Step by Step from Environmental Samples to Preservation Vials
Guan Jie Phang
Abstract
This protocol shows how to generate a MICROBES ISOLATION FROM ENVIRONMENTAL SAMPLES into LAB COLLECTION. Please follow the instructions and do not modify the protocols by yourself. If any changes are needed please inform the lab manager or admin.
Steps
Isolate Primary Culture from Environmental Samples
Once you received your environmental samples.
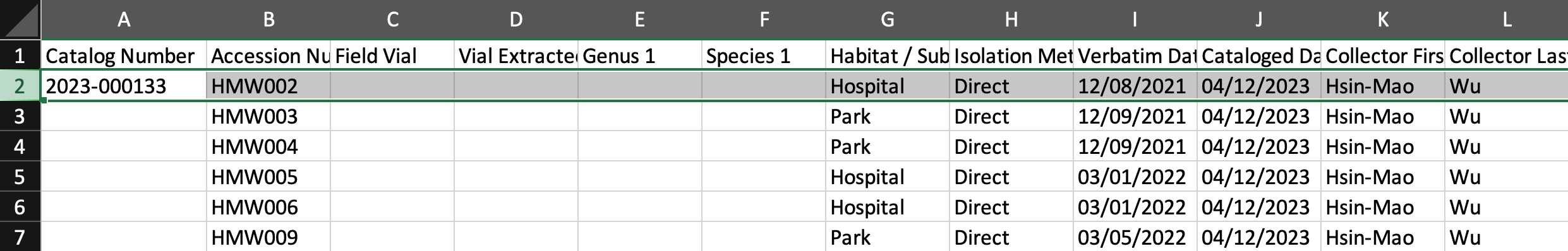
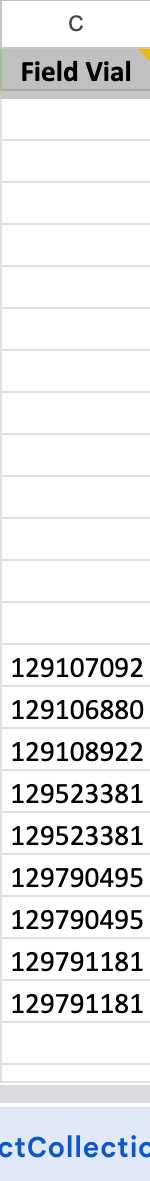
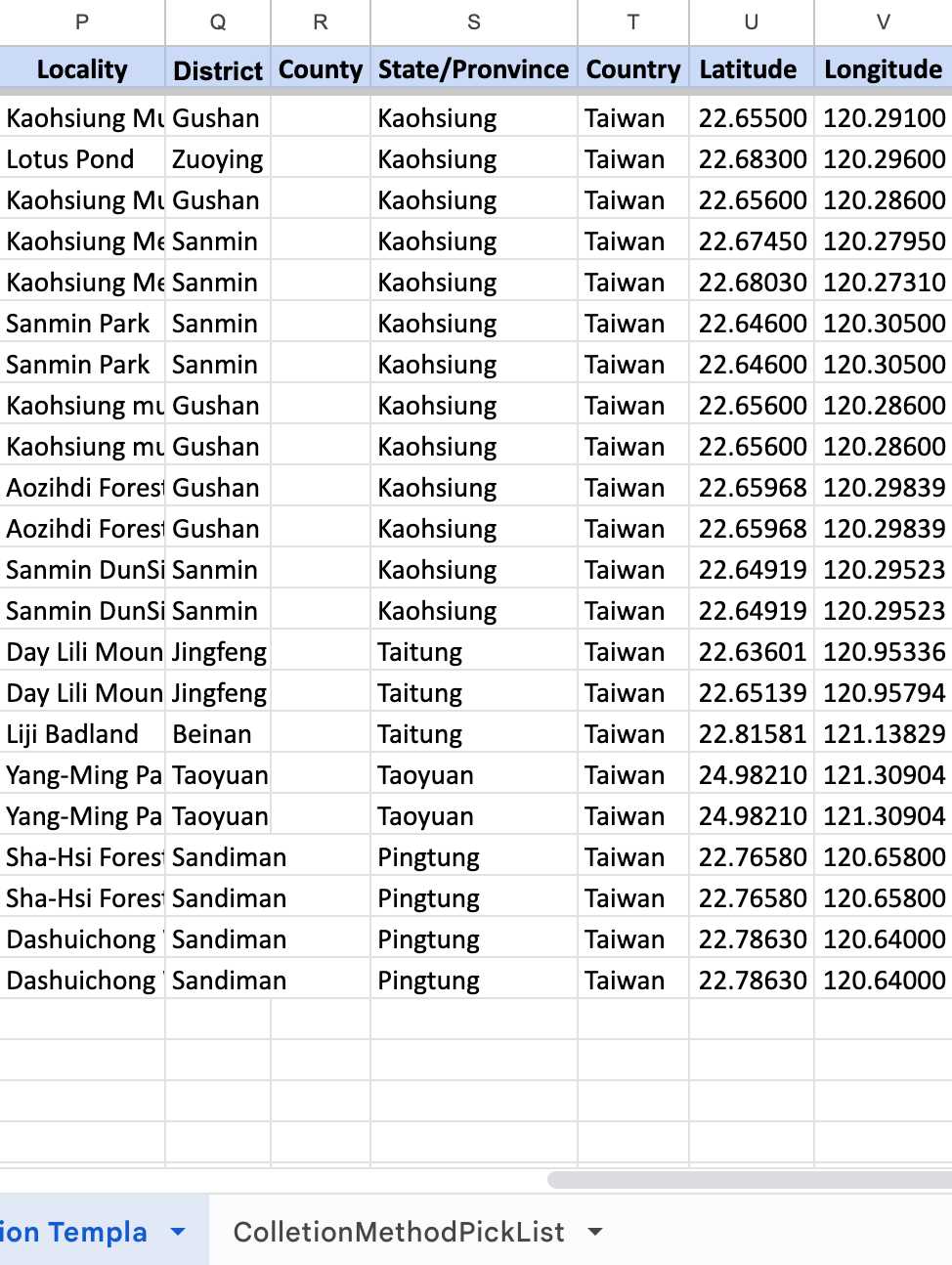
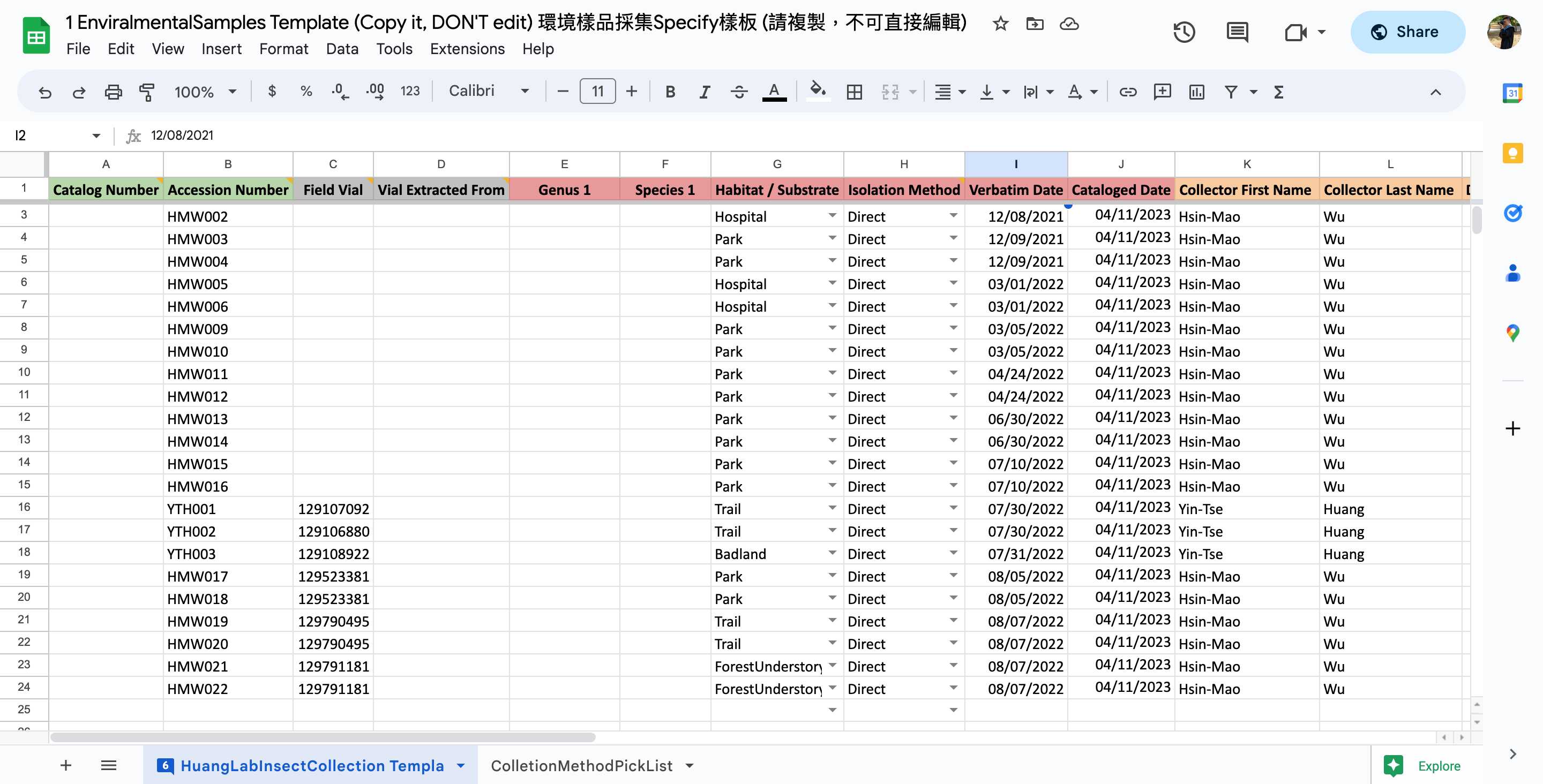
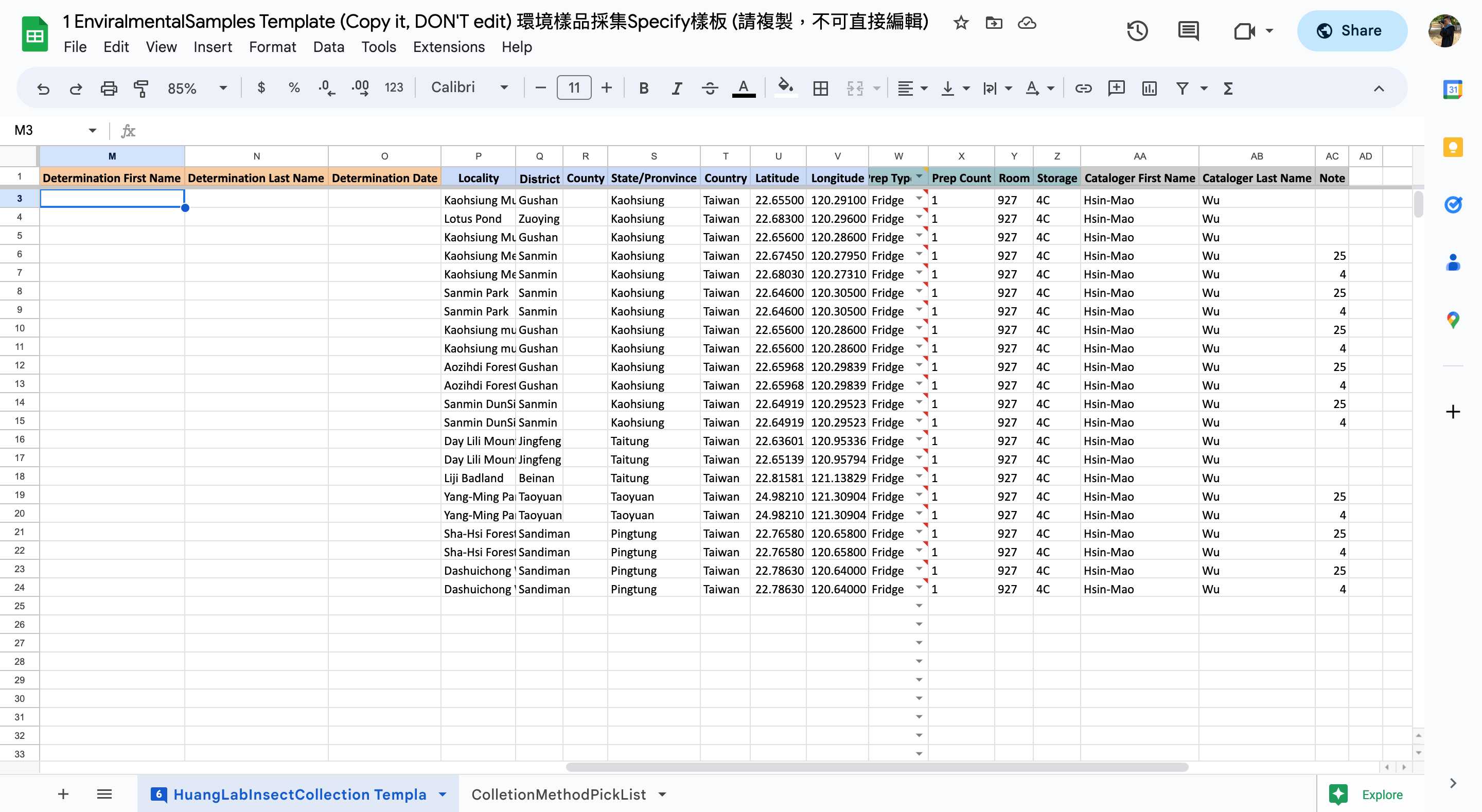
Please catalog them in your Environmental Samples spreadsheet.
