2-step PCR mixture and conditions (Barcoded-head primers for seqs pooling)
Yin-Tse Huang, Tsu-Chun Hung
Abstract
PCR mixture and condition (2X SUPERGREEN PCR MASTER MIX)
Steps
Wear glove, clean up the working bench w. 1% bleach
For 1' PCR head-primers
Prepare 1' PCR master mixutre for head-primers ( prepare 1.2X of solutions for pipetting error if needed )
PCR mixture for head-primers for each reaction
| A | B | C | D |
|---|---|---|---|
| Component | Volume | Volume (1.2X) | Final conc. |
| Forward Primer (10 µM) | 1.6 μl | 1.9 μl | 1 µM |
| Reverse Primer (10 µM) | 1.6 μl | 1.9 μl | 1 µM |
| 2X Supergreen PCR Master Mix | 7.8 μl | 9.4 μl | - |
| ddH20 | 4.1 μl | 4.9 μl | - |
| Total volume | 15 μl | 18 μl | - |
Mix the 1' PCR master mixture gently by pippeting. Quick spin the tube.
Transfer 15µL 1' PCR master mixutre in 8-strip PCR tubes.
Add 0.6µLDNA template in 8-strip PCR tubes, resulting in a 15.6µL reaction mixture for 1' PCR.
Negative control contains only 15µL master mixture but not DNA template
Mix the reaction mixture gently by tapping the tubes. Quick spin the tubes.
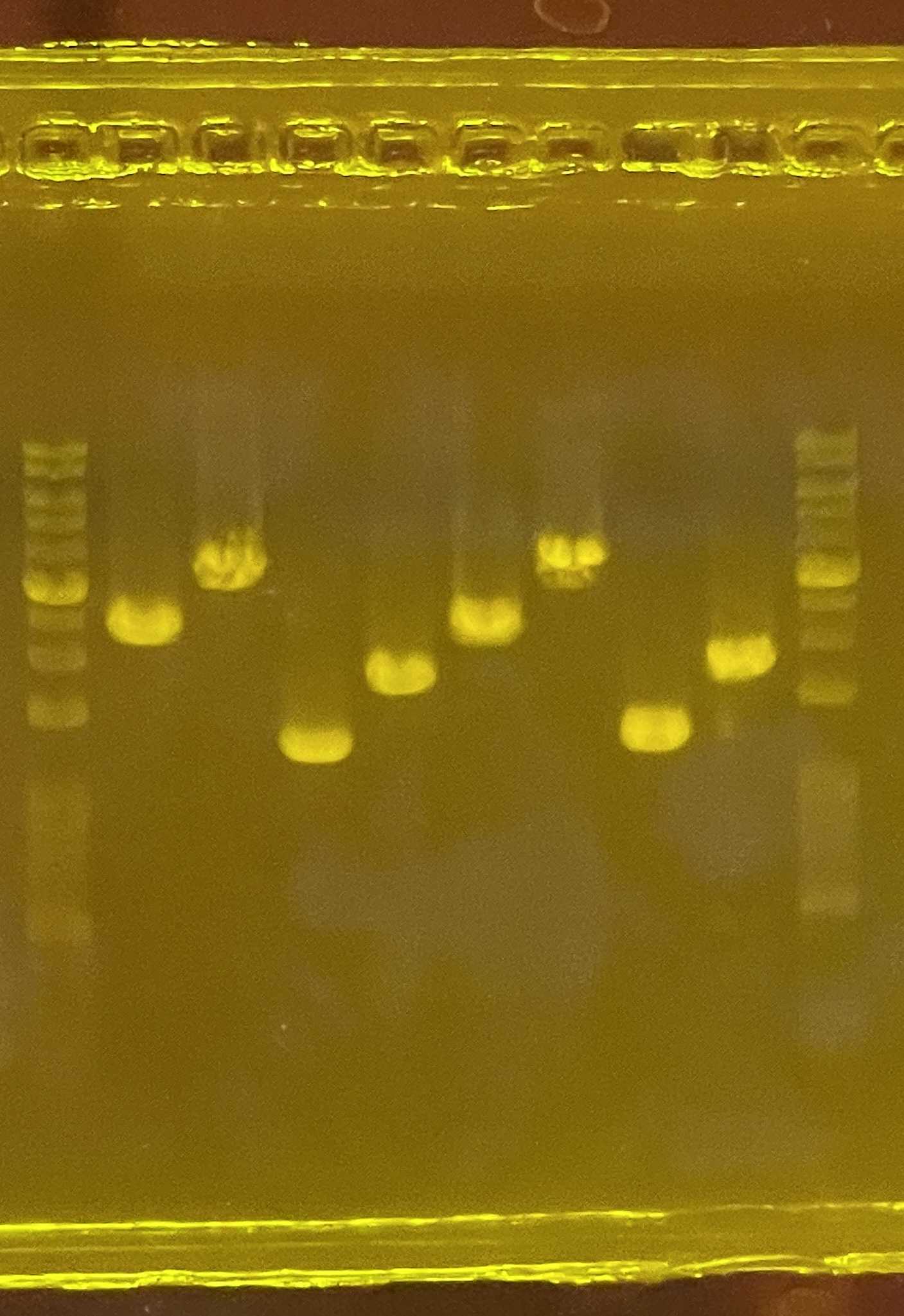
Carry out PCR using the following condition:
1' PCR condition for head-primers
| A | B | C | D |
|---|---|---|---|
| Step | Temp | Sec | Cycle |
| Initial denaturation | 95 ºC | 30-180 (a) | |
| Denaturation | 98 ºC | 15 | 20-25 cycles |
