Short-Term Freezing of Anopheles stephensi (Mosquito) Larvae
Michael C. Young1, Jackson Watkins, Gabriela Ramirez, Emily N. Gallichotte, MaKala Herndon, Elisha Xiao-Kim, Madison Stoltz, Maria Alexandra Marquez, Gaukhar Iskakova, Jennifer Barfield, Karen M. Dobos, Gregory D. Ebel
Disclaimer
DISCLAIMER – FOR INFORMATIONAL PURPOSES ONLY; USE AT YOUR OWN RISK
The protocol content here is for informational purposes only and does not constitute legal, medical, clinical, or safety advice, or otherwise; content added to protocols.io is not peer reviewed and may not have undergone a formal approval of any kind. Information presented in this protocol should not substitute for independent professional judgment, advice, diagnosis, or treatment. Any action you take or refrain from taking using or relying upon the information presented here is strictly at your own risk. You agree that neither the Company nor any of the authors, contributors, administrators, or anyone else associated with protocols.io, can be held responsible for your use of the information contained in or linked to this protocol or any of our Sites/Apps and Services.
Abstract
Mosquito insectaries must be maintained around the clock without interruption, due to the lack of preservation procedures. Long-term maintenance of mosquito colonies can lead to population bottlenecks, laboratory adaptation, loss of genetic
markers, etc., leading to colonies that are attenuated and different from their wildtype counterparts. Cryopreservation is an essential tool to combat these challenges that has been developed in other insects, including silkworms, fruit flies, and honey bees. Unfortunately, there is no robust method of cryopreservation for mosquitoes. This protocol details the short-term freezing of Anopheles stephensi (Mosquito) larvae; an initial step towards establishing a cryopreservation method for mosquito larvae.
Attachments
Steps
Phase I: Establish and maintain mosquito colony
Phenotypic Analysis of developing mosquitoes (Figure 1)
- Larvae develop from eggs to first instar larvae (L1) around 2-3 days after transferring egg paper.
- L1 develop into L2/L3 over the next 4 days (approximately). During that time, they show the ability to hatch, feed and further develop. L4 transition into pupa and emerge as adults
approximately 2 days after pupation
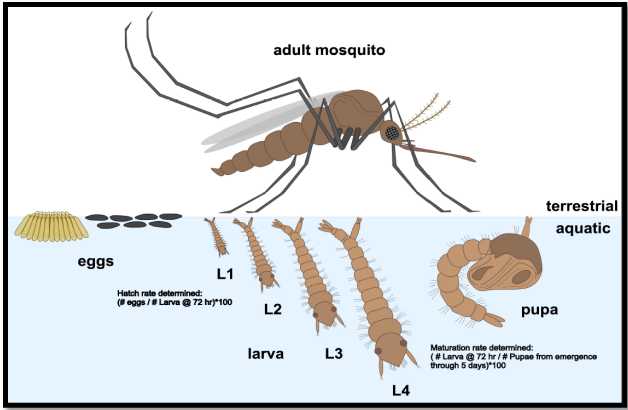
Husbandry (modified from MR4 Methods in Anopheles Research. MR4 Methods in Anopheles Research Laboratory Manual, BEI Resources, 2015) )
1. Hatch eggs
- Obtain egg paper with referenced eggs from BEI or other qualified source.
- Take clean small hatch bin, label with date, and fill with ~
500mLof distilled water. Dispense water slowly to avoid bubbles (eggs will hatch asynchronously if bubbles are present). - Put on gloves and place the egg paper into the water.
- Add a pinch of fish food and cover the bin.
- Place bin in insect chamber and maintain under controlled conditions of temperature (
28°C), relative humidity (70%) and light (12:12 L:D diurnal cycle).
2. Larvae splitting and feeding
- Slowly fill a large hatch bin with water. Tap or distilled water can be used.
- Move larvae into a larger bin using a strainer, roughly 24 hours after eggs are hatched.
- Make sure to thoroughly rinse out the small bin where eggs were hatched to ensure all larvae are transferred to the large bin.
- For the following days, make sure that bins are not over saturated with larvae (~60% saturation), or else the larvae will stop maturing; split larvae between current bin and new bin as much as necessary.
- Add food and cover bin to any new bins prepared.
- Changing the water may be necessary to avoid mold and other microbial growth; Use strainers and transfer larvae into a fresh bin if necessary.
3. Collecting pupae
- Pour an ounce of tap water into a clear, plastic, disposable 2oz (about 59.15 ml) cup.
- Use a transfer pipette or a small mesh-made-scoop to collect the pupae into the cup.
- Transfer the cup into an insect rearing cage by placing cup through top port. Add sugar and water cups through the port.