<img src="https://static.yanyin.tech/literature_test/protocol_io_true/protocols.io.n2bvj8mk5gk5/Screen%20Shot%202023-04-15%20at%205.08.41%20PM-min.jpg" alt="Make a copy of the template for yourself. The template "1 EnvironmentalSamples Template" marked with blue box is available in HuangLab Collection." loading="lazy" title="Make a copy of the template for yourself. The template "1 EnvironmentalSamples Template" marked with blue box is available in HuangLab Collection."/>
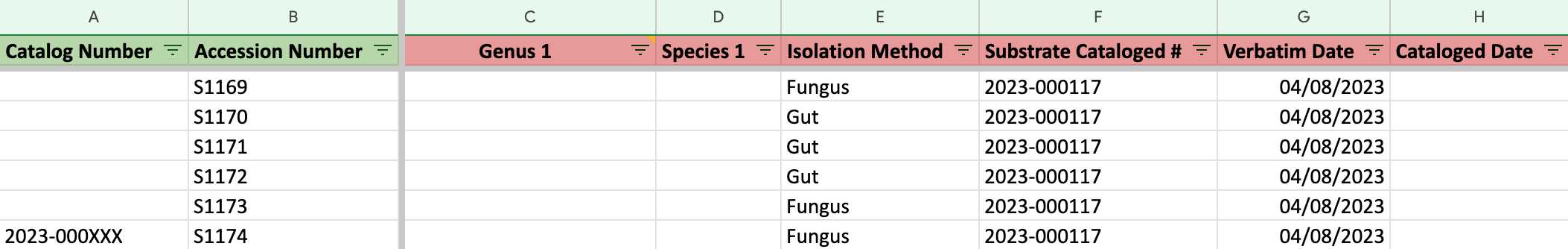
Fill in the columns of the spreadsheet step by step (As shown in the following steps 2.1-2.15)
*In the template, some template data are there for your reference, please delete them in your copy and fill in only your data.
- Remember to scroll to the right and fill in as much as information you can.
For Column E, F, G, G:
If your samples are SOIL SAMPLES , please follow this example.
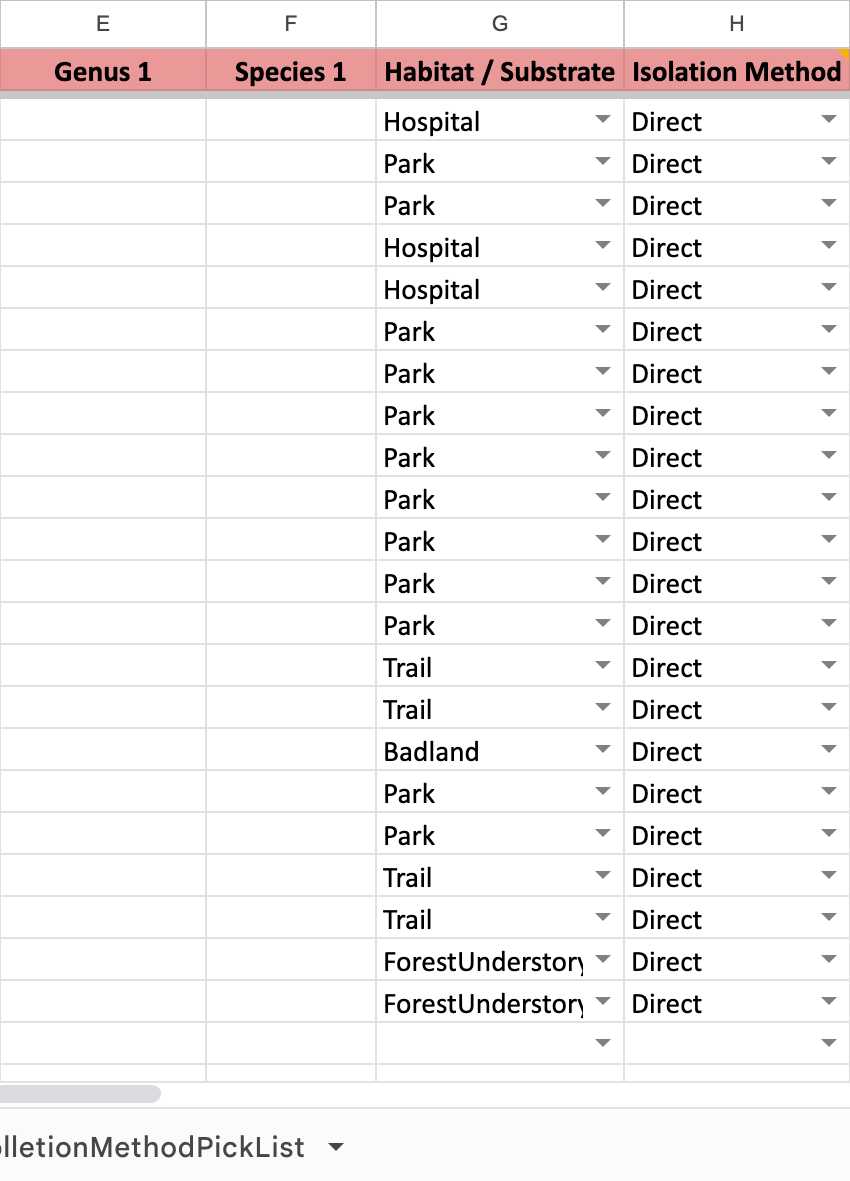
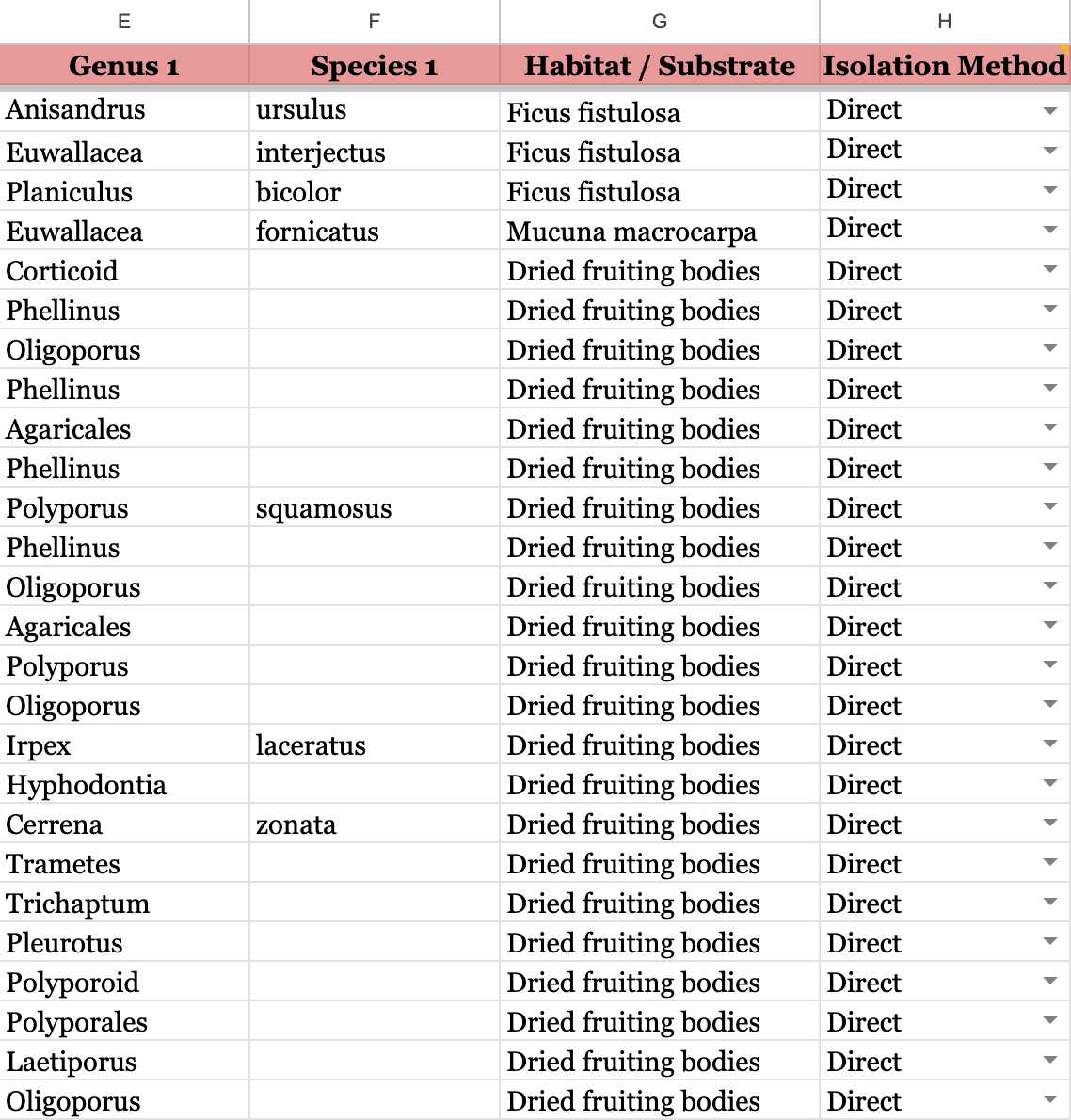
If your samples are ENVIRONMENTAL SAMPLES , please follow this example.
