F/2 medium added soil extract from estuary water at 27 PSU of salinity
Estelle Bigeard, Laure Guillou
Abstract
F/2 medium (Guillard’s Marine Water Enrichment Solution) is used to maintain in culture microalgae (for example, Ddinoflagellates) and their parasites (Alveolates, Syndiniales & perkinsids, Fungi Dinomyces ).
Most of these strains have been sampled from an estuary, at 27 PSU of salinity.
Adding soil extract is sometimes necessary to improve their growth.
Since 2007, Roscoff Parasites Culture Collection was maintained in this medium.
Since October 2022, Amoebophrya Culture Collection was maintained in Red Sea F/2 medium (see protocol
dx.doi.org/10.17504/protocols.io.n92ldzyrxv5b/v11)
Steps
Water Sampling
Sea water is sampled in the Penzé Estuary from June to July (during the dinoflagellate blooms), at a salinity of 27 PSU.
The sea water is stored at dark during three months (minimum two months).
Installation and Sterilization Procedures for Filtration System
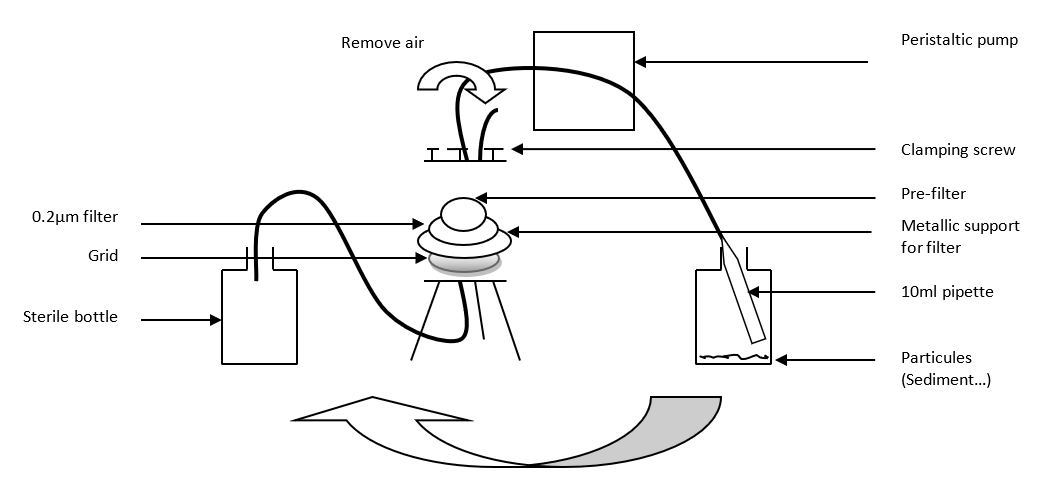



On the tripod, place :
-
First the grid, upward spirale (see figure 2)
-
The metallic disc
-
The filter (diameter 90 mm, porosity 0.2µm)
-
A glass fiber prefilter (since estuary water contains a lot of particles, sediments, etc.)
Close the system with the 3 screws.
-
For the first filtration (seawater filtration), use the Allen key to properly close the system.
-
For the second filtration (F/2 medium filtration), do not close completely, wrap the system with aluminium foil and place it in autoclave at 121°C for 20 minutes.
Medium preparation
Filtration of Seawater in Non-Sterile Conditions
On the bench,
Place the sterile 10L carboy, the vacuum pump and the filtration system.
Perform a filtration of 40 liters of old sea water through a 0.22µm filter.
As the medium will be used to maintain a Culture Collection of parasite, this medium will be tested first using replicated cultures. This is a cumbersome step; better to prepare the largest volume of medium as possible (40 liters if possible).
Before this filtration, check the filtration system:
-
Check the tubings
-
Break the end (with cotton) of a plastic 10ml pipette.
-
Attach the pipette to the tubing connected to the top of the system and place it in the "water to filtrate" bottle.
-
This tubing is placed in the peristaltic pump
-
Place the second tubing connected to the bottom of the system into a transparent sterile bottle (Nalgene).
Turn on the pump. Remove air, and adjust the speed (not too fast).
Switch off the pump when the filtration is completed.
NB: Replace the filter (and the pre-filter) by a new one every 10-20 liters.
Autoclave the filtered sea water at 121°C for 20 minutes.
Filtration of the F/2 medium in Sterile Condition
The Day before :
-
Thaw the F/2 solution at +4°C.
-
Prepare the filtration system (Fig. 1 to 4)
-
Autoclave the system at 121°C 20 min.
On the Day of filtration, Under a laminar flow cabinet (Sterile Conditions):
-
Add 20mL of the F/2 solution (50x concentration) per liter of sterile seawater.
-
Remove the aluminum foil covering the tripod. Securely close the tripod using the key (3 screws).
-
Prepare the Tubing
-
Break the end (with cotton) of a 10mL plastic pipette,
-
Attach the intake tubing to the tapered end,
-
Place the pipette in the 10L carboy containing the medium to be filtered.
-
Position the outflow tubing (filtrate side) into a new 10L carboy.
-
-
Open the bleeding valve before starting the pump.
-
Start the pump and adjust medium flow rate.
-
Close the bleeding valve once sample water comes out without air.
-
When all the medium is filtered, stop the pump.
Storage of F/2 medium
Medium is stored at room temperature, ideally in a culture room.
Preparation of the F/2 medium with 5% Soil Extract
Add 50mL of soil extract solution per liter of F/2 medium.
Comment: it is recommended to use F/2 medium that was prepared several days before in order to remove aggregates, as precipitates can be formed at this step.
For this filtration, use sterile filtration units with a 0.2µm pore size (Millipore) with a capacity of 1L and transfer the filtered medium into Nalgene 1L bottles.
Storage of F/2 medium with 5% Soil Extract
The medium should be stored at room temperature, ideally in a culture room.
Validation test of the F/2 medium added 5% soil extract
Prior the use of the Medium for the Parasites Cultures Collection, it is Important to test it:
To accomplish this, select different parasitic strains, having different host and/or transfer time.
Transfer first the host in the new medium, three times consecutively. Then, transfer the parasite cultures into their respective hosts cultivated in the new medium for a period of 4 to 8 weeks.
Monitor the growth of the hosts, ensuring they reach the correct density without any clumps of dead cells. Additionally, assess the quality of the infection, checking for infection regularity and the absence of encystment.
If the result is satisfactory in all aspects, the medium can be used for the Culture Collection.

