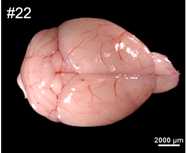
Sectioning and HE staining of Mouse Brain and embryo by Cryostat
Yuting Fu, jiashikai, Xiaodong Liu
Disclaimer
The protocol content here is for informational purposes only and does not constitute legal, medical, clinical, or safety advice, or otherwise; content added to protocols.io is not peer reviewed and may not have undergone a formal approval of any kind. Information presented in this protocol should not substitute for independent professional judgment, advice, diagnosis, or treatment. Any action you take or refrain from taking using or relying upon the information presented here is strictly at your own risk. You agree that neither the Company nor any of the authors, contributors, administrators, or anyone else associated with protocols.io, can be held responsible for your use of the information contained in or linked to this protocol or any of our Sites/Apps and Services.
Abstract
This protocol describes how to use the cryostat to prepare and slice mouse brain and embryo sections for HE staining and following spatial transcriptomic experiments.
Steps
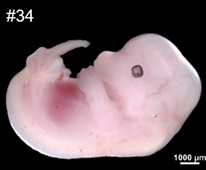
Preparation Methods for E12.5 mouse-embryo-eye
E12.5 pregnant female mice was anesthetized with carbon dioxide, the whole uterus was
collected and washed 3 times in ice-cold DPBS;
The yolk sac was collected to extracted DNA for genotyping (identification of sex);
Using dust-free paper to gently wiped the liquid on the surface of the embryo, the
embryo was rinsed with ice-cold Tissue-Tek OCT (Sakura, 4583), and then moved
to encapsulation box with ice-cold OCT;
Mouse embryos were collected from pregnant C57BL/6J female mice at embryonic day 12.5
(E12.5). Mouse brain was dissected from 8-week-old C57BL/6J male mice;
The location of embryonic eye was circled, and marked the orientation of embryo,
then tissues was transferred to a -80℃ freezer for snap-frozen and storage;
Embryos of average size and normal phenotype were selected for subsequent cryosection
and sequencing (note: the embryos used in our benchmarking analysis came from a
litter of mice);
The tissue block was smoothly glued to the sample head, so that the embryo was
sectioned in sagittal position. If necessary, the angle can be fine-tuned so
that the blade section is strictly parallel to the cross-section of the tissue
block;
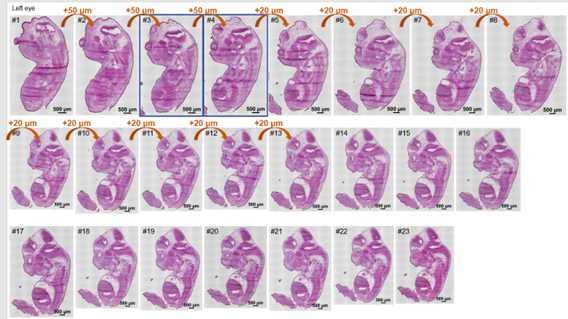
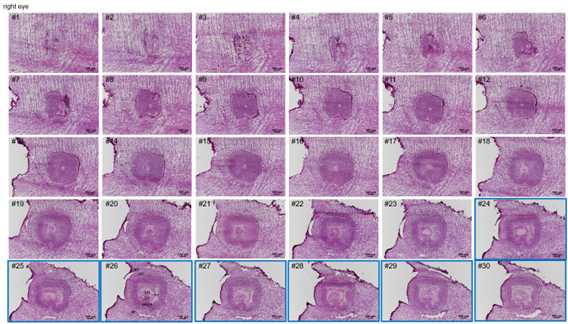
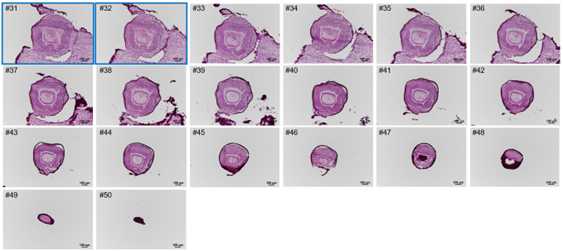
Cryosections were cut at a thickness of 10 μm, both left eye and right eye can be collected;
HE staining procedure: cryosections were balanced at room temperature for 30 min,
and then fixed with 4% PFA for 3 min. Then, the sections were washed with dd22O
for 2 min, stained with hematoxylin for 6 min, washed with d2H2O,
stained with eosin for 2 min, washed with 2dH2O. After that, sections were gradient dehydrated (75% ethyl alcohol for 1 s, 85% ethyl alcohol for 1 s, 95% ethyl alcohol for 1 s, 100% ethyl alcohol for 1 s, 100% ethyl alcohol for 1 min), cleared (xylene for twice), and sealed with Permount TM Mounting Medium after airing. Finally, the figure was scanned using Motorized Fluorescence Microscope (Nikon, Ni-E).