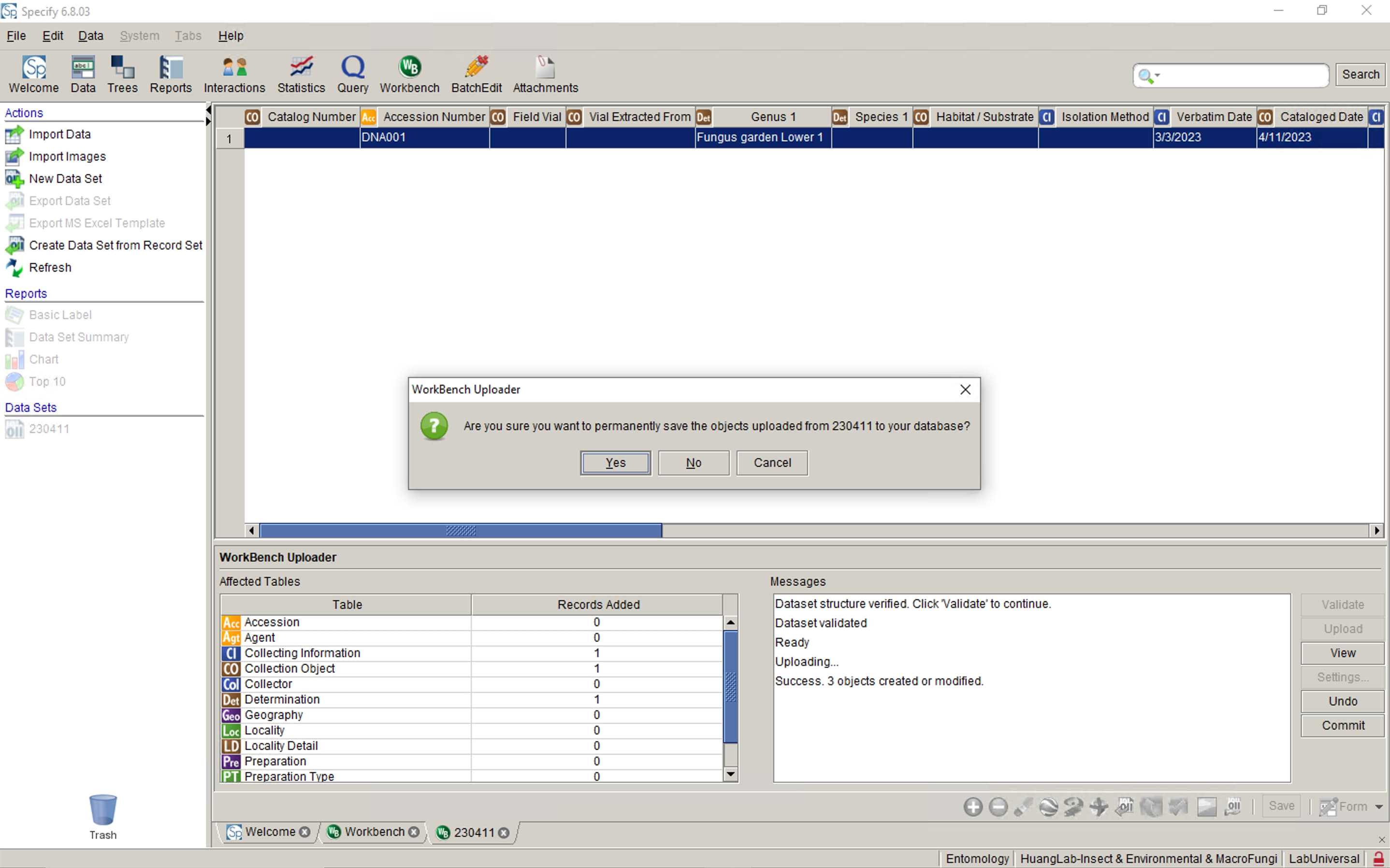
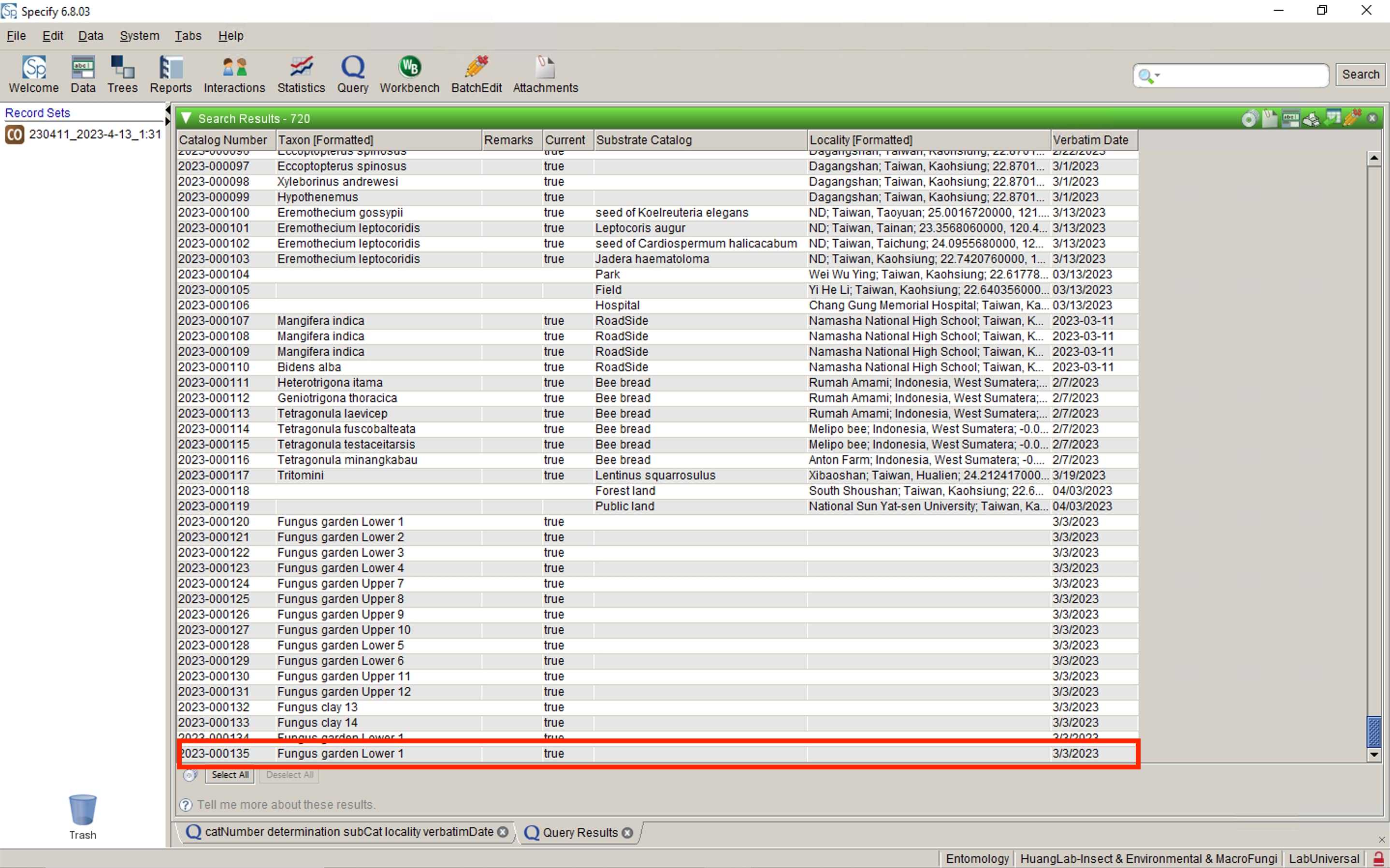
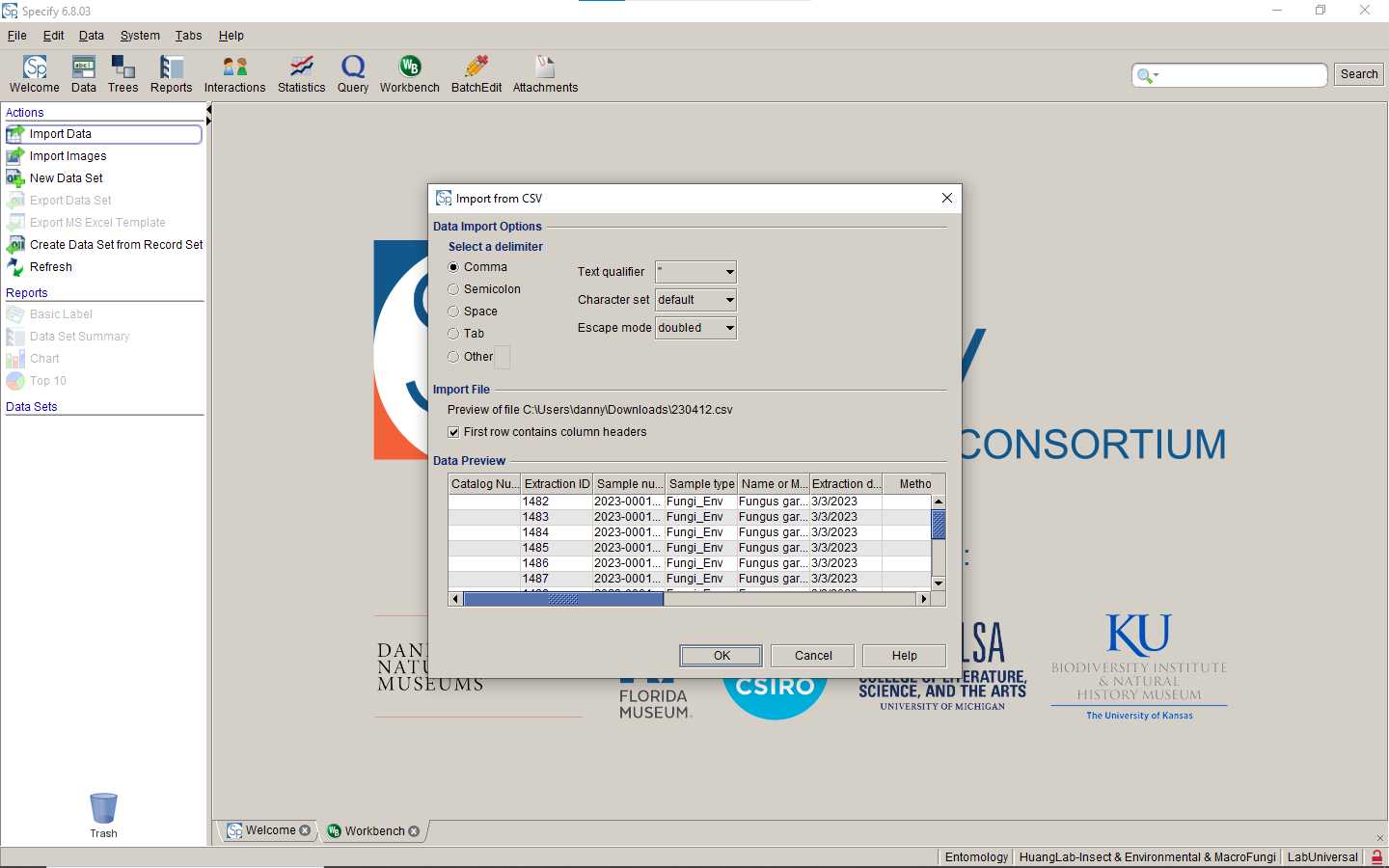
Upload your datasheet to Specify database
Paste the Catalog Number of uploaded data back to your spreadsheet.
Isolate the microbes from your environmental samples
Now, move the equipment and materials for microbes isolation back to the sterilized area.
