Preparation of large biological samples for high-resolution, hierarchical, synchrotron phase-contrast tomography with multimodal imaging compatibility
J. Brunet, C. L. Walsh, W. L. Wagner, A. Bellier, C. Werlein, S. Marussi, D. D. Jonigk, S. E. Verleden, M. Ackermann, Peter D. Lee, Paul Tafforeau
multimodal imaging
large biological samples
synchrotron phase-contrast tomography
dehydration
mounting protocol


















Extended
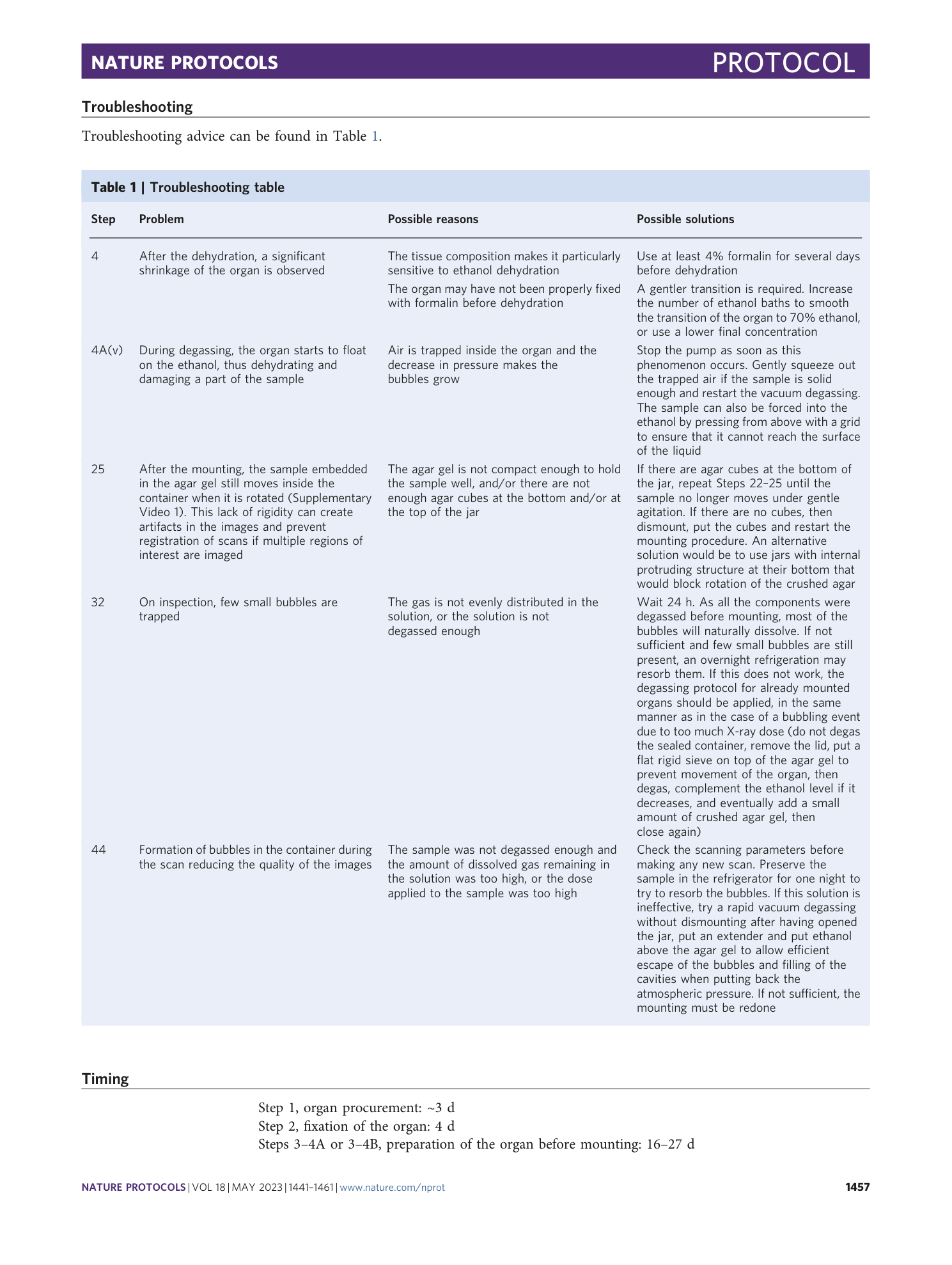
Extended Data Fig. 1 Challenges faced during the development of the technique and their solutions.