Preparation Methods for male mouse brain
8-week-old male mice was anesthetized with carbon dioxide and decapitated;
The air bubbles were carefully removed with the syringe, and the brain was placed
properly with tweezers;
The location of hippocampus was circled, and marked the orientation of brain, then
tissues was transferred to a -80℃ freezer for snap-frozen and storage;
Brain samples of average size and normal phenotype were selected for subsequent cryosection
and sequencing;
The tissue block was smoothly glued to the sample head, and the cerebellum was oriented towards the experimenter, so that the brain was sectioned in coronal position. If necessary, the angle can be fine-tuned so that the blade section is strictly parallel to the cross-section of the tissue block;
Cryosections were cut at a thickness of 10 μm;
The structure of the sequenced cryosections were shown in the followed image:
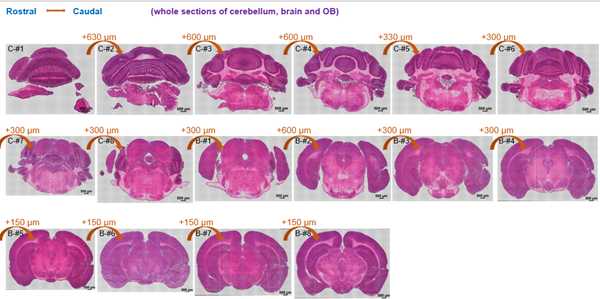
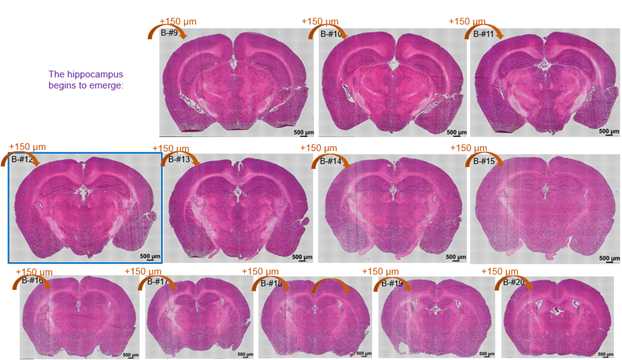
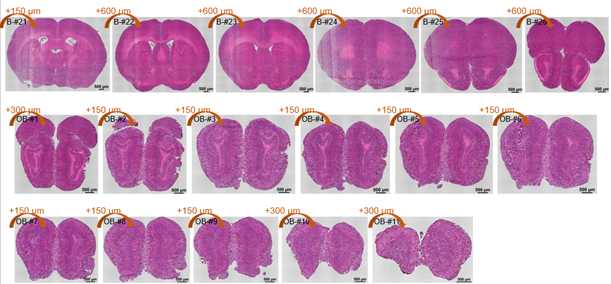
HE staining of cryosections. The distance of cryosections was marked.
HE staining procedure: cryosections were balanced at room temperature for 30 min,
and then fixed with 4% PFA for 3 min. Then, the sections were washed with dd22O
for 2 min, stained with hematoxylin for 6 min, washed with d2H2O, stained with eosin for 1 min, washed with d2H2O. After that, sections were gradient dehydrated (75% ethyl alcohol for 1 s, 85% ethyl alcohol for 1 s, 95% ethyl alcohol for 1 s, 100% ethyl alcohol for 1 s, 100% ethyl alcohol for 1 min), cleared (xylene for twice), and sealed after airing. Finally, the figure was scanned using Motorized Fluorescence Microscope (Nikon, Ni-E).