Before isolation, key the information about your isolation (how many plates you are going to isolate, what kind of media you will use, etc) into the 1 Fungi Isolation Plates (Direct edit) 菌株分離培養基編號 (直接編輯) spreadsheet first
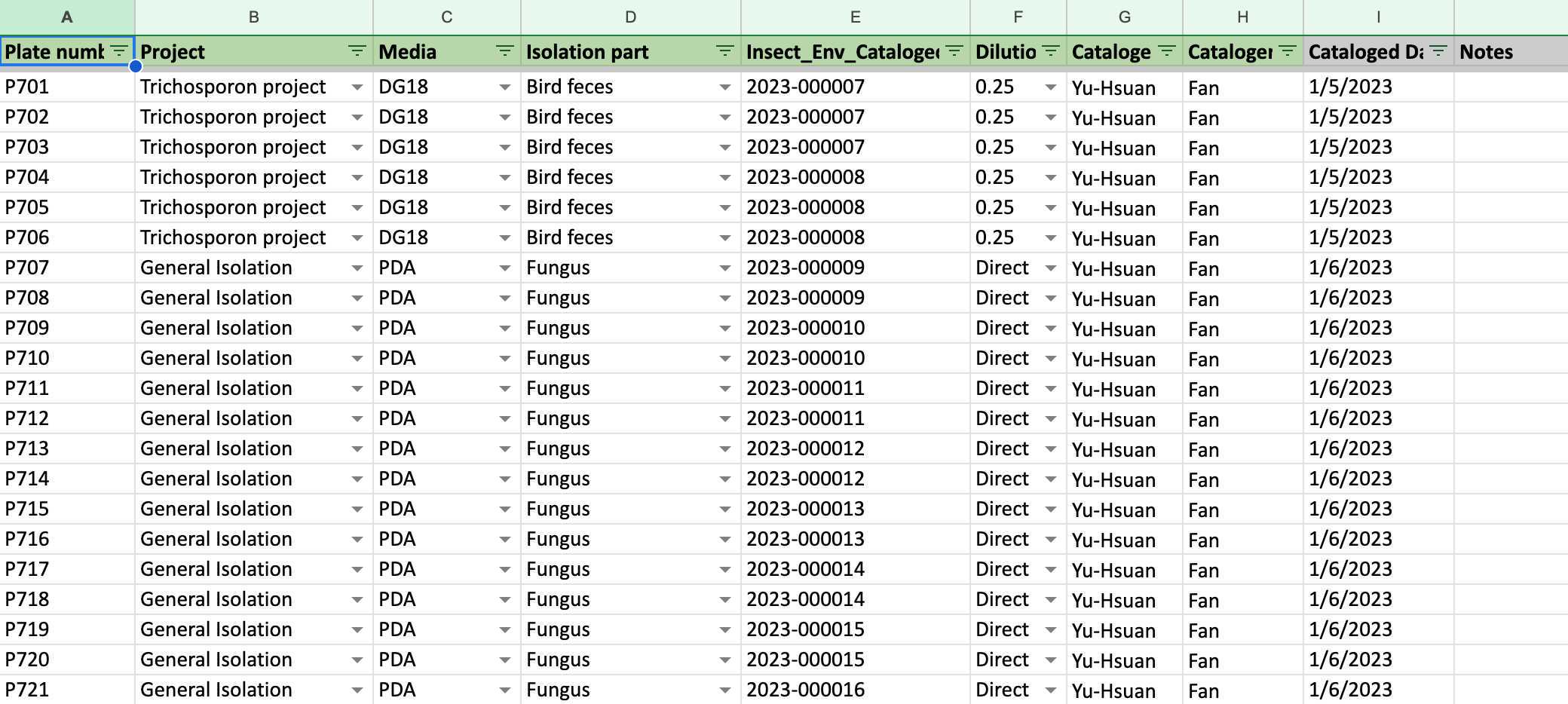
Make sure that you sterilize every tool before using them for each isolation:
-
Dip the scalpel, tweezer, or spreader into 95% ethanol cooling bottle, and flame it immediately.
-
Repeat the step(dip and flame) THREE times for sterilization of equipment.
Now, you can start to isolate microbes from your environmental samples. We called them primary cultures.
Isolation method varied depend on the type of sample, check published papers or consult with seniors if needed