This protocol was developed in an iterative manner overcoming all the different challenges related to soft tissue imaging, dose deposition and local tomography.
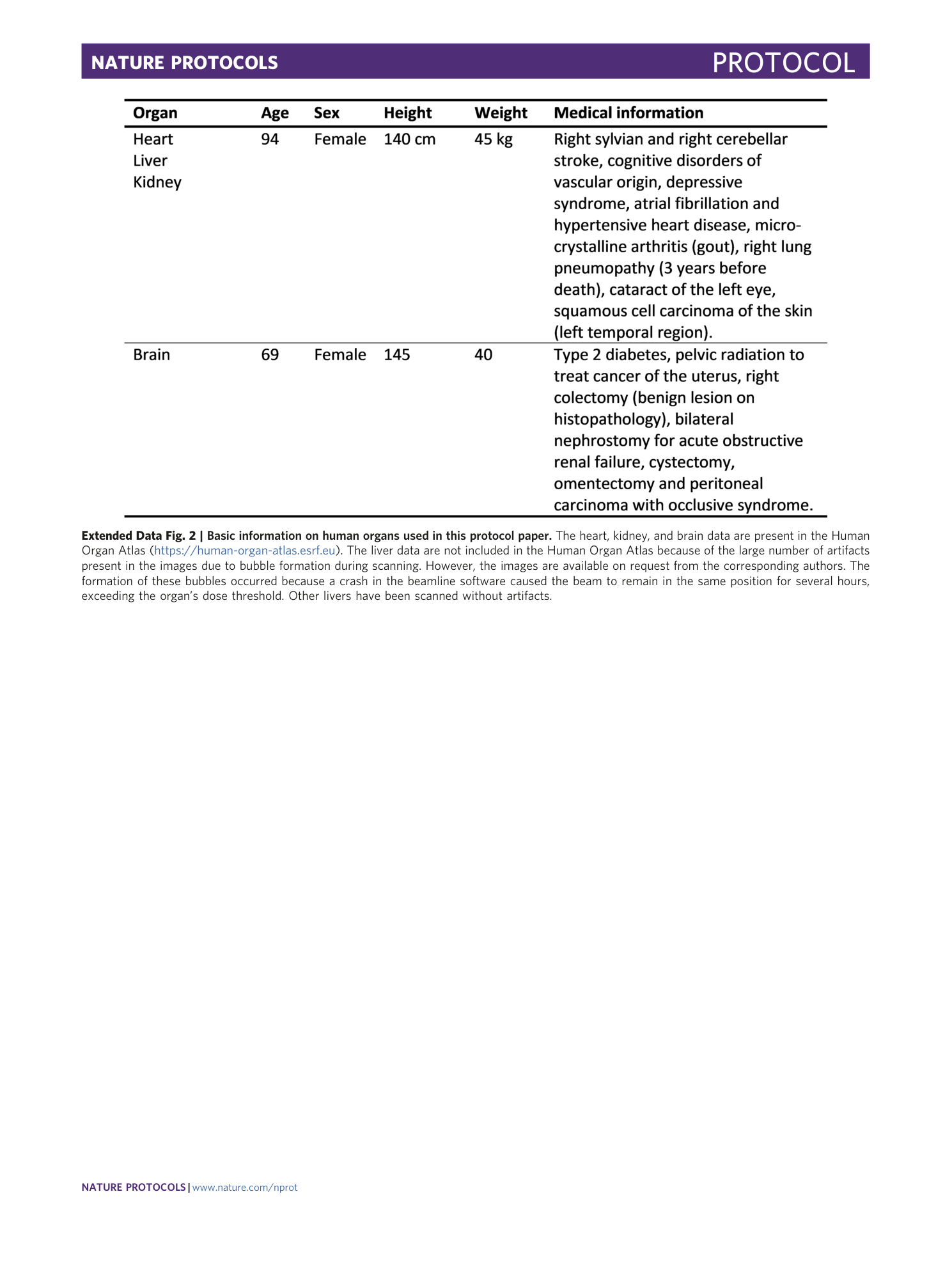
Extended Data Fig. 2 Basic information on human organs used in this protocol paper.