- Males will emerge first, so it is important to pick up pupae every day for several days (~
120h 0m 0s) to ensure females emerging from pupae are also picked.
4. Blood feeding mosquitoes (glass feeder protocol)
- The optimal time to blood feed mosquitoes is 3-5 days post-eclosion.
- Remove sugar and water 12-16 hrs prior to blood feed.
- Fill water basin with tap water and attach heating element/pump. Turn on and pre-warm water to
37°C–42°C. - Prepare glass feeders. Use scissors to cut the length of parafilm or hog gut sufficient to cover mouth of glass feeder.
- Briefly turn off the water pump and use plastic tubing to connect all feeders in a circuit that leads back into the water bath. Wet each end of each piece of plastic tubing before use to ensure easier fit. Turn pump back on and ensure A) a steady flow of water around circuit and B) no dripping. Do not, however, place feeders on top of rearing cages yet, as mosquitoes may begin to drink and fill up on water.
- Briefly turn off the water pump and use plastic tubing to connect all feeders in a circuit that leads back into the water bath. (Wet each end of each piece of plastic tubing before use to ensure easier fit.) Turn pump back on and ensure
- a steady flow of water around circuit and
- no dripping. Do not, however, place feeders on top of cartons yet, as mosquitoes may begin to drink and fill up on water.
- Use transfer pipette to measure appropriate amount of blood into well of glass feeder.
1mL-2mLper feeder is more than enough. - Place feeders on top of cartons and begin feeding. Optional: tear off a long strip of tape to hold glass feeder firmly in place against roof of mosquito carton.
- Visually inspect mosquitoes to see if they are feeding. Look for females clustered around feeder with bright red full bellies. Ensure that the temperature of the water is approximately
37°C. Some variability in temperature is permissible but note that parafilm may melt/deform if bath is run too hot. - Allow mosquitoes to feed for up to
1h 0m 0s. Return all mosquito cartons to insectary and disassemble water bath apparatus. - Let glass feeder and membrane soak in 10% bleach solution for at least
0h 15m 0s. Dispose of membrane and rubber band in trash. Wash glass feeder and let dry. - Eggs are laid roughly 48 hrs after the blood meal. Collect eggs onto filter paper and continue the process to maintain colony.
C. Obtaining and counting larvae
At 16- 18 hrs post-egg hatching, remove An. stephensi larvae hatch bin.
Transfer a small amount of water from the hatching habitat to a clean bin lid.
Cut the tip of a plastic bulb pipette with scissors, approximately 1cm above the tip, to widen the inlet area of the pipette.
Remove 7-20 larvae at a time by pipetting, transfer to water on clean bin lid, and count the larvae collected into the water drops. Continue quickly, until all larvae are counted, or the number needed for experiments are collected.
Transfer to a clean bin lid containing hatch bin water.
After larvae are counted, transfer them back to the original habitat, along with the water from the new hatch bin.
D. Phenotypic Characterization of Larvae at each Instar by Advanced Stereo Microscope (Larval Microscopy) )
Cut the tip of a plastic bulb pipette with scissors, approximately 1cm above the tip, to widen the inlet area of the pipette.
Remove 5-7 larvae from their hatch bin, and transfer in one drop to a glass microscope slide.
Analyze larvae using an Olympus SZX16 Advanced Stereo Microscope, at variable magnification settings and determine (a tabulation might help):
- Microscope settings
- Photographic and descriptive phenotypic characterization of larvae at each instar:
- Number of larvae at first instar (L1)
- Characteristics of L1 larvae and on through pupation
Phase 2: Freezing and Recovery of L1 Larvae
Prepare cryoprotectants (or Cryoprotective Agents, CPAs) in nano pure water Room temperature and determine toxicity of cryoprotectants to the L1 larvae. Potential cryoprotectants are listed below.
Potential Cryoprotectants
1.5Molarity (M)ethylene glycol (EG),1.5Molarity (M)methanol (ML),1.5Molarity (M)dimethyl sulfoxide (DMSO), or1.5Molarity (M)methyl acetamide or methyl formamide (MA or MF)
B. Determination of the Toxicity of Cryoprotectants
Collect 50 or more L1 larvae from the insectary bin using a transfer pipette with a blunt end and transfer onto a filter dish.
Lift filter basket with larvae out of the water, blot the sides of the basket to remove excess water, and submerge the basket into a Petri dish containing 1.5Molarity (M) EG, 1.5Molarity (M) ML, 1.5Molarity (M) DMSO, or 1.5Molarity (M) MA/MF, or water alone (control) and hold for 1, 2, 3, 4, 5, 6, or 8h 0m 0s at Room temperature(22°C-25°C).