Pack all the plates using a PP bag (no. 8, 10, or 12 depending on how many plates you have). Incubate the plates in your slot in the incubator for 7~21 days (depending on the growth rate of the microbes).
no. 8: 8 plates
no. 10: 24 or 32 plates
no. 12: 48 or 56 plates

Check the growth condition of the primary cultures every day. Make sure they are not overgrown.
Once they are matured, you can start to subculture them.
Record and upload the data of primary cultures
-
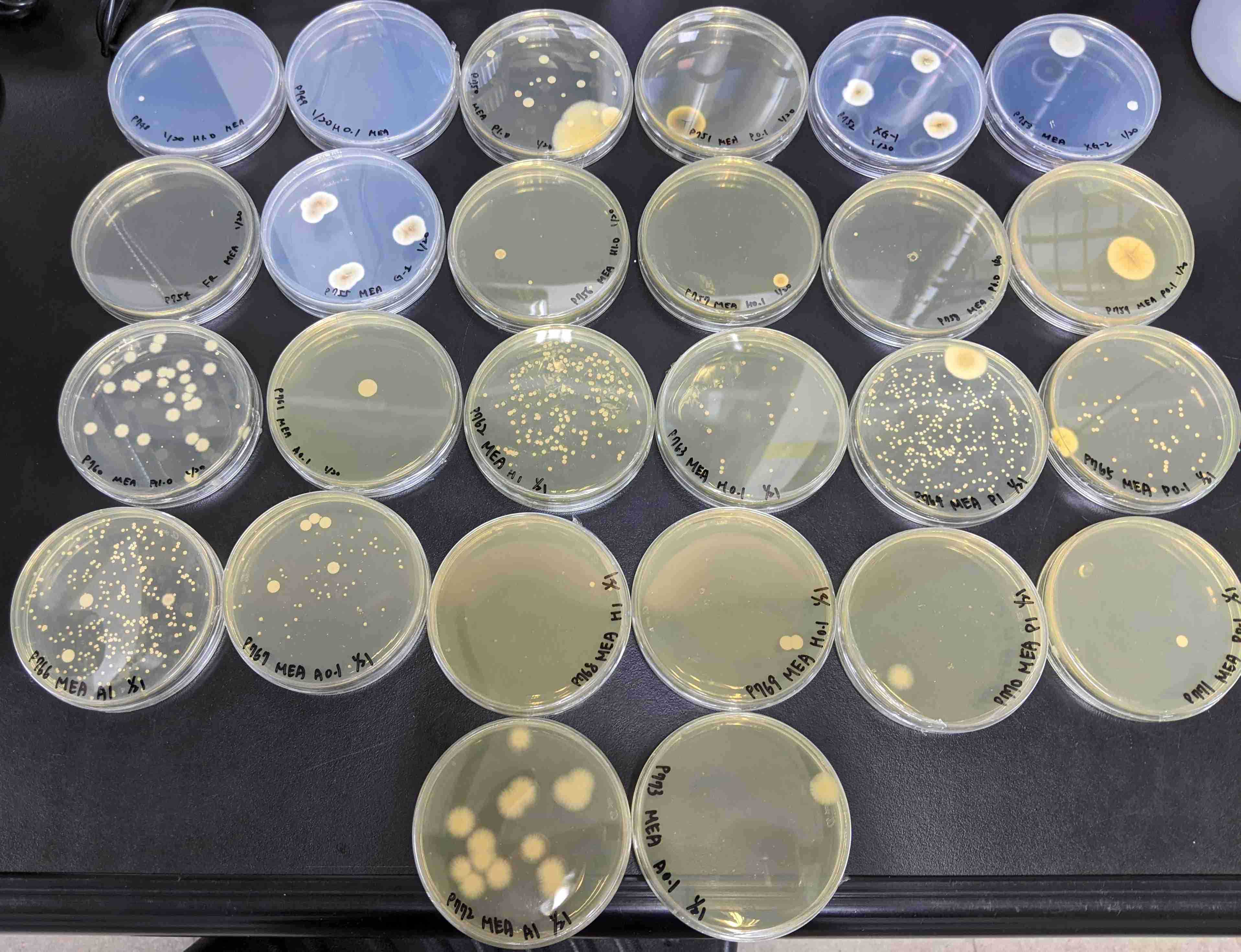
Examine the microbes colonies under a dissecting microscope.
-
Choose your desired colonies, which are your targeted colonies.
-
Mark the targeted colonies with their later corresponding secondary plate number.
-