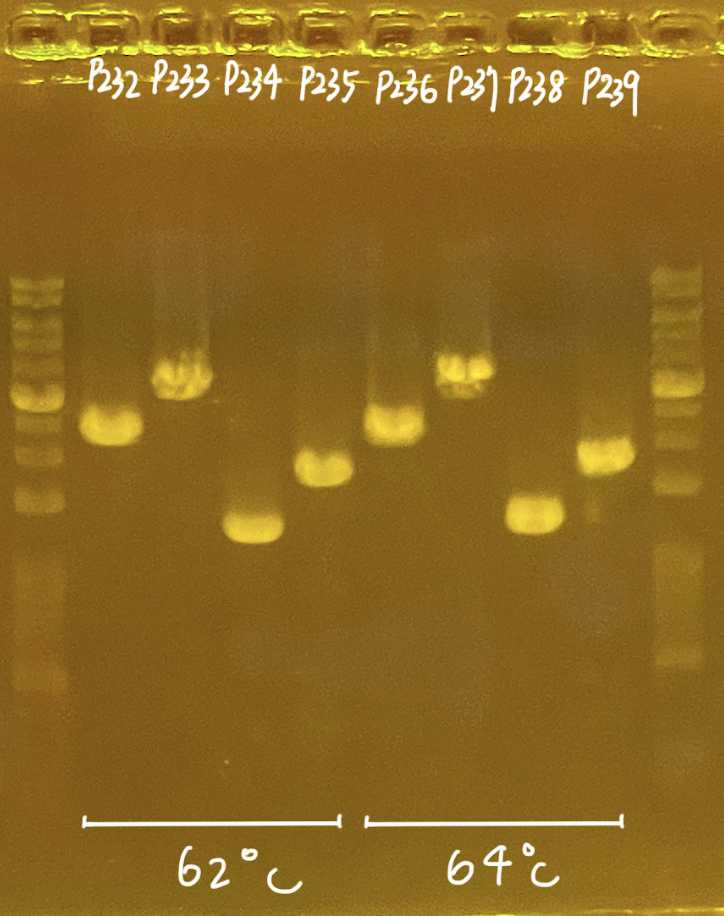
| Annealing | 64-68 ºC varied (b) | 15 | |
| Extension | 72 ºC | 60-180 (c) | |
| Final extension | 72 ºC | 210 | |
| Preservation | Preservation | 4 ºC | ∞ |
a. Varied depend on template complexityb. Annealing varied, 62-68C is working; Refer to 1' PCR primers for annealing temperaturec. 1kb ~ 1min extension; enough time allow full extension of sequence
1' hear-primers used in Huang lab
| A | B | C | D |
|---|---|---|---|
| Name | Sequence | Tm°C | CG% |
| NS1B1ngs_H1 | GCTATGCGCGAGCTGCcctngttgatyctgccagt | 71.7 | 60 |
| ITS4ngs_H1 | GCTATGCGCGAGCTGCtcctscgcttattgatatgc | 69 | 55.6 |
| LR5_H1 | GCTATGCGCGAGCTGCtcctgagggaaacttcg | 70.2 | 60.6 |
| EF1-526F_H1 | GCTATGCGCGAGCTGCgtcgtygtyatygghcaygt | 71 | 59.3 |
| EF1-1567R_H1 | GCTATGCGCGAGCTGCachgtrccrataccaccratctt | 70.6 | 56 |
| EF1-2218R_H1 | GCTATGCGCGAGCTGCatgacaccracrgcracrgtytg | 72.2 | 60.3 |
| Ben2f_H1 | GCTATGCGCGAGCTGCtccagactggtcagtgtgtaa | 70.5 | 56.8 |
| Bt2b_H1 | GCTATGCGCGAGCTGCaccctcagtgtagtgacccttggc | 74.5 | 62.5 |
| T22_H1 | GCTATGCGCGAGCTGCtctggatgttgttgggaatcc | 70.3 | 56.8 |
| RPB2-3bF_H1 | GCTATGCGCGAGCTGCggwggwtayttyatyatyaatgg | 65.6 | 48.7 |
| RPB2-7cR_H1 | GCTATGCGCGAGCTGCcccatrgcttgyttrcccat | 72.3 | 59.7 |
| fRPB2-11aR_H1 | GCTATGCGCGAGCTGCgcrtggatcttrtcrtcsacc | 71.7 | 60.8 |
For 2' PCR barcoded-head primers
Prepare 2' PCR master mixutre for barcoded-primers ( prepare 1.2X of solutions for pipetting error if needed )