After the appropriate exposure time, lift the basket out of the respective CPA dish and rinse larva by immersion in tap water while they remain in the filter basket.
Repeat step three two more times, or empirically based on loss of glossy sheen on the L1 larvae.
Culture the L1 larvae in water with fish food (as described in Step 2.1) and assess for survival based morphological changes from L1-L4 larvae.
Determine the percent survival (Percent survival = # of pupae collected/initial egg count) for each time point for a given CPA solution.
Record a minimum of three replicates for each CPA to assess toxicity.
C. Freezing larvae
In this example, a mixture of 7Molarity (M) methyl formamide (MF) in 0.5Molarity (M) of trehalose was chosen as the optimum CPA. Further, our studies with newly emerged versus late L1 stage larvae supported using 14–24-hour old L1 larvae for freezing. A diagram of the protocol for this process is provided ( Figure 2 ).
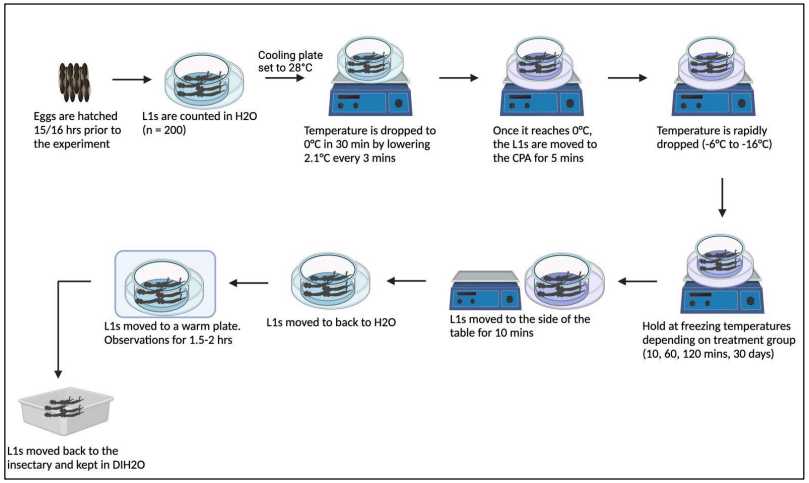
Prepare the CPA solution of 7Molarity (M) MF in 0.5Molarity (M) trehalose in a petri dish and place dish on cooling table set at 28°C.
Place 14-20 hr emerged L1 larvae in clean water. Count larvae.
Transfer larvae to a nylon mesh basket submerged in clean water that is in a square culture dish set on the cooling table. Use ~ 200 L1 larvae per condition group for optimum data sets.
Begin slow cooling on the cooling plate from 28°C to 0°C over 0h 30m 0s.
Lift the basket from the water, blot dry the sides of the mesh basket to remove excess water, and transfer the basket to the Petri dish containing the CPA solution cooled to 0°C. Hold for 0h 5m 0s at 0°C
Begin rapid cooling of L1 Larvae on cooling table, and cool to -15°Cusing a rate of 0.65-0.7 degrees / min.
Leave L1 larvae on cooling table (10 min – 2 hr), or transfer to -20°C freezer (longer time periods) until warming.
D. Warming larvaae
The current warming method is described below. We note that additional warming trials may need to be conducted for further optimization; viability using this method and maintaining freezing conditions for 0h 10m 0s is 60%.
Remove the petri dish from the cooling table, and hold on a shelf in the refrigerator at 4°Cfor 0h 10m 0s.
Remove the mesh basket and submerge into a petri dish containing water held at 4°C and incubate for an additional 0h 5m 0s.
After this time, collect the L1 larvae using a clipped transfer pipette and transfer them to a square dish with water held at 28°C for 1-2h 0m 0s. Perform initial viability assessment by watching for movement (including mid-gut movement) using a dissecting microscope.
Return L1 larvae to husbandry conditions for recovery and development through life stages ( Figure 1 ).
Determine recovery and analysis of viability as described below.
E. Viability studies
Determine the number of pupae that emerge from each freezing incubation time point; calculate the percent recovery as done for percent survival, based on number of L1 larvae from each time point.
Determine the percentage of recovered pupae that survive and develop into adults.
Determine the ratio of males to females (sexual differentiation) in adult mosquitoes.
Perform phenotypic similarity analyses (via microscopy; section I.C. of this protocol)
Determine the number of eggs subsequently produced from adults maturing after recovery from cryopreservation.
Determine the number of larvae (F2) hatching from eggs produced by adults that matured after recovery from freezing to confirm the colony has returned to healthy, normal husbandry conditions.