If there is more than one morphotype targeted colony in a certain plate, you need to record them into a distinct plate number, e.g., TWO morphotypes will have TWO distinct secondary plate numbers from the same primary plate number.
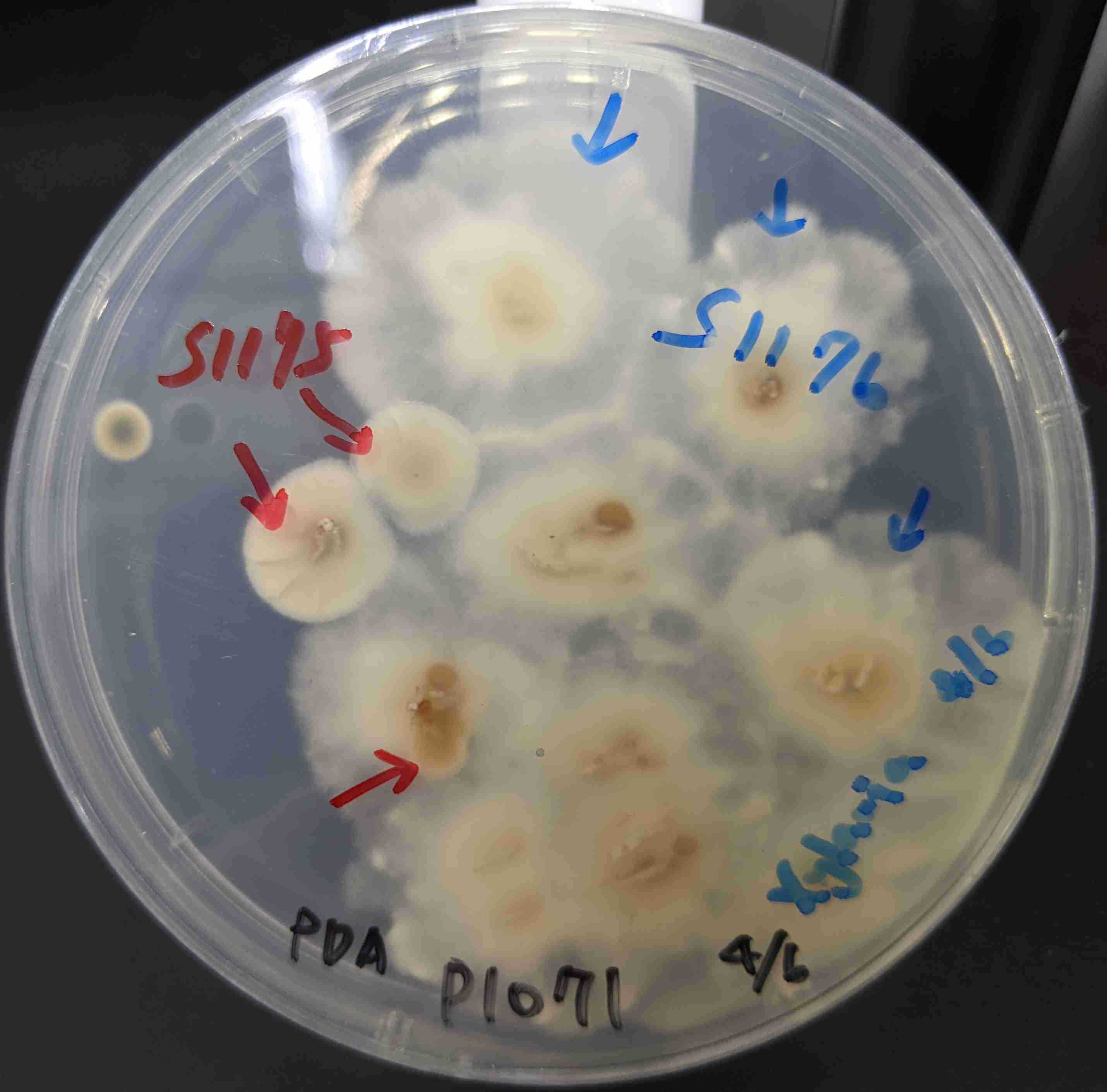
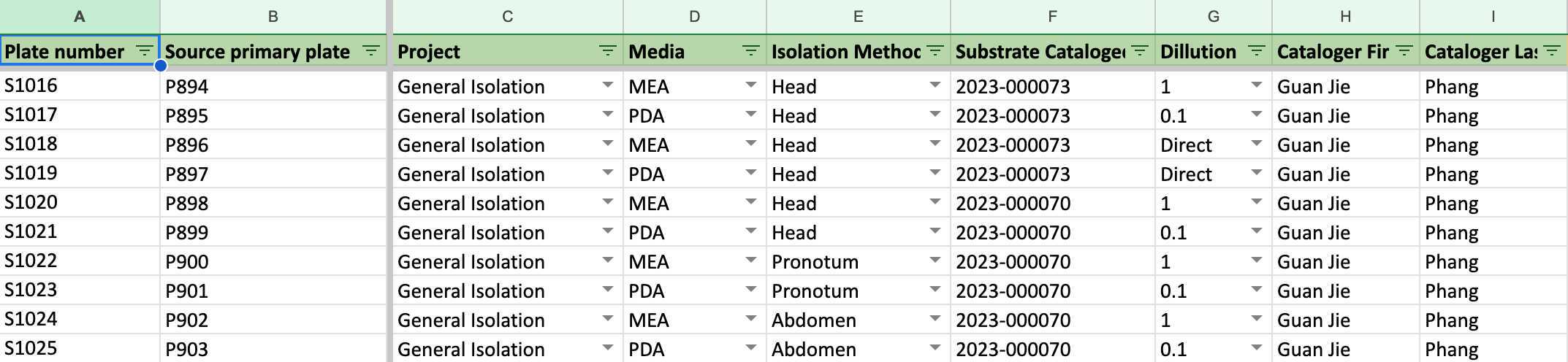
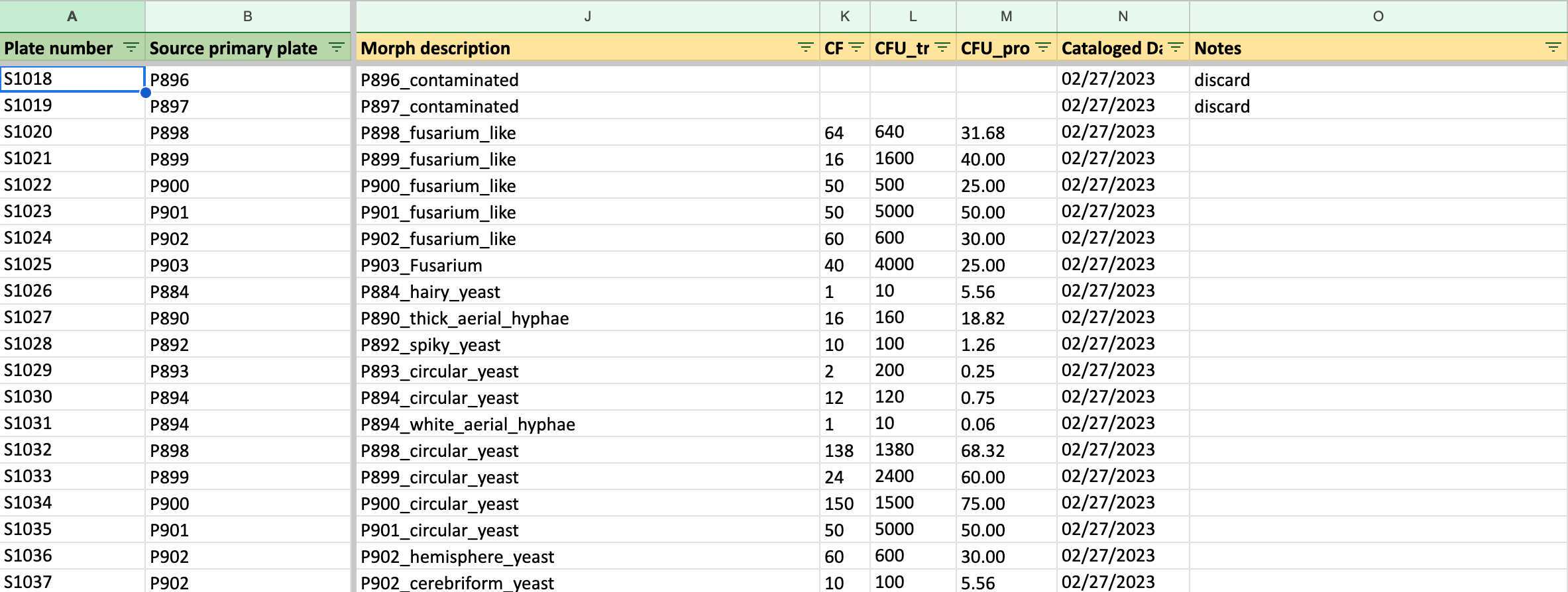
Record all the data of primary cultures to the second worksheet: Secondary Isolate PDA (in 1 Fungi Isolation Plates (Direct edit) 菌株分離培養基編號 (直接編輯)).
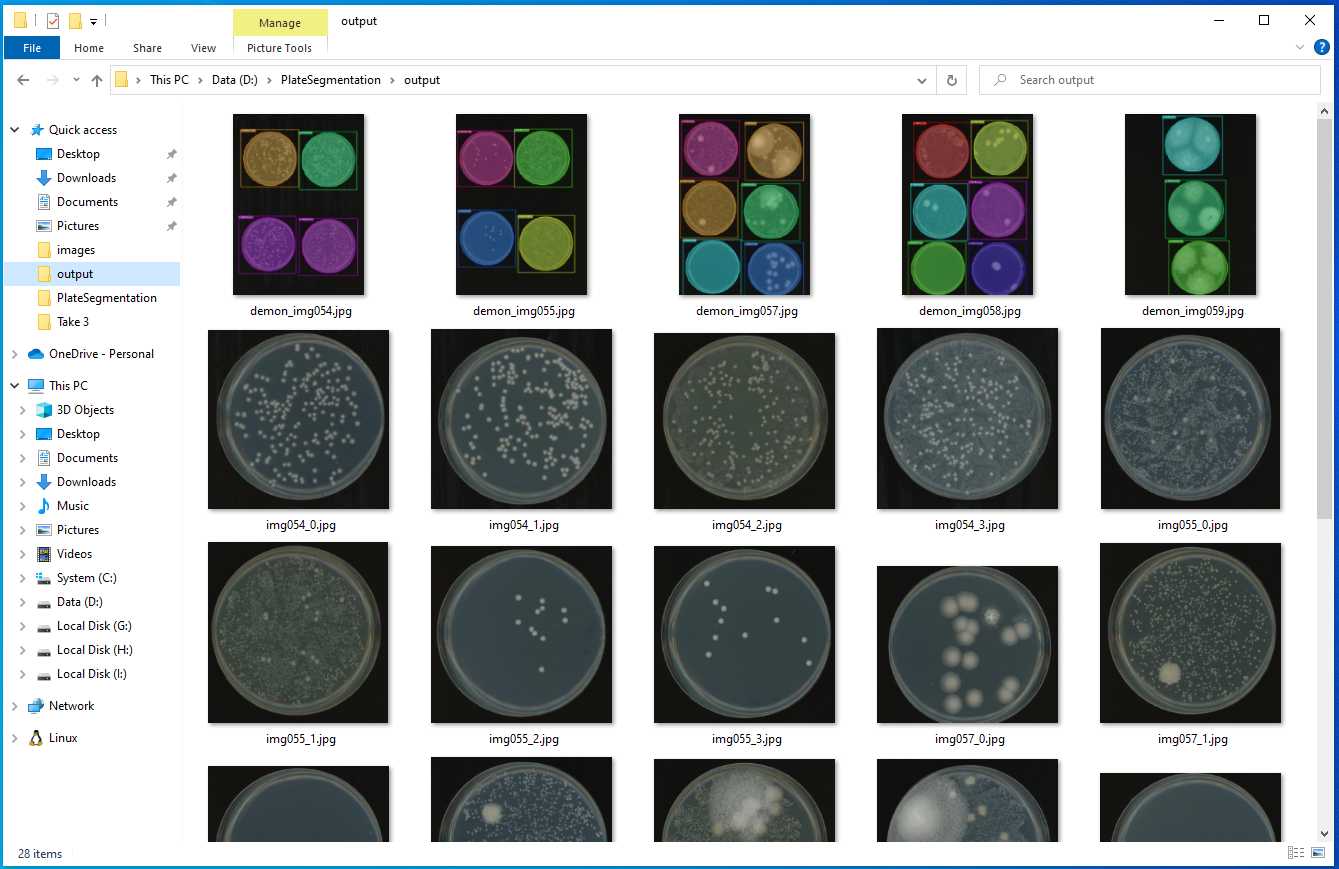
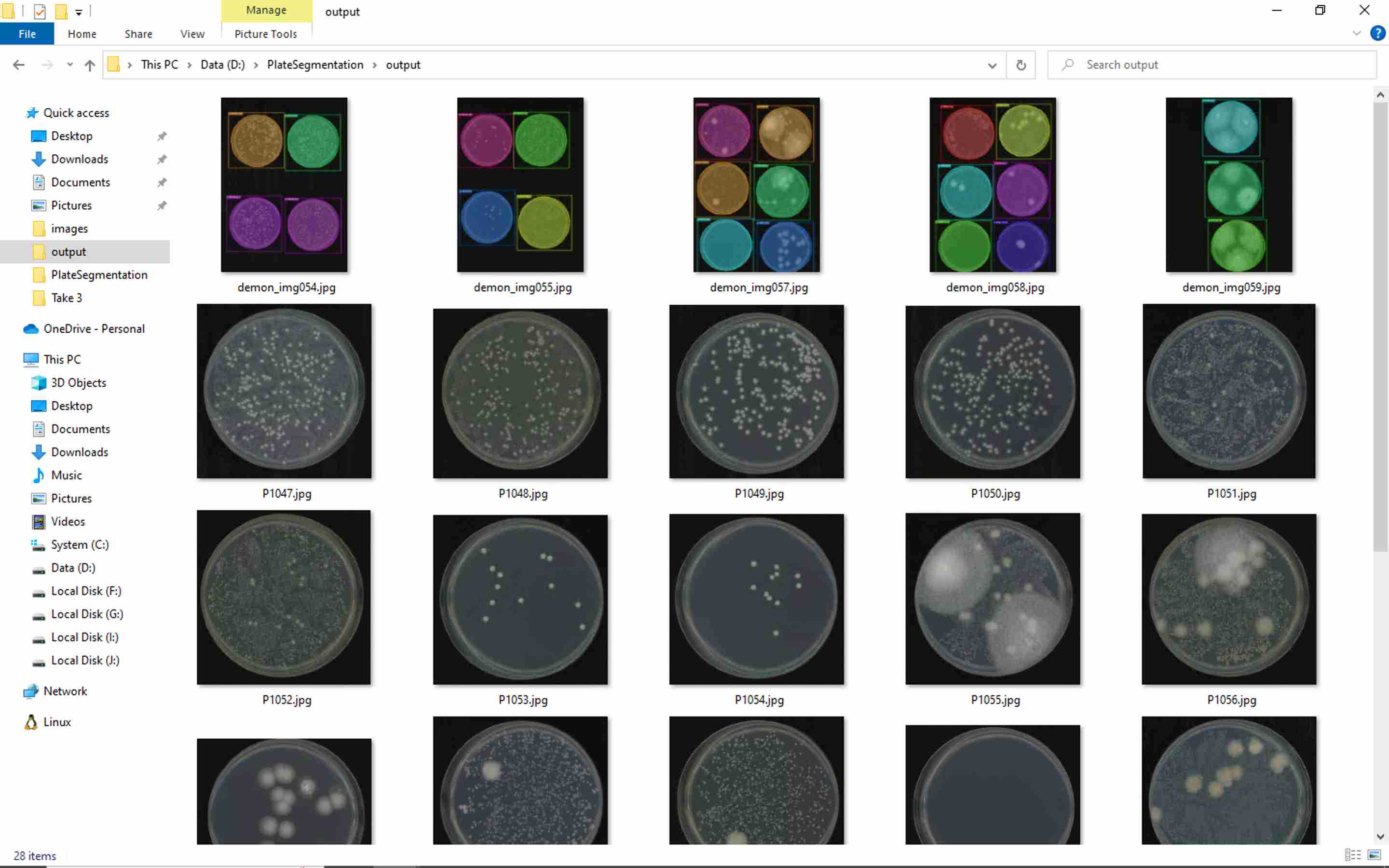
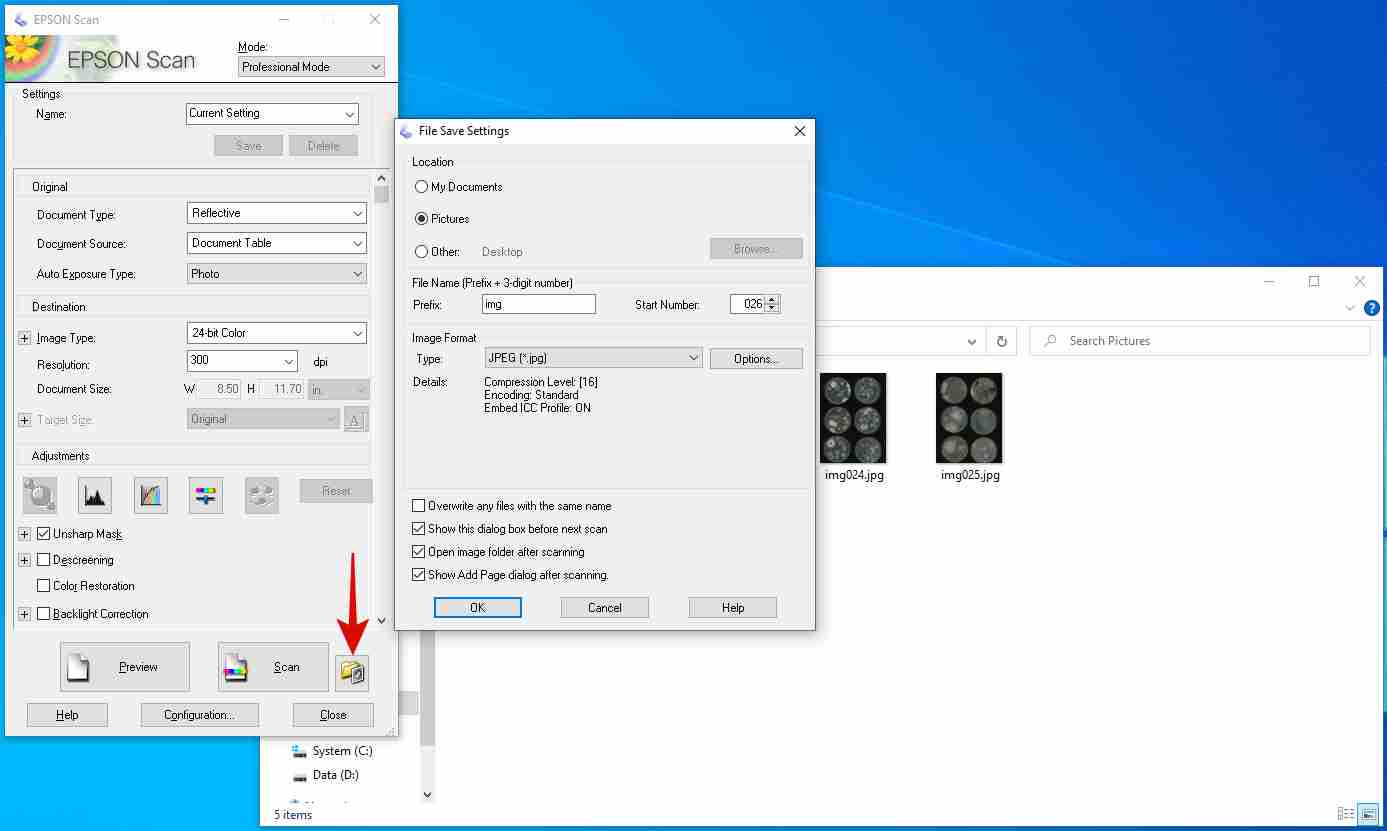
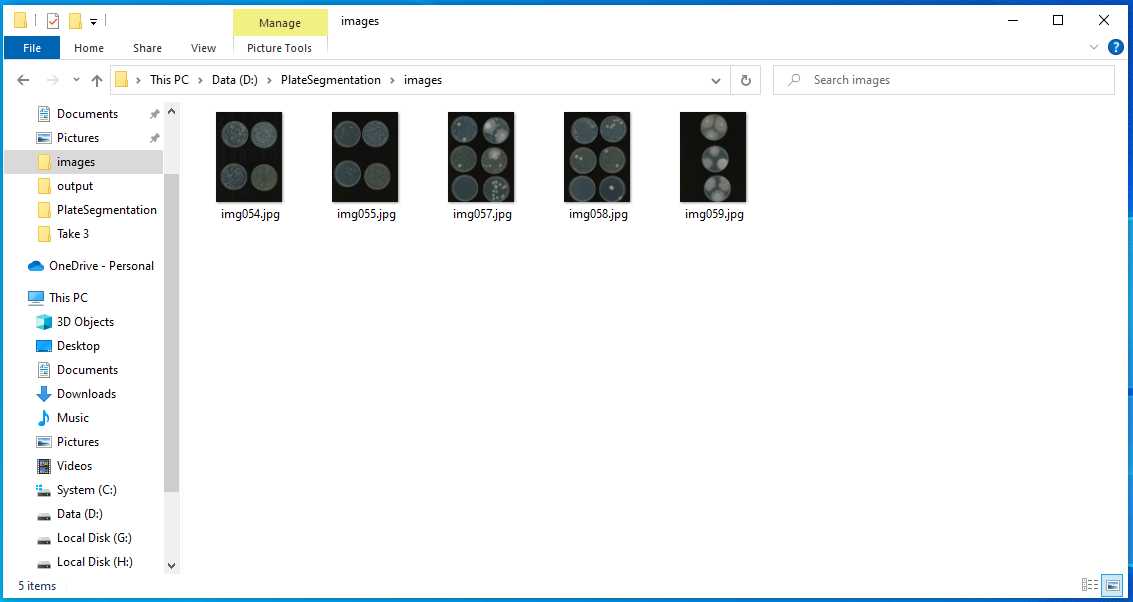
Fill in all the columns following the photos and instructions shown below.
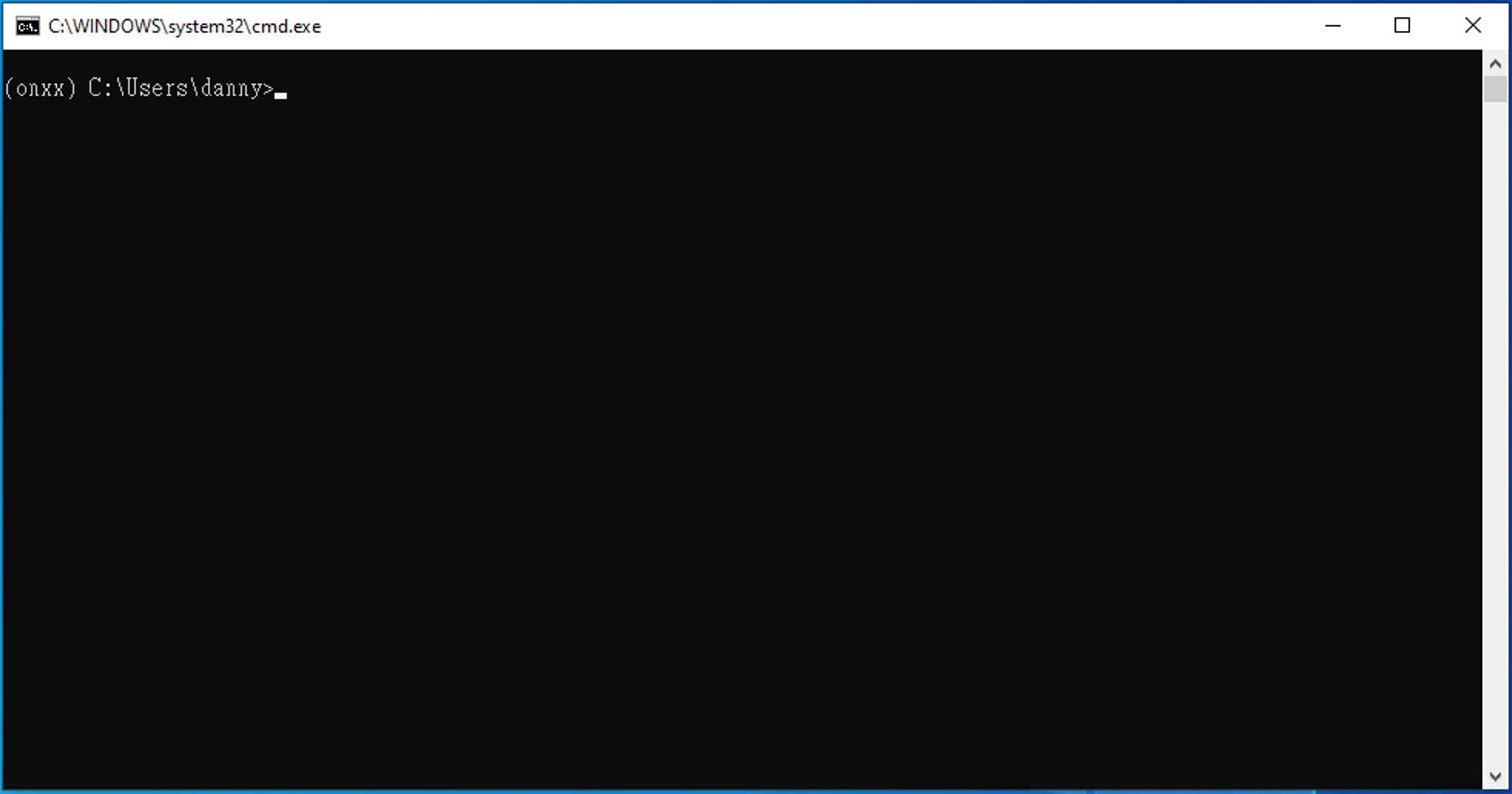
Follow the commands, and you will have the similar output as the photos shown below.
