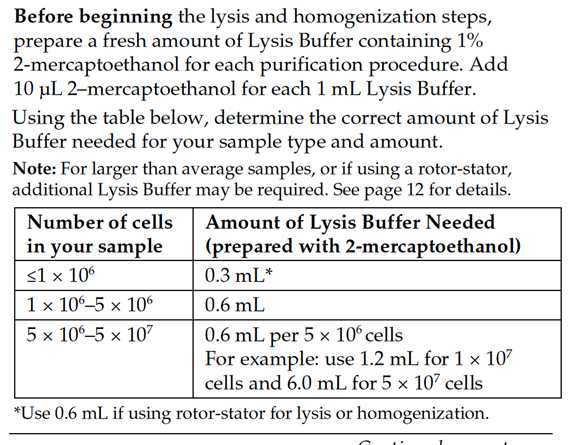
RNA extraction using the PureLink® RNA Mini Kit
Enrico Bagnoli, Miratul Muqit
Abstract
This Protocol details the extraction of total RNA using the PureLink® RNA Mini Kit.
Steps
Prepare lysates from ≤5 × 106 monolayer cells:
Remove the growth medium from the cells.
Proceed with one of the following homogenization options atRoom temperature:
Transfer the lysate to a homogenizer inserted in a collection tube and centrifuge at 12000x g. Remove the homogenizer when done, or
Transfer the lysate to a 1.5 mL RNase–free tube and pass 5–10 times through an 18-21 gauge needle attached to an RNase-free syringe
Centrifuge the homogenate at ∼ , then transfer the supernatant to a clean RNase-free tube 2600x g, then transfer the supernatant to a clean RNase-free tube
Transfer the lysate to an appropriately sized RNase-free tube and homogenize using a rotorstator homogenizer at maximum speed for at least 0h 0m 45s.
Centrifuge the homogenate at ∼2600x g, then transfer the supernatant to a clean RNase-free tube.
Proceed to Binding, Washing, and Elution
Add one volume 70% ethanol to each volume of cell homogenate (prepare the sample as described in specific protocols.
Vortex to mix thoroughly and to disperse any visible precipitate that may form after adding ethanol.
Transfer up to 700µLof the sample (including any remaining precipitate) to the Spin Cartridge (with the Collection Tube).
Centrifuge at 12000x g .
- Discard the flow-through, and reinsert the Spin Cartridge into the same collection tube.
Repeat Steps 6–7 until the entire sample is processed.
Add 700µL wash buffer I to the Spin Cartridge.
- Centrifuge at
12000x g. Discard the flow-through and the Collection Tube. - Place the Spin Cartridge into a new Collection Tube.
Add 500µLWash Buffer II with ethanol to the Spin Cartridge.
Centrifuge at 12000x g.
- Discard the flow-through and reinsert the Spin Cartridge into the same collection tube.
Repeat Steps 10–11 once.
Centrifuge the Spin Cartridge at 12000x g,0h 0m 0s for 0h 1m 0s-0h 2m 0s to dry the membrane with attached the RNA. Discard the collection tube and insert the Spin Cartridge into a recovery tube.
Add 30µL–3 × 100µL RNase–Free Water to the center of the Spin Cartridge (see Elution Parameters).
Incubate at Room temperature for 0h 1m 0s.
Centrifuge the Spin Cartridge for 0h 2m 0s at ≥ 12000x g,0h 0m 0s to elute the RNA from the membrane into the recovery tube.
Store your purified RNA or proceed to Analyzing RNA Yield and Quality or to DNase I Treatment after RNA purification.