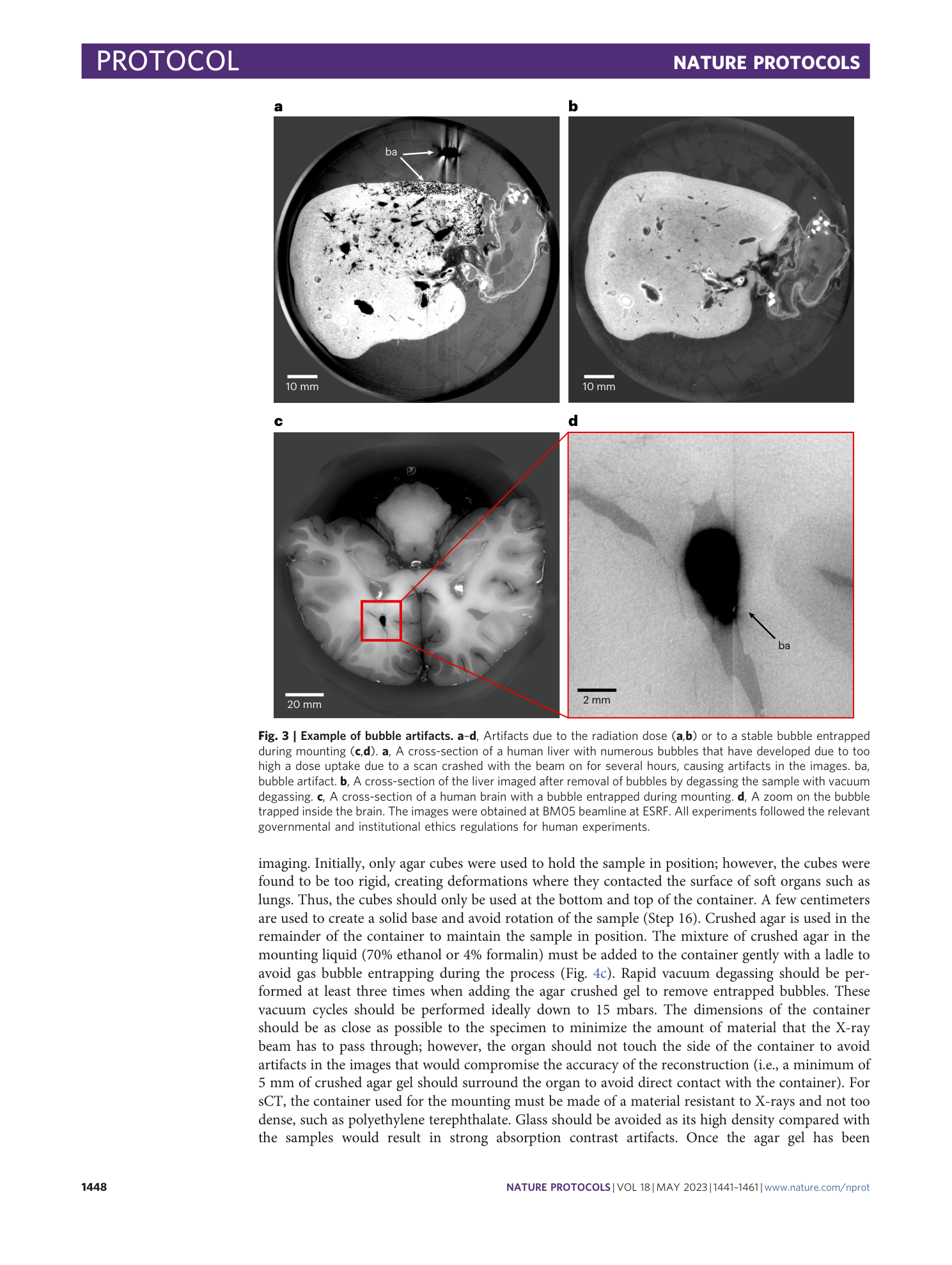
The heart, kidney, and brain data are present in the Human Organ Atlas ( https://human-organ-atlas.esrf.eu ). The liver data are not included in the Human Organ Atlas because of the large number of artifacts present in the images due to bubble formation during scanning. However, the images are available on request from the corresponding authors. The formation of these bubbles occurred because a crash in the beamline software caused the beam to remain in the same position for several hours, exceeding the organ’s dose threshold. Other livers have been scanned without artifacts.