Type D: , then press enter
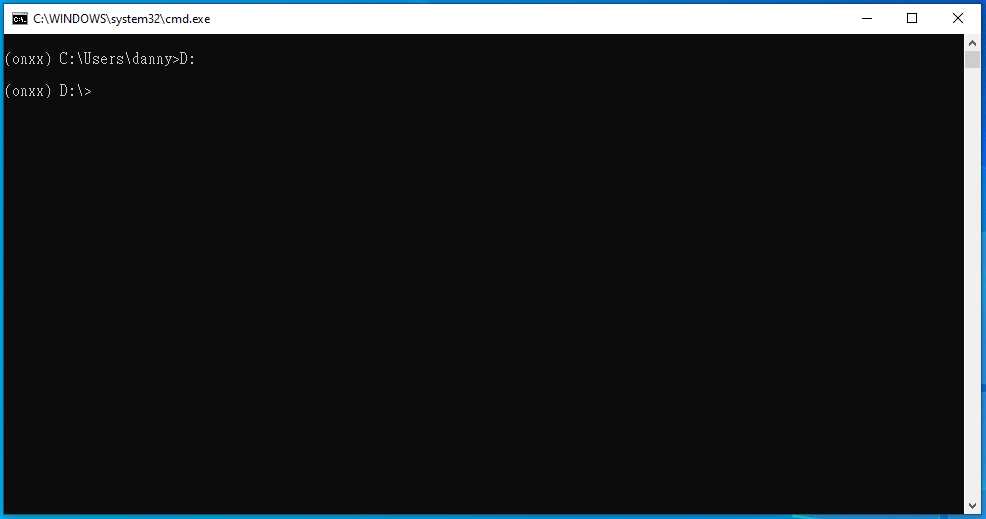
Type cd PlateSegmentation , then press enter
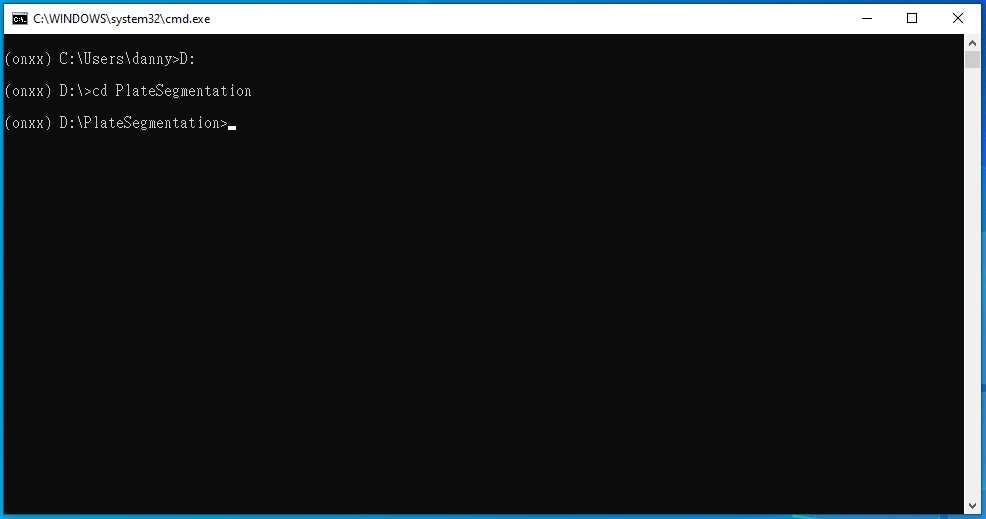
Type python ONXX_predict.py , then press enter
Press enter again and again. You will see a _ flashing.
Then, a new command line comes out, which represent the program execution was complete.
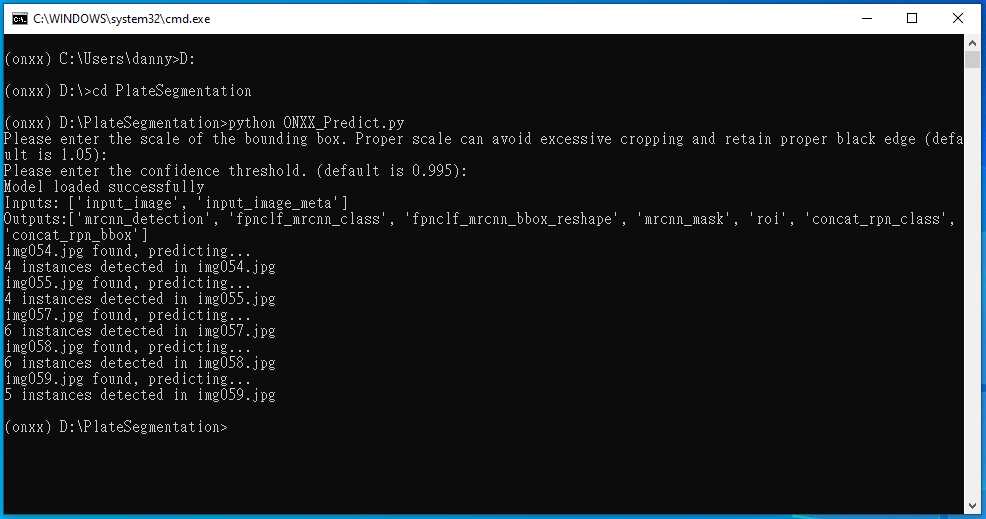
Subculture the primary cultures
Follow to for the subculture of primary cultures. Now, we called them secondary cultures .
You may need to keep the primary cultures for a while before the secondary plate are all secured.
Ready for preservation
After 7-21 days (depending on the growth rate of your targeted microbes), the secondary cultures are ready to be preserved. If the secondary cultures were contaminated, please redo the subculture from the primary cultures.
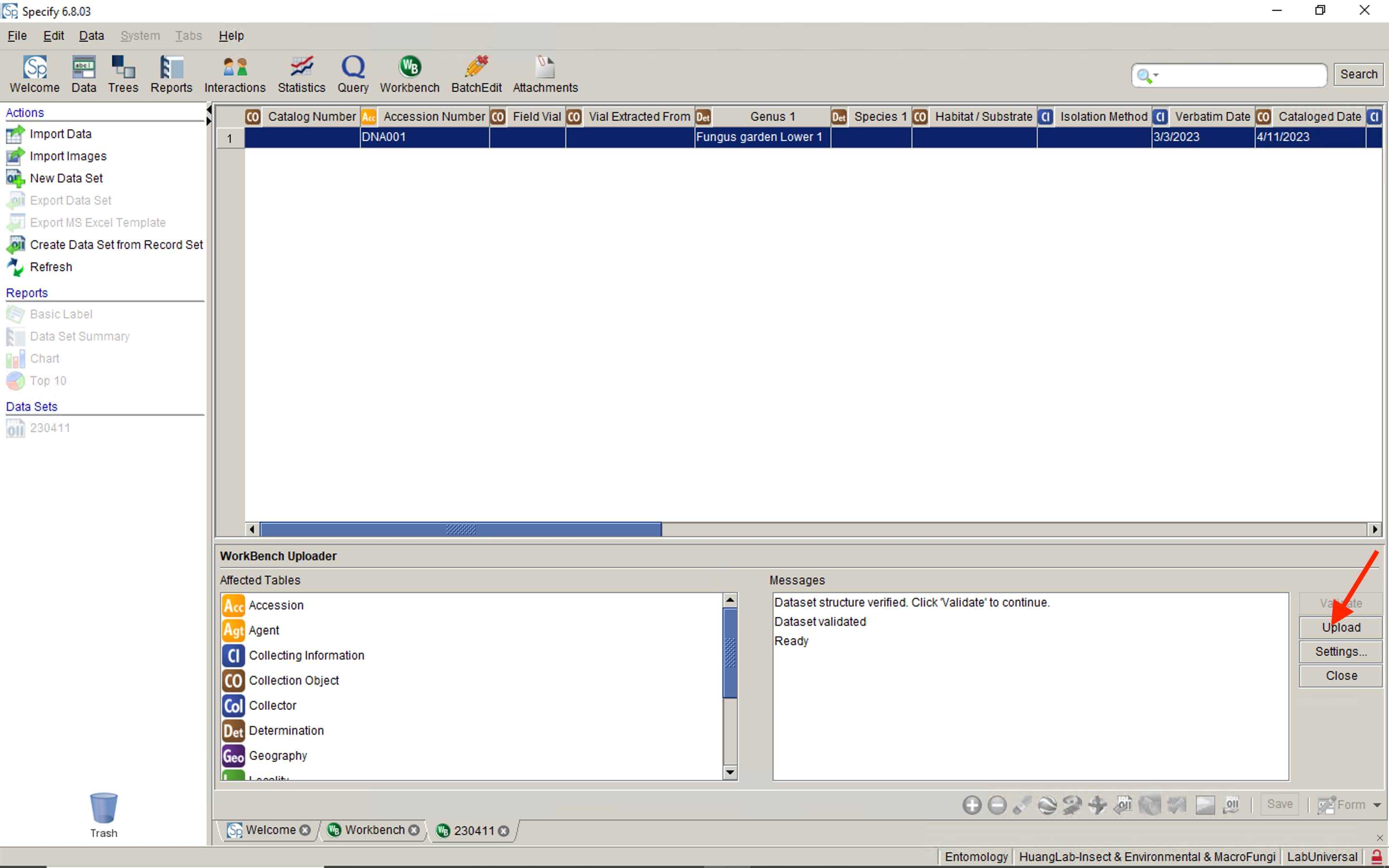
Before prepare preservation vials, the For Specify cateloging COPY to MODIFY!!! worksheet need to be filled.
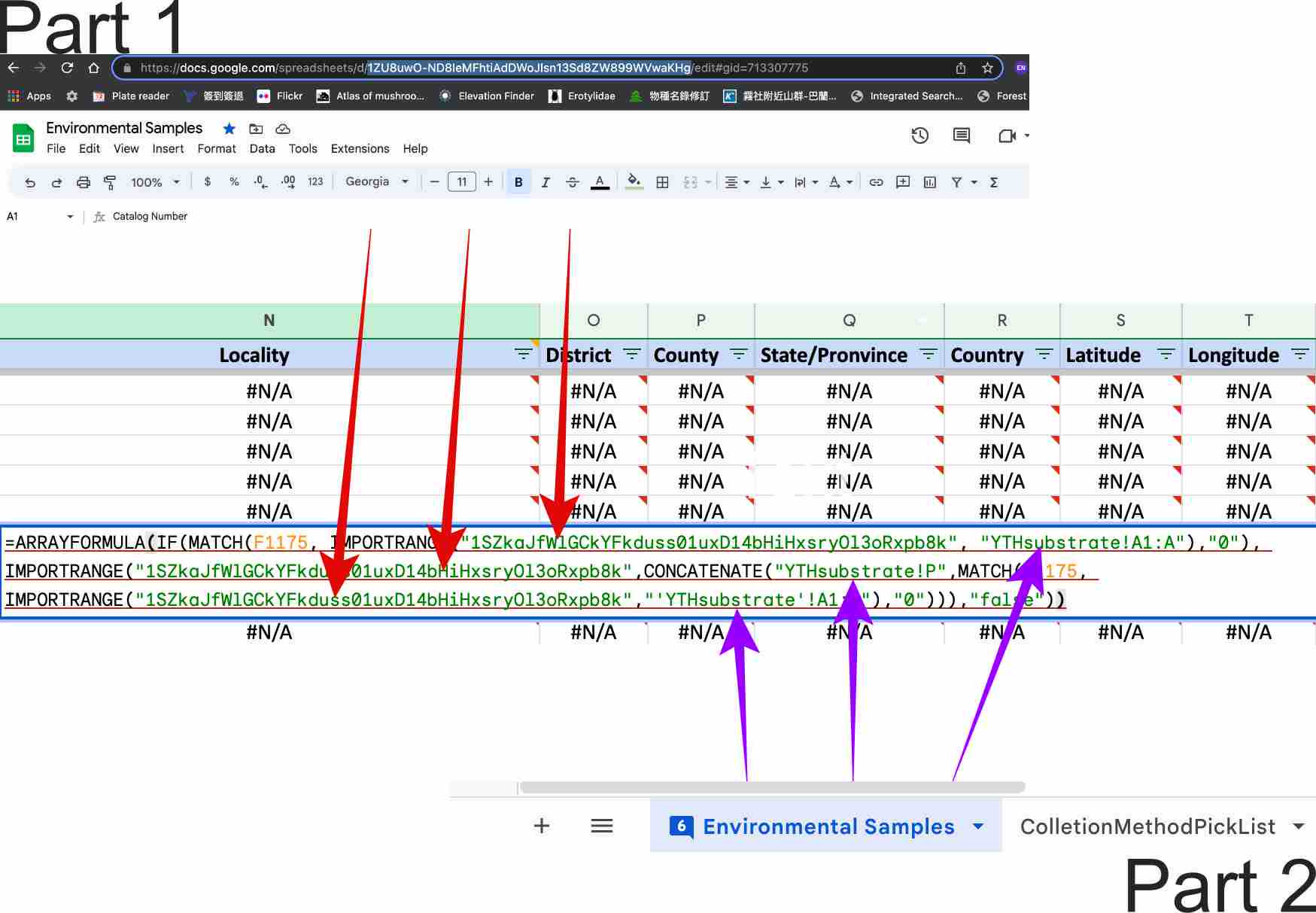
There is 2 part for all the locality information, you need to replace the each information: spreadsheet code (red arrow pointed), worksheet name (purple arrow pointed) with your own spreadsheet information.