PCR mixture for barcoded-primers for each reaction (NO PRIMERs at this point!!)
| A | B | C | D |
|---|---|---|---|
| Component | Volume | Volume (1.2X) | Final conc. |
| 2X Supergreen PCR Master Mix | 10.75 µL | 12.9 µL | - |
| ddH20 | 10.75 µL | 12.9 µL | - |
| Total volume | 21.5 µL | 25.8 µL | - |
Mix the 2' PCR master mixture gently by pippeting. Quick spin the tube.
Transfer 21.5µL of the 2' PCR master mixture to 8-strip PCR tubes.
Add 2.5µL pre-mixed barcoded-head primers (Forward + Reverse) to each PCR tubes.
Add 1µL of 1' PCR product as template , resulting in 25µL reaction mixture for 2' PCR.
Negative control contains only 24µL master mixture and premixed barcoded-head primers but not DNA template
Mix gently by tapping the tubes. Quick spin the tubes.
Carry out 2' PCR using the following condition:
2' PCR condition for barcoded-head primers
| A | B | C | D |
|---|---|---|---|
| Step | Temp | Sec | Cycle |
| Initial denaturation | 98 ºC | 30 | |
| Denaturation | 98 ºC | 15 | 10-15 cycles |
| Annealing | 64-68 ºC varied (a) | 15 | |
| Extension | 72 ºC | 60 (b) | |
| Final extension | 72 ºC | 210 | |
| Preservation | Preservation | 4 ºC | ∞ |
a. Annealing varied, 65 C is working based on test on 220531; Refer 2' PCR primers for annealing temperatureb. 1kb ~ 1min extension; enough time allow full extension of sequence