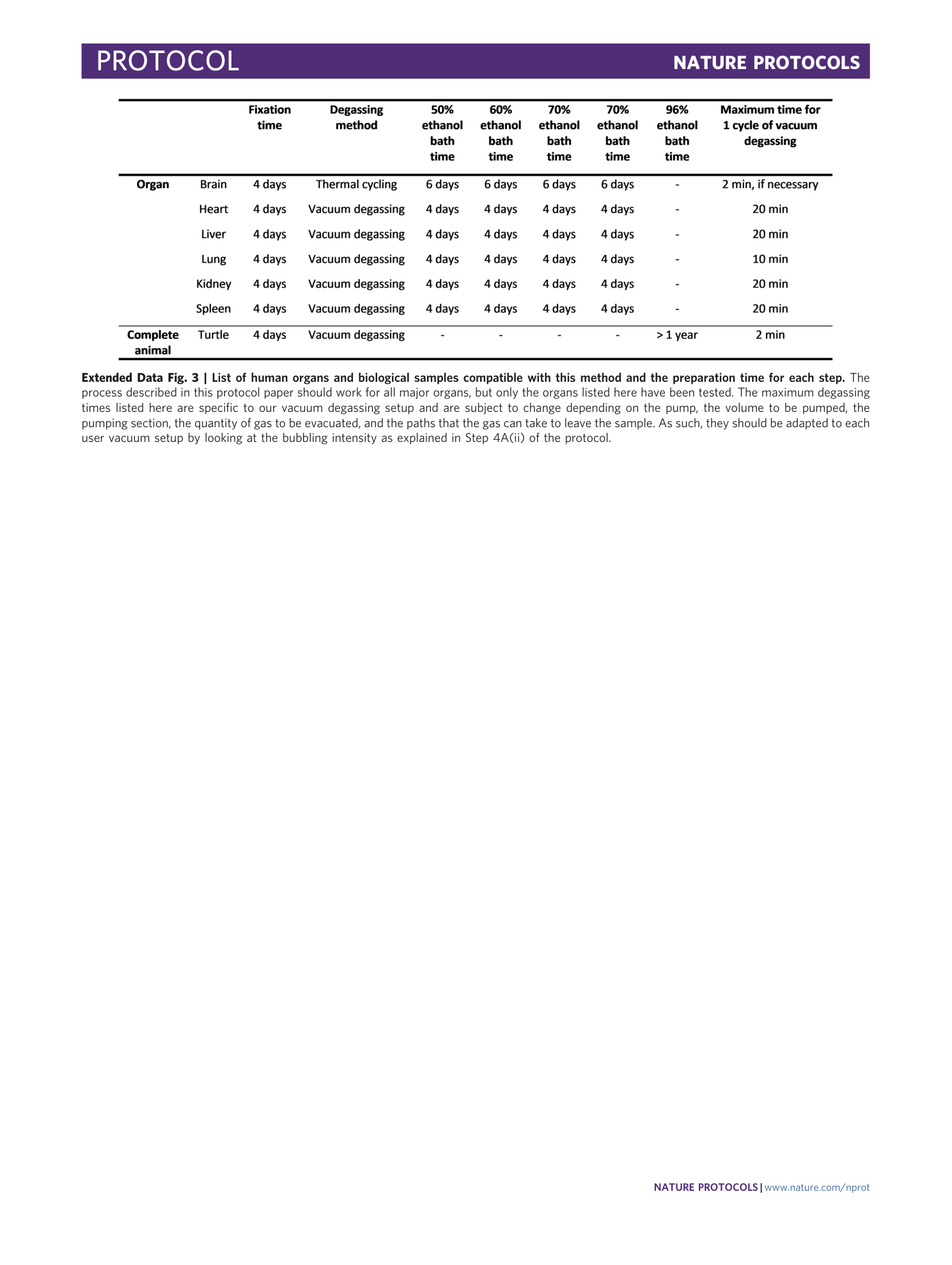
Extended Data Fig. 3 List of human organs and biological samples compatible with this method and the preparation time for each step.
The process described in this protocol paper should work for all major organs, but only the organs listed here have been tested. The maximum degassing times listed here are specific to our vacuum degassing setup and are subject to change depending on the pump, the volume to be pumped, the pumping section, the quantity of gas to be evacuated, and the paths that the gas can take to leave the sample. As such, they should be adapted to each user vacuum setup by looking at the bubbling intensity as explained in Step 4A(ii) of the protocol.
Extended Data Fig. 4 The two types of large leak-proof container in PET in the custom-made container holders.