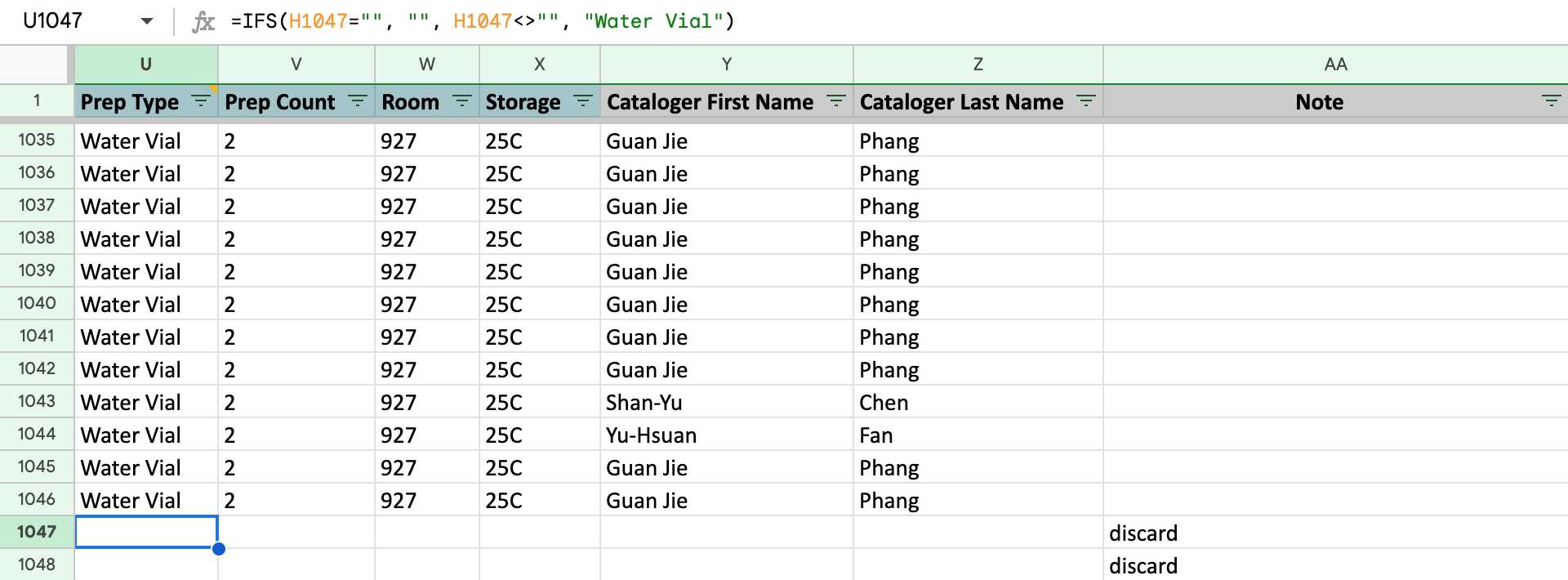
If the Cataloged Date is filled, the Column U to Column Z is auto-filled with the written formula. Do not change it unless it needed. The Column AA is synchronized with the Column O of the Secondary Isolate PDA worksheet.
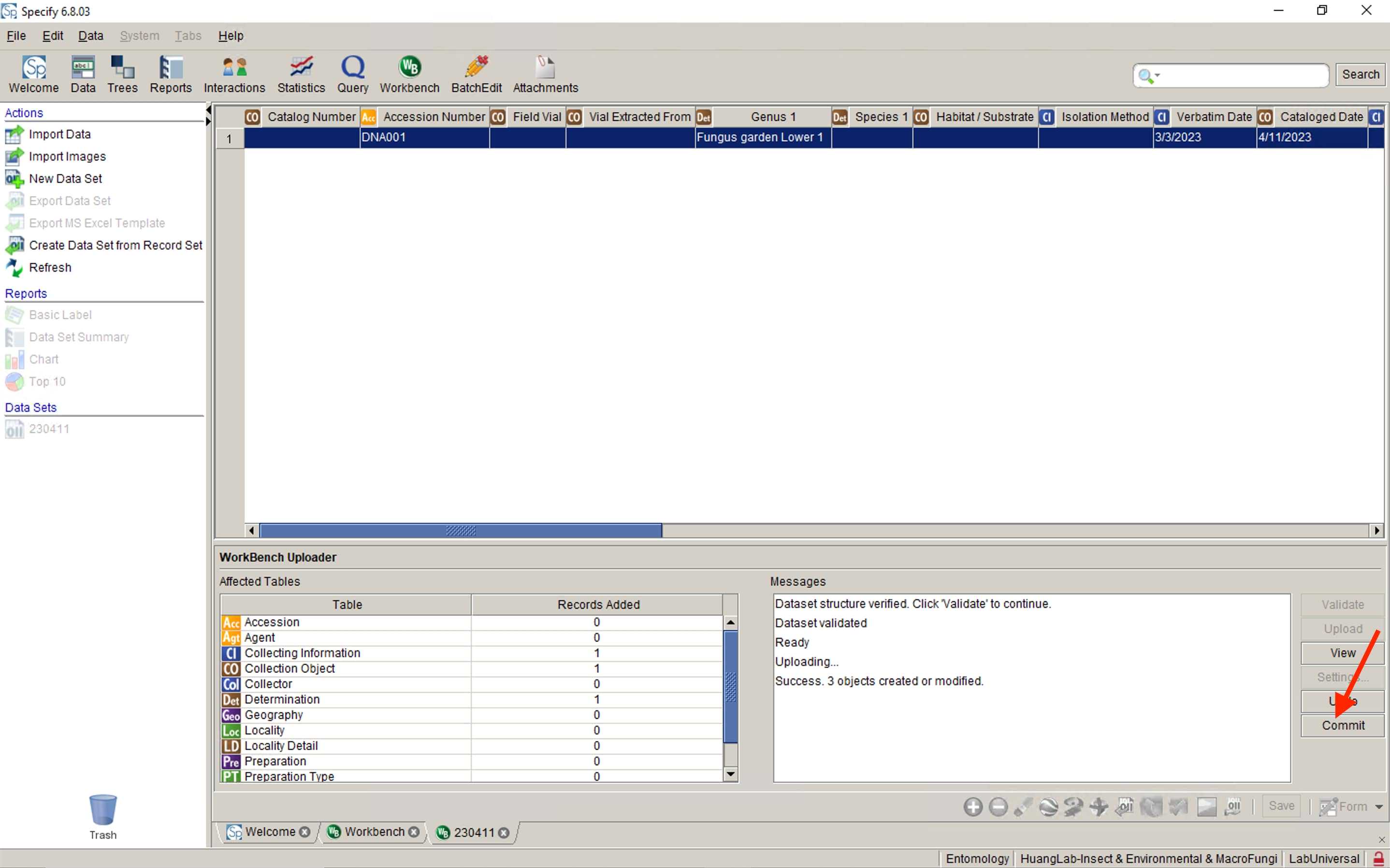
Follow to for preservation vials data upload.
For each Catalog Number , you must prepare 2 water vials and 2 glycerol vials. You also need a 2 ml microtube with lysis mixture and beads (for details, check DNA extraction protocols ).
Each tube needed a sticker with its corresponding Catalog Number on top of the cap. The label should be written with a black color marker pen or a technical pen alternative.
Please follow the example in the photo shown below.

The lysis mixture microtubes that are ready for use will look like this.

Operate UV sterilization of the laminar flow for 15 minutes. After UV sterilization, you need to ethanol-sterilize the materials that are going into the laminar flow.
Light up the alcohol lamp and flame the material.
The materials you need are the same as , but you only need , but you only need a scalpel instead of a tweezer and a spreader.
Culture preservation
Preserve the plates with one water and one glycerol vial for one colony. The rest is for DNA extraction. Make sure you flame-sterilize the scalpel every time before slicing the agar.
The details of culture preservation are not provided here.
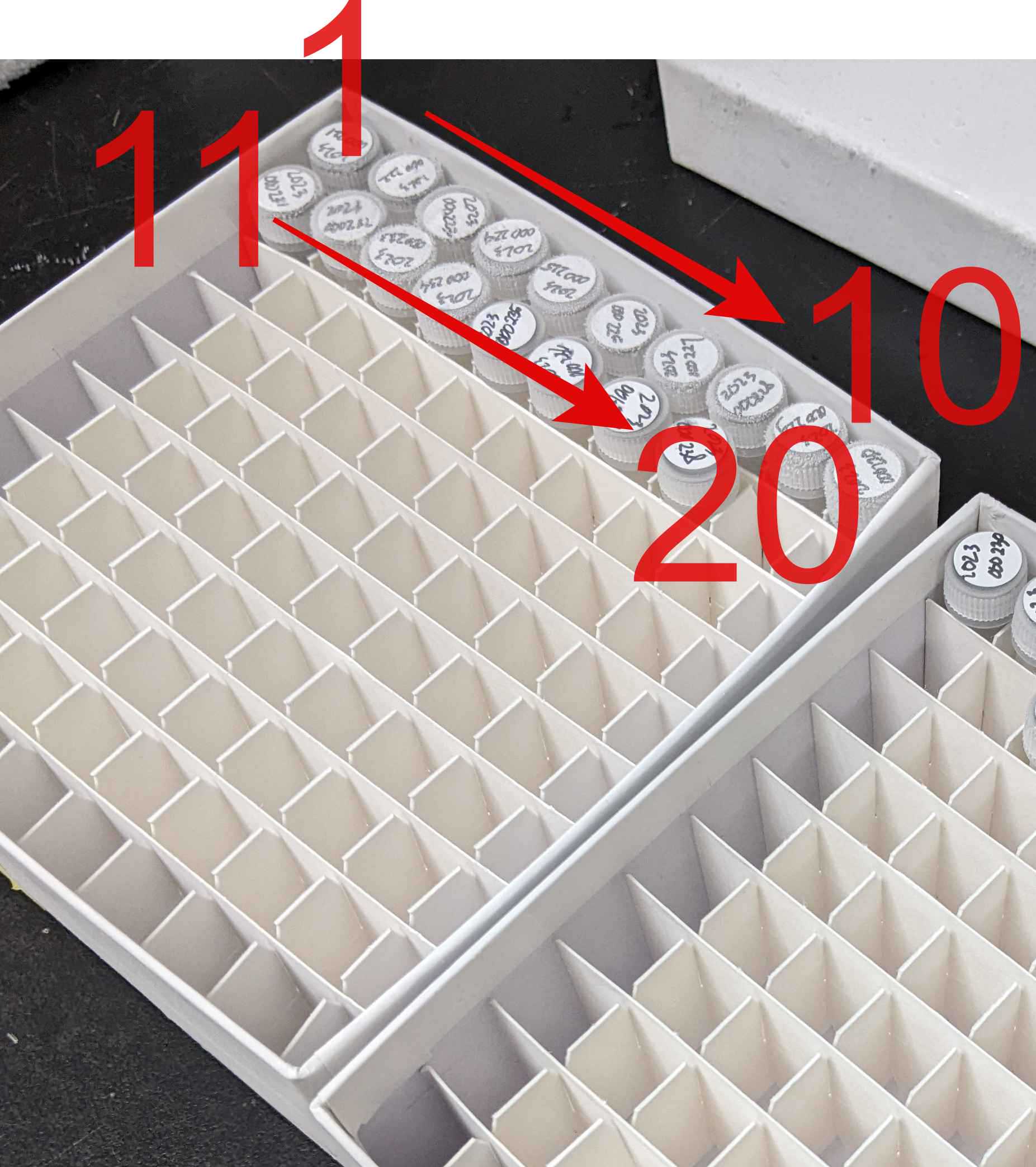
Store the preservation vials in their corresponding freezing box.
For water vials, you can keep them directly.
For glycerol vials, you need to keep them in a freezing container and then freeze them inside a -20ºC fridge for 1 day before keeping them in the -80ºC fridge collection.
The freezing boxes of water vials are kept in the steel cabinet in front of Prof. Huang's office; the freezing boxes of glycerol vials are kept in the -80ºC fridge outside N927