Pre-extraction sample processing for CALIBER project
James JN Kitson
Abstract
This is a document that outlines the steps needed to prepare field-collected samples during the CALIBER project for archiving and further processing.
Steps
Setup
Source steel beads (ball bearings) for tissue grinding (Tungsten beads are not usually necessary). We use hardened carbon steel or stainless steel bearings from simplybearings.co.uk. This protocol requires two beads per sample tube.
Beads are usually shipped coated in manufacturing oil (especially the carbon steel beads). To remove this, place beads in a borosilicate glass beaker or Duran bottle with the pouring lip and lid removed then bake for at least 12 hours at 250 oC.

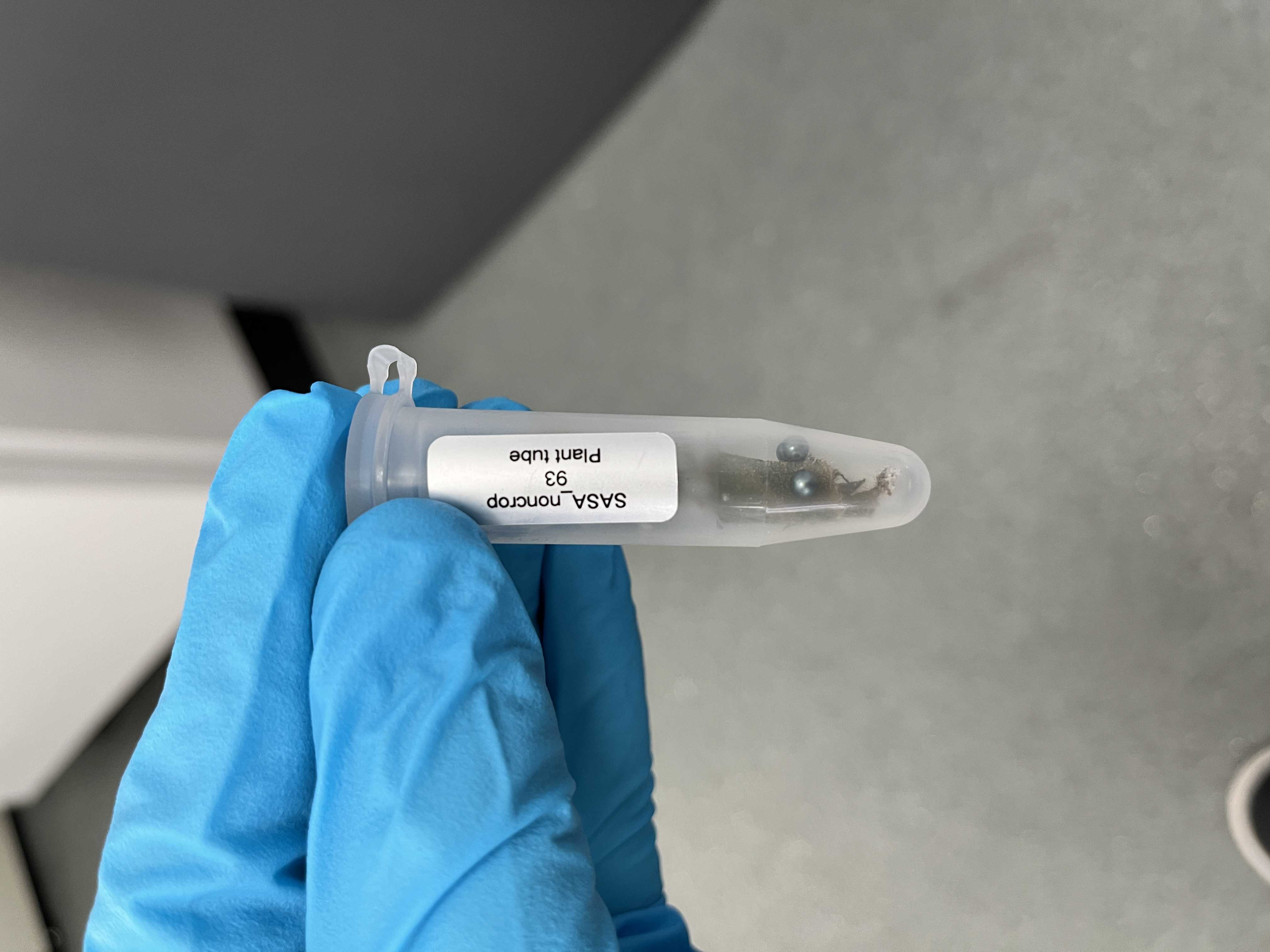
Prepare 5 ml screwcap collection tubes (e.g. Starlab #E1450-1100) containing three 3 mm hardened steel beads in batches of 96 tubes.

Insect sample processing
Using clean forceps, pick off all insects and place in a screwcap tube of the appropriate size (2ml/5ml/15ml) then fill the tube with a solution of 95% Ethanol and 5% Glycerol.
Archive plant sample processing
Into this envelope, place approximately 2-5 g of leaf and chopped stem material.
The archive sample is ready for long term storage at room temperature.
DNA extraction plant sample processing
Using a clean scalpel, slice and place ~ 50 mg of plant material into the pre-prepared collection tubes containing the hardened steel ball bearings (from step 3).
Store at -20 oC until ready to proceed with DNA extraction.
Cleanup and reset
The remaining plant material and packaging can be disposed of in a clinical waste bin.
Wash forceps and scalpel in 1x chemgene.
Rinse forceps and scalpel in molecular grade water.
Rinse forceps in 100% Isopropanol and leave to dry.
Wipe down dissection area with blue roll and 1x chemgene.
Proceed with the next sample.