The left leak-proof container is a Medline Scientific container (cat. no. 129-0592) of 3 L. The right leak-proof container is a Lock & Lock container (cat. no. INL-403) of 2.1 L. Both are inside a custom-made container holder described in Extended Data Fig. 5 .
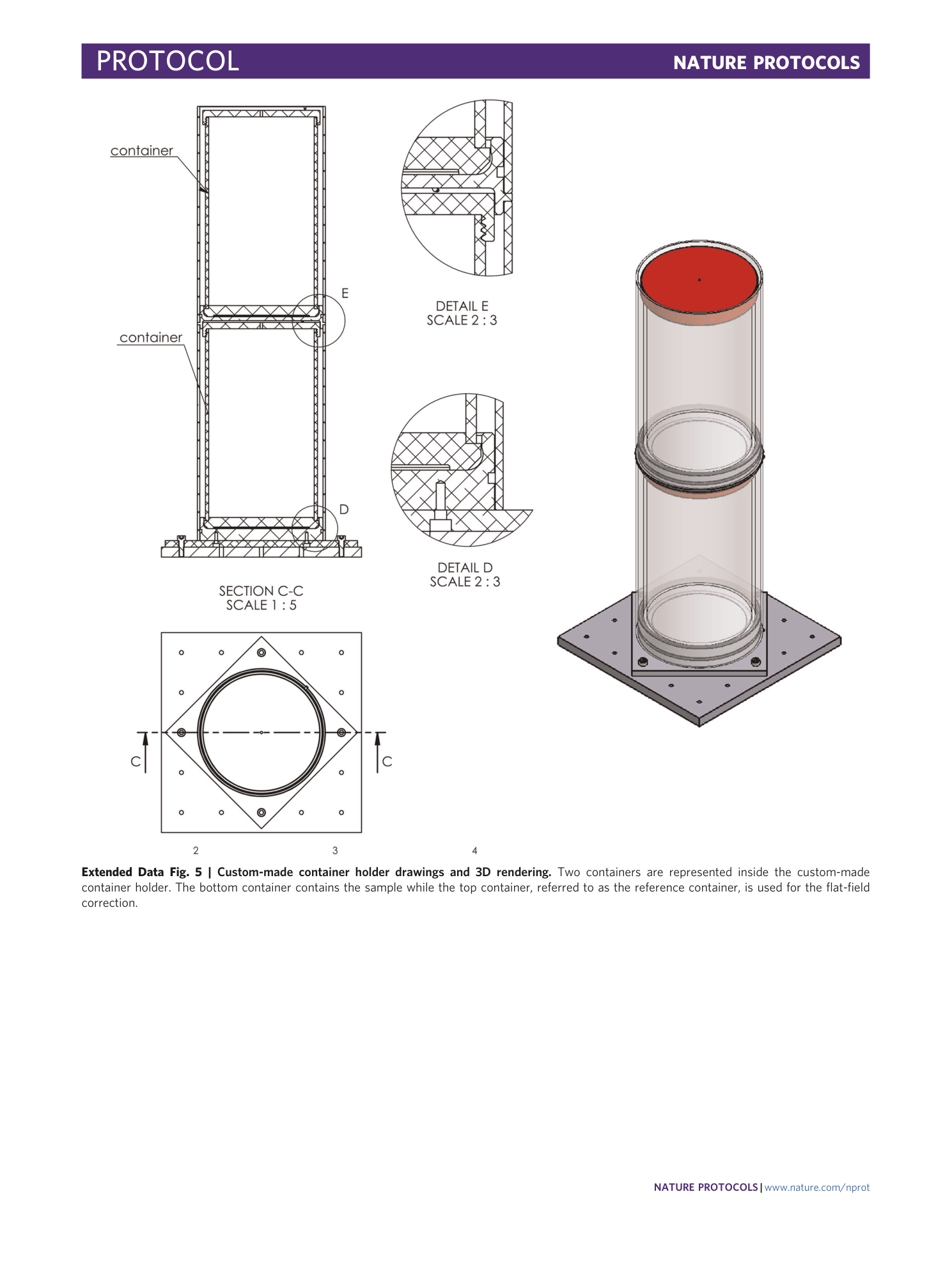
Extended Data Fig. 5 Custom-made container holder drawings and 3D rendering.
Two containers are represented inside the custom-made container holder. The bottom container contains the sample while the top container, referred to as the reference container, is used for the flat-field correction.
