Optogenetic Stimulation of superior mesenteric ganglion in a model of septic shock
Kaitlin Murray, Jessica Sladek, Colin Reardon
Abstract
Protocol details how to use optogenetics to activate neurons in the superior mesenteric ganglion of a mouse.
Before start
Steps
Optogenetic Stimulation
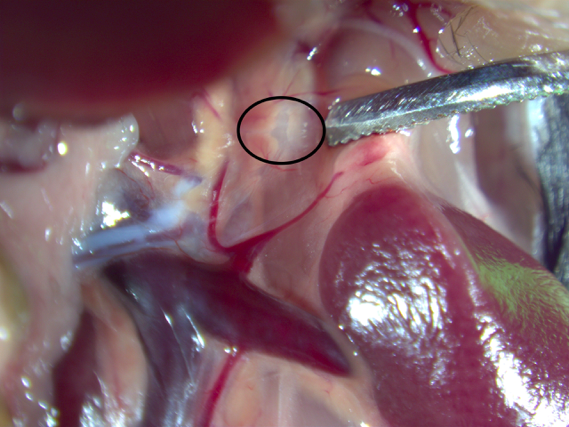
Place anesthetized mouse supine on heating pad under stereo dissecting scope
Make a 1-inch incision on the right side of the mouse, directly under the rib cage
Begin optogenetc stimulation (250mA, 10Hz, 2ms) of superior mesenteric ganglion (SMG) by placing the fiber 1-2mm over SMG
Stimulate for 10 minutes
Retro-orbital IV injection of 4mg/kg LPS
Continue optogenetic stimulation of SMG for another 10 minutes
Keep tissues moist with continual application of sterile 1X PBS and cover wound with sterile gauze
Allow mouse to rest (under anesthesia) for 50 minutes
Blood Collection
Perform a cardiac puncture to draw blood serum and collect in serum separator tube
Euthanize mouse
Centrifuge blood at 15000rpm for 5 mins, 4C
Remove top layer and store at -80C
ELISA Protocol
Coat Corning Costar 9018 ELISA plate with 100ul/Well of capture antibody buffer
- Add 48ul capture antibody to 12ml 1x Coating Buffer
- Seal plate, incubate overnight 2-8C
Aspirate wells and wash 3x with >250ul/well washing buffer
- wash buffer: 1x PBS, 0.05% Tween 20
- Allow ~1 minute soak between washing
- Blot on absorbent paper
Dilute 5x ELISA/ELISAPOT diluent with 4 parts DI water and block wells with 200ul/well of 1x ELISA/ELISAPOT diluent
- 10ml of 5x ELISA/ELISAPOT to 40ml DI water
- Incubate RT for 1 hour
Optional: aspirate and wash with wash buffer
Reconstitute lypohilized standards, sit for 15 minutes with gentle agitation prior to diluting further
- Add 0.8ml DI water to vial
Dilute reconstituted standard with 1x ELISA/ELISAPOT diluent
- Add 100ul of standard to 1400ul of 1x ELISA/ELISAPOT diluent
- Add 100ul/well of top standard concentration to appropriate wells
- Perform 2-fold serial dilutions of the top standards to make a standard curve of a total of 8 points: Add 100ul/well
- Include at least 2 wells with 100ul/well of 1x ELISA/ELISAPOT diluent as blanks
- Seal plate and incubate at RT for 2 hours (or overnight 2-8C)
Aspirate and wash for 3-5 washes
Add 100ul/well of detection antibody diluted in 1x ELISA/ELISAPOT diluent
- 48ul detection antibody to 12ml of 1x ELISA/ELISAPOT diluent
- seal plate and incubate 1 hour at RT
Aspirate and wash for 3-5 washes
Add 100ul/well of Avidin-HRP diluted in ELISA/ELISAPOT diluent
- 48ul enzyme to 12ml 1x ELISA/ELISAPOT diluent
- seal and incubate 30 minutes at RT
Aspirate and wash, soak wells in wash buffer 1-2 minutes prior to aspiration
- repeat 5-7 washes
Add 100ul/well of 1x TMB solution to each well
- Incubate at RT 15 minutes, or until fully developed
Add 50ul of stop solution to each well
Read plate at 450nm- if λ subtraction is available, subtract the values of 570nm from those of 450nm and analyze