tvi B_P HEX - TGGATGCCGAAGAGGTAAGACGAGA-BHQ1;
sta G ___ F 5’- CGCGAAGTCAGAGTCGACATAG-3’;
sta G ___ R 5’-AAGACCTCAACGCCGATCAC-3’;
sta G ___ P FAM-5’-CATTTGTTCTGGAGCAGGCTGACGG-3’-BHQ1
The reaction mixture contain 0.65 µl of tviB_F (20µM), 0.75 µl each of tviB_R (20µM), staG_F(20µM),and staG_R (20µm), 0.5 µl each of the probe tviB_P (10µM) and staG_P (10 µM), 12.5 µl of SsoAdvanced Universal Probes Supermix (Bio-rad), and 5 µl of DNA in a final volume of 25 µl. The PCR reaction conditions include initial denaturation at 95oC for 5 min, followed by 45 cycles of 95oC 30 sec, 64oC 30 sec, 72oC 10 sec, and final extension at 72oC for 5 min.
Detection of S . Paratyphi A
S . Paratyphi A Is detected using primers and probe targeting SPA2308 (Nga et al., 2010)
SPA2308_F 5’- ACGATGATGACTGATTTATCGAAC-3’;
SPA2308_R5’-TGAAAAGATATCTCTCAGAGCTGG-3’;
SPA2308_PCY5 - CCCATACAATTTCATTCTTATTGAGAATGCGC-BHQ2
The reaction mixture containing 1 µl of each primer (10µM), 0.4 µl of probe (10µM), 200µM of dNTPs, 5mM of MgCl2, 5U of HotStar Taq DNA polymerase (Qiagen), and 5 µl of DNA in a final reaction volume of 25 µl. The PCR reaction conditions include initial denaturation at 95oC for 5 min, followed by 45 cycles of 95oC 30 sec, 60oC 30 sec, 72oC 30 sec, and final extension at 72oC for 10 min.