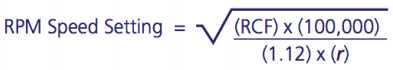CPT Processing
Clemens Scherzer, Bradley Hyman, Charles Jennings
Abstract
This protocol explains the Standard Operating Protocol for processing CPT.
Steps
Processing Protocol
***Prep Steps:
Label the appropriate number of cryopreservation vials with program and sample name, cell type, and date. Put On ice.
Prepare before processing CPTs:
- FBS (heat shocked/heat inactivated) and put
On iceto pre-cool. - Create solution to equal
10% (v/v)in90% (v/v)(heat shocked/heat inactivated) and putOn iceto precool. - FBS is usually stored at 9 mL in -20C. The 10% DMSO/90% FBS mixture is referred to as Freeze Media.
The BD Vacutainer CPT Tube with Sodium Citrate should be at Room temperature (18-25°C) and properly labeled for patient identification.
Collect blood into the tube using the standard procedure.
After collection, store tube upright at room temperature until centrifugation. Blood samples should be centrifuged within 2 hours of blood collection for best results.
Centrifuge tube/blood sample at 1500rcf.
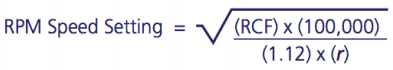
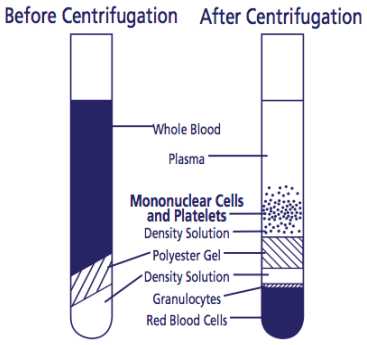
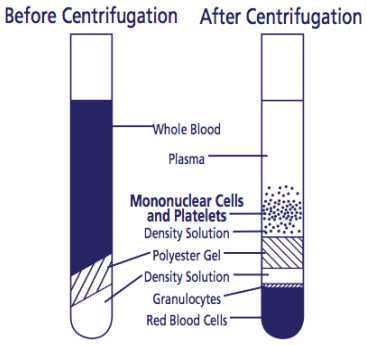
After centrifugation, mononuclear cells and platelets will be in a whitish layer just under the plasma layer (see figure). Transfer all the plasma and cell layer into a 15 mL Falcon tube.
Alternatively, if processing tubes within 24 hours, resuspend cells into plasma by inverting unopened tube gently 5 to 10 times. The sample can be stored upright in room temperature for up to 24 hours after centrifugation. Before collecting cells, remix tubes by gently inverting 5-8 times. To collect the cells, pipette entire contents of tube above the gel into a 15 mL size conical centrifuge tube with a cap
Washing Protocol
Slowly add DPBS to bring volume to 14-15 mL (15 mL if processing within 24 hours) by tilting serological pipette tip to wall of tube. Cap tube. Mix cells by gently pipetting using serological pipette.
Centrifuge at 300rcf. Gently decant as much supernatant as possible without disturbing cell pellet.
Resuspend cell pellet by gently pipetting.
Slowly add DPBS to bring volume to 9-10 mL (10 mL if processing within 24 hours). Cap tube. Mix cells thoroughly using serological pipette. Be careful of bubbles.
Remove 30µL for counting (see following section after Cryopreservation of PBMC’s Protocol for Counting Cells protocol).
Centrifuge at 300rcf. Gently decant as much supernatant as possible without disturbing cell pellet. Resuspend the pellet using finger until no clumps are visible. Pelleted cells will start dying if not promptly resuspended.
Cryopreservation of PBMC’s Protocol
Following Step 13 under Washing Protocol, resuspend cells in 1mL and gently mix to resuspend cells:
Centrifuge at 300rcf. Gently decant as much supernatant as possible without disturbing cell pellet. Resuspend the pellet using finger until no clumps are visible. Pelleted cells will start dying if not promptly resuspended.
Gently swirling tube, add drop-wise another 1mL (per tube of blood) and immediately place On ice.
Immediately dispense 1mL per vial.
Place vial in Room temperature freezing container previously equilibrated to 4°C and place immediately into -80°C.
After 24 hours, remove vial from both -80C and freezing container and place in separate box. Transfer into liquid nitrogen (LN2) for long-term storage.
In designated excel file, note the date and time the blood draw was performed, the time the sample underwent the first centrifugation, the time the sample went to the -80C freezer, and the time the sample was transferred to liquid nitrogen.
Counting Cells Protocol
To get an equal cell distribution, mix cell suspension prior to adding stain and again just before loading hemacytometer.
To prepare hemacytometer, first clean hemacytometer with H2O and then with 70% ETOH. Dry off with Kimwipe.
Staining cells with Trypan Blue: In microcentrifuge tube, combine 30µL with 6µL (5:1). Mix and allow dilution to incubate for 0h 5m 0s at Room temperature (15-30C).
Loading Hemacytometer: Place hemacytometer on counter. Center a cover glass over the hemacytometer chambers.
Inject 10µL into one chamber. Be careful not to overfill. Allow cell suspension to settle in hemacytometer for at least 10 sec before counting.
Observing and Counting Cells: Place hemacytometer on the stage of microscope and adjust focus using 10X magnification, then change to 20X and refocus if necessary.
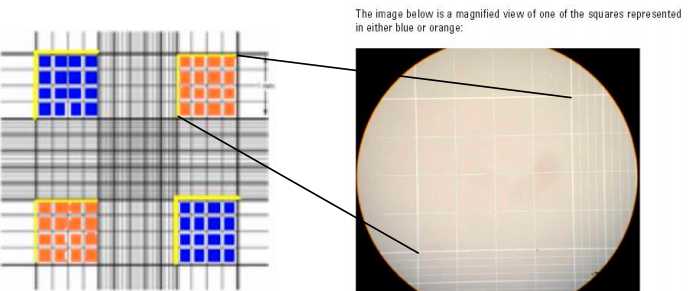
Count live cells in the four large corner squares. Include cells that touch either the top line or left vertical perimeter line of any corner square. Do not count any cells that touch either the bottom line or right vertical perimeter line of any corner square. Blue-stained cells are dead and clear are alive. COUNT ONLY VIABLE CELLS. It may help to use a hand-held counter if available. See figures below.
Formula to Determine Cell Counts: Viable cells/mL = (Total # viable cells/squares counted) x 104 x dilution factor 4 x dilution factor (dilution factor is 1.2 since 5:1 of cell suspension:stain)
To calculate total viable cells: Total viable cells = viable cells/mL x volume of original cell suspension in mL (volume of original cell suspension is 9-10 mL: See Steps 11-12 under Washing Protocol)
To clean, rinse hemacytometer and cover slide with H2O and 70% ETOH and wipe dry.
Thawing of PBMCs Protocol
Set aside media or DPBS at Room temperature before thawing procedure to wash out DMSO.
Add desired amount of room temperature media or DPBS in 50 mL falcon tube.
When cryovial containing cells are thawed at room temperature for 1-2 min, transfer cells to 50mL falcon tube containing room temperature media or DPBS.
Centrifuge cells at 1500rcf. Decant supernatant and gently finger flick tube to break up pellet.
Resuspend in desired volume of media.
Determine cell number and viability.
Adjust cell volume for functional assay.