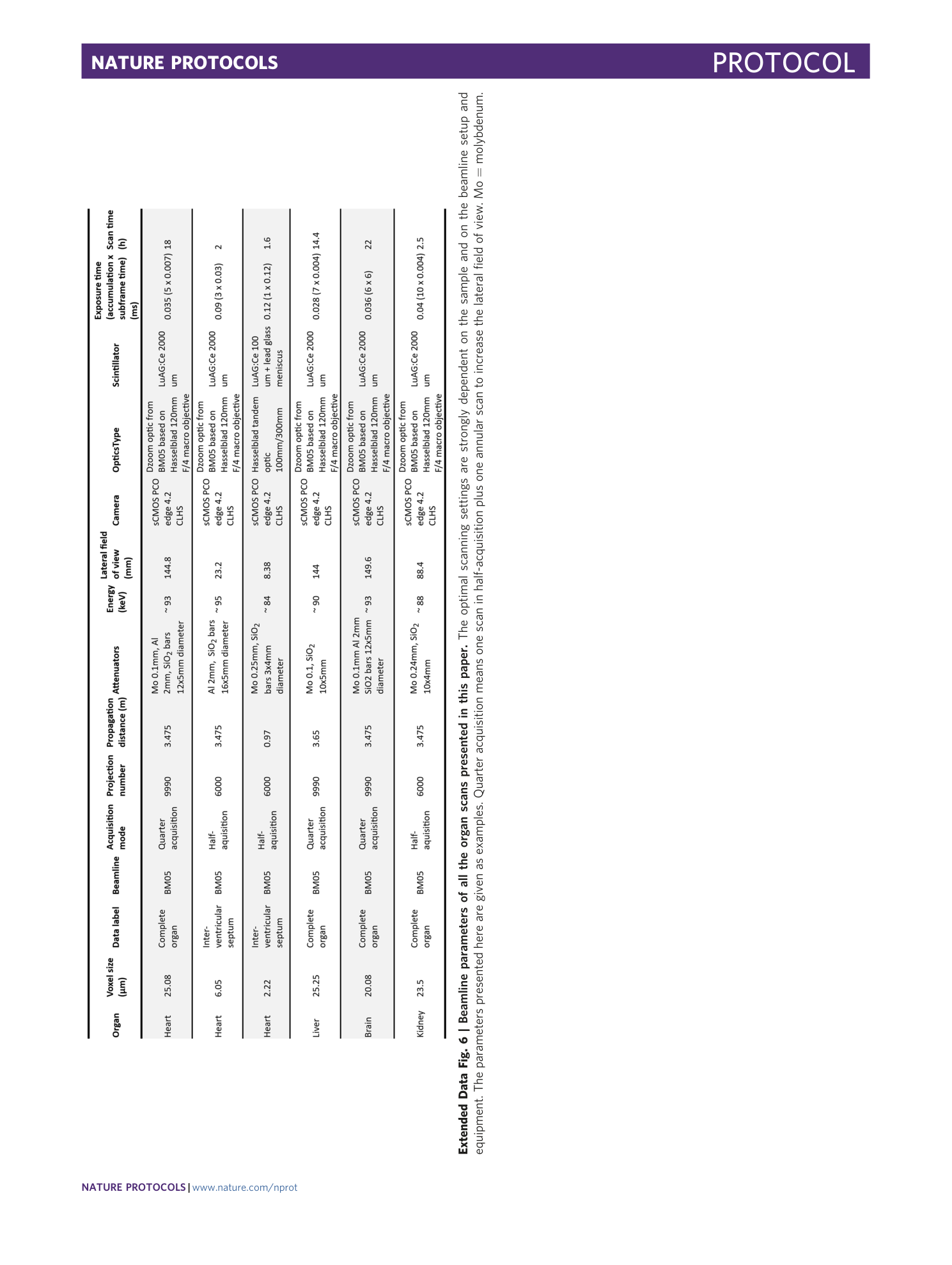
Extended Data Fig. 6 Beamline parameters of all the organ scans presented in this paper.
The optimal scanning settings are strongly dependent on the sample and on the beamline setup and equipment. The parameters presented here are given as examples. Quarter acquisition means one scan in half-acquisition plus one annular scan to increase the lateral field of view. Mo = molybdenum.
Supplementary information
Supplementary Video 1
Example of a sample mounted with insufficiently compacted agar, allowing rotation upon a slight movement.

