Prepare agarose pad for microscopy (2022 iGEM)
Team Fudan iGEM
Abstract
This protocol describe the steps to perform fluorescence microscopy for E. coli
Before start
Culture cells in liquid media
Steps
Cell growth
Grow cells to late-log phase density (e.g. OD600 > 0.6).
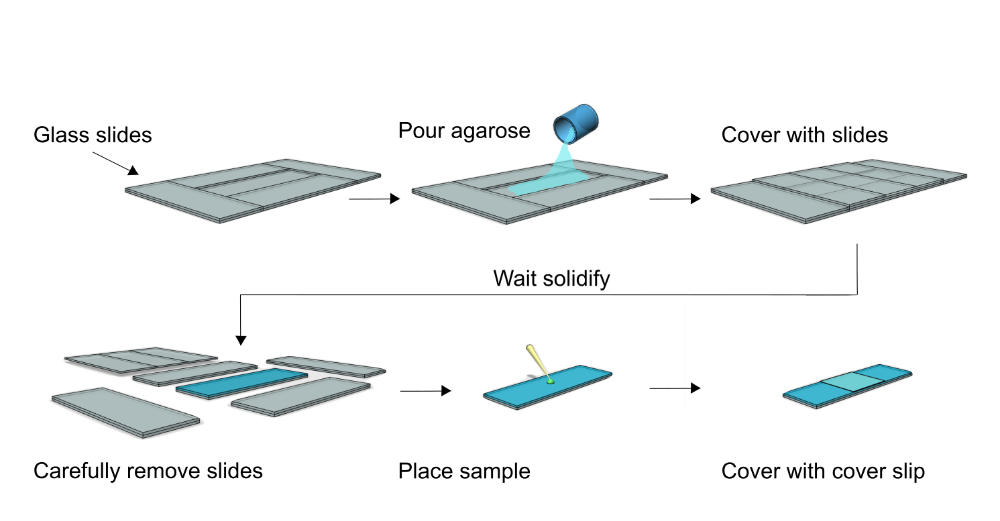
Agarose pads
Prepare a glass slide with a double adjacent slides barrier (see below), forming a wall around the pad slide. Add slides on top of the poured agarose solution (1-1.2 mL) to allow formation of a smooth surface. It takes time for 1% agarose solution to cool down to ~40 degree, and the solution is viscous but not runny.
Wait for 45-60 minutes. Carefully remove adjacent and top slides, leaving the bottom slide.
Cut the pad into 15x15 mm square pierces, and carefully transfer the pad pierces to other cleared slide (left one agarose pad on the existing glass slide, if it was clean).
Add 2-5 µL bacteria culture onto the agarose pad, and cover it was a 18x18 mm #1.5 square coverslip. Seal with wax if needed.
Microscopy GFP
Place the slide onto the microscope stage, with the coverslip facing the objective.* Use a laser at 488 nm to excite GFP.
- Record images.
Analyze the images. For our microscope with 150x objective, the pixel size is 0.0806 µm.