Intracellular Staining of PUMA in Primary PBMC Lymphocytes
Dennis Juarez, David Fruman
Disclaimer
DISCLAIMER – FOR INFORMATIONAL PURPOSES ONLY; USE AT YOUR OWN RISK
The protocol content here is for informational purposes only and does not constitute legal, medical, clinical, or safety advice, or otherwise; content added to protocols.io is not peer reviewed and may not have undergone a formal approval of any kind. Information presented in this protocol should not substitute for independent professional judgment, advice, diagnosis, or treatment. Any action you take or refrain from taking using or relying upon the information presented here is strictly at your own risk. You agree that neither the Company nor any of the authors, contributors, administrators, or anyone else associated with protocols.io, can be held responsible for your use of the information contained in or linked to this protocol or any of our Sites/Apps and Services.
Abstract
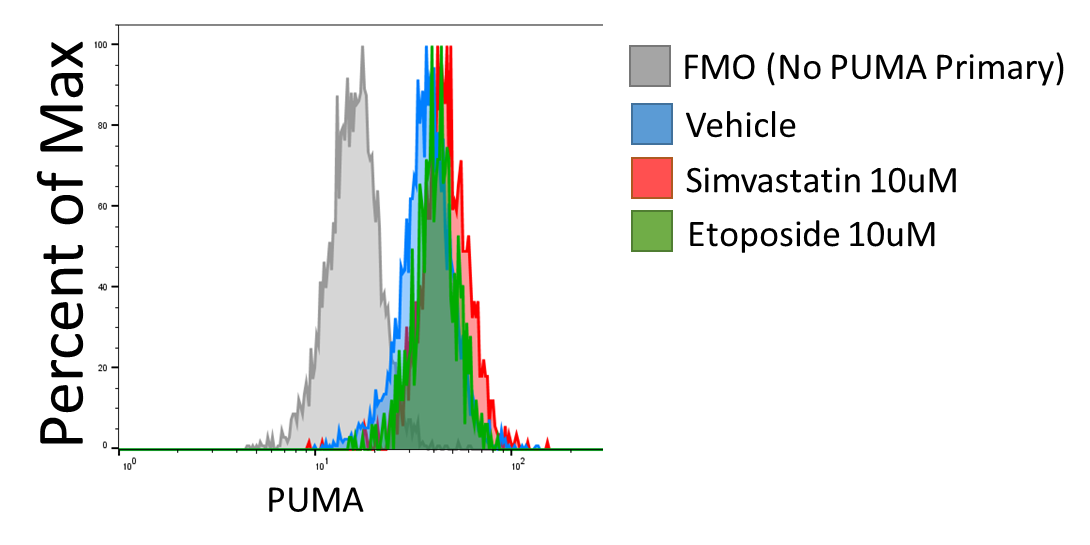
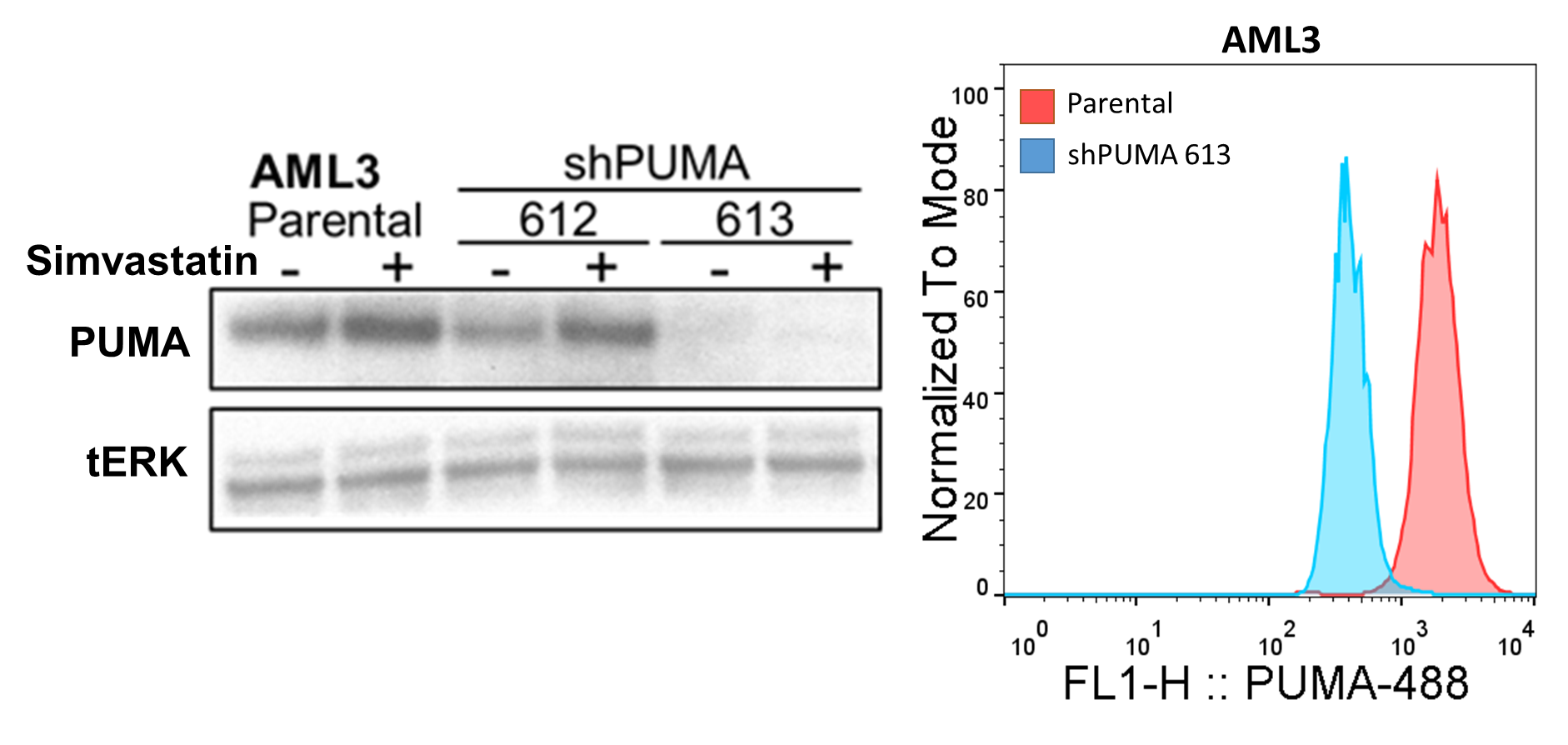
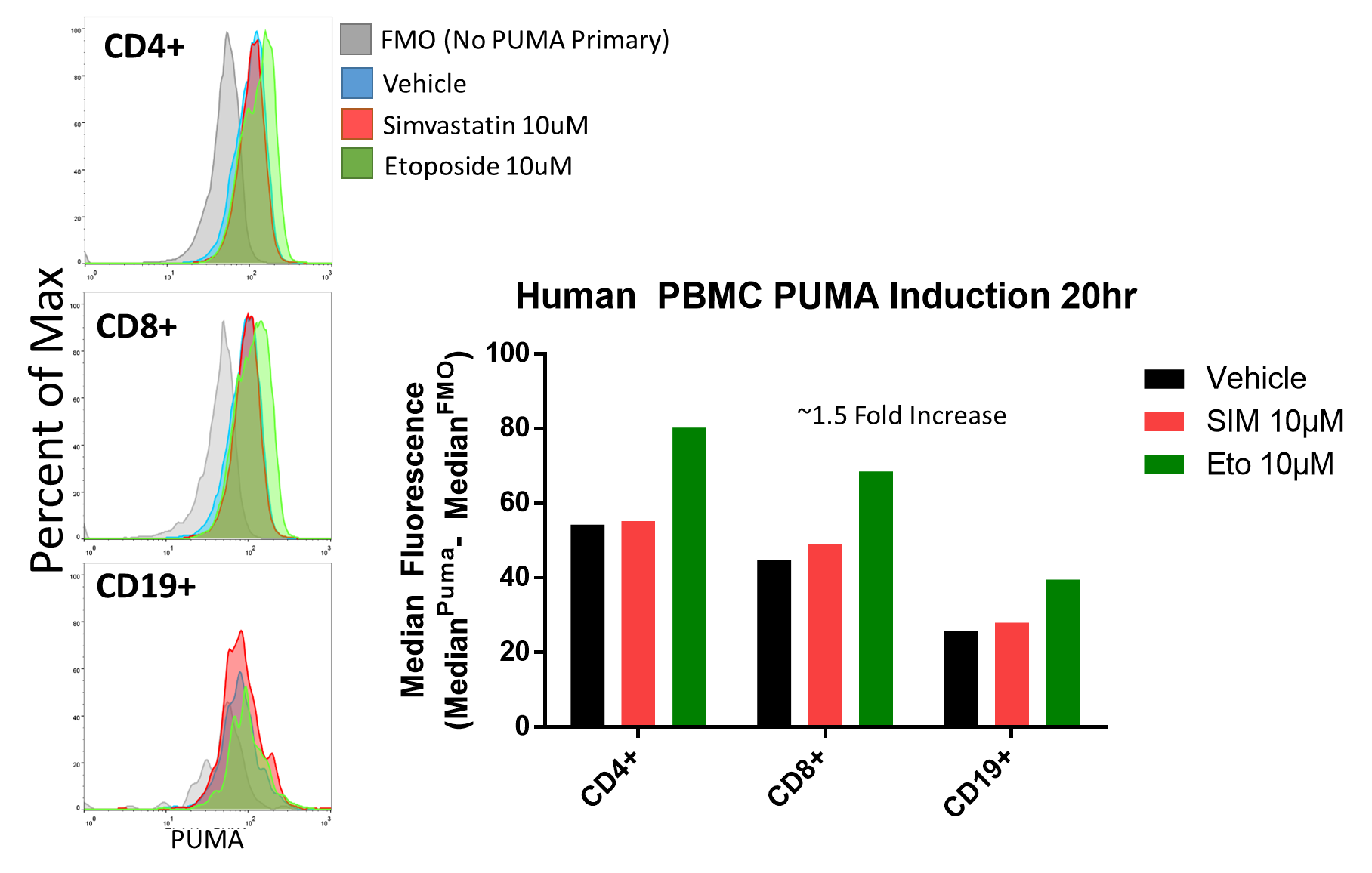
Flow cytometric assessment of Intracellular PUMA levels is useful when wanting to assess PBMC subsets but are limited by sample as with primary patient samples. Here I describe a protocol for intracellular PUMA staining validated with PUMA knockdown and induction with PUMA inducing treatments.
Steps
Staining
Treat at least 1 million PBMCs per sample, including flow cytometry controls (unstained, single stain controls, and FMO controls)
Harvest cells at 20 hours and wash with PBS.
Stain cells with anti-CD3, anti-CD4 and anti-CD19 for 20 minutes at 4 degrees in the dark. Create unstained, single stain controls, and FMO controls.
After staining, wash with PBS and resuspend 1 million cells in 100uL of PBS-diluted 2% formaldehyde for 10 minutes at RT.
Add 1mL of PBS and spin down the cells at 800xg for 5 minutes.
Wash two times with 1x intracellular staining buffer (see materials).
Stain for intracellular PUMA in 1x intracellular staining buffer: 50ul staining volume per sample with 1:50 final dilution Puma (Cell signaling D30C10) Rabbit mAb
Stain at 4 degrees for 1 hour in the dark.
Spin down and wash two times with 1x intracellular buffer.
Prepare the secondary Invitrogen’s anti-Rabbit IgG 647 #A-21244 at Final Dilution 1:500 in 1x staining buffer. Be sure to stain the PUMA FMO with the secondary antibody.
Stain for 1 hr in the dark at RT.
Wash twice with 1x intracellular buffer and once with PBS. Resuspend in 100-150uL PBS and samples are ready to run.
Validation