Sample Cutting/Processing Protocol
Oksana Polesskaya, Abraham Palmer, Khai-Minh H Nguyen, Katarina A Cohen
Abstract
Procedure for preparing a 96 sample plate for extraction on the EPmotion 5075 (can also do by hand). This protocol is created for Agencourt's DNAdvance extraction Kit.
Before start
Make sure you have dry ice available (~2 pounds is good enough).
All consumables and specific equipment are detailed in materials section.
Steps
Logging New Samples in Excel
- Place 16 samples on tube rack in two rows of 8.
- Leave them to thaw for
0h 15m 0s - While you allow them to thaw, proceed to the next steps
Download new Barcode Processing Template Bar_Code Processing Template.xlsx
Create a DNA plate code name
- We typically do "PI Name ##"
- Ex. John03
- This name will be assigned to this plate. Make sure you create a new name for each new plate.
- This will be the name that you will write on the following plates used in the protocol.
Rename the newly downloaded Barcode processing template excel sheet with the DNA plate code name
- EX. John 03 Barcode Processing
In Column A "Origin", enter the name of the box in which the samples were shipped in.
Scan and enter transponder IDs
- Enter "no transponder" in comments column if transponder chip is not scanning.
- The first row of 8 samples will be entered into A1-H1
- The second row of 8 samples will be entered into A2-H2
- Ensure the order is maintained throughout the protocol
Scan Barcodes
- Enter "no barcode" in comments column if barcode label is missing.
- Note the written code on tube if barcode label is missing
- Scan barcodes in the same order in which you scanned the transponders (ex. first row will be entered into A1-H1)
If barcode is a truncated version of the transponder ID, use the CONCACTENATE function
- e.g This will concatenate the first three zeroes "000" of the transponder ID to determine if it matches the scanned barcode ID.
=IF(TEXT(C2, "0")=CONCATENATE("000", TEXT(D2, "0")), "PASS", "FAIL")
Check for mismatches, missing transponder chips or barcodes.
- If "Match" Column shows as "FAIL" then mark sample as mismatch.
Enter tissue type in column J
- We typically extract from spleen or tail
Preparing Workstation
- Fill insulated foam box with dry ice and place empty tube rack to chill.
Fill ice pan with ice and chill metal cutting blockOn ice
Add 50µL of
- Use reagent reservoir and a multichannel pipette to add lysis buffer to all wells
- Place deepwell plate
On ice. - Write the DNA plate code (the code in which you named the file ex. John03) on the side of the plate
- Write the date and your initials on the side of the plate
Fill wash bottle with 70% EtOH. Clean tweezers, razor and metal cutting block with 70% EtOH and Kimwipes.
Processing Samples
Cut a small section of sample using your razor and place into corresponding well of the deepwell plate with your tweezers
- Spleen tissue = 5-6 mg
- Tail = 8-10 mg
- NOTE - Maintain the same order in which you scanned your samples into the barcode processing sheet (Check well numbers A1, B1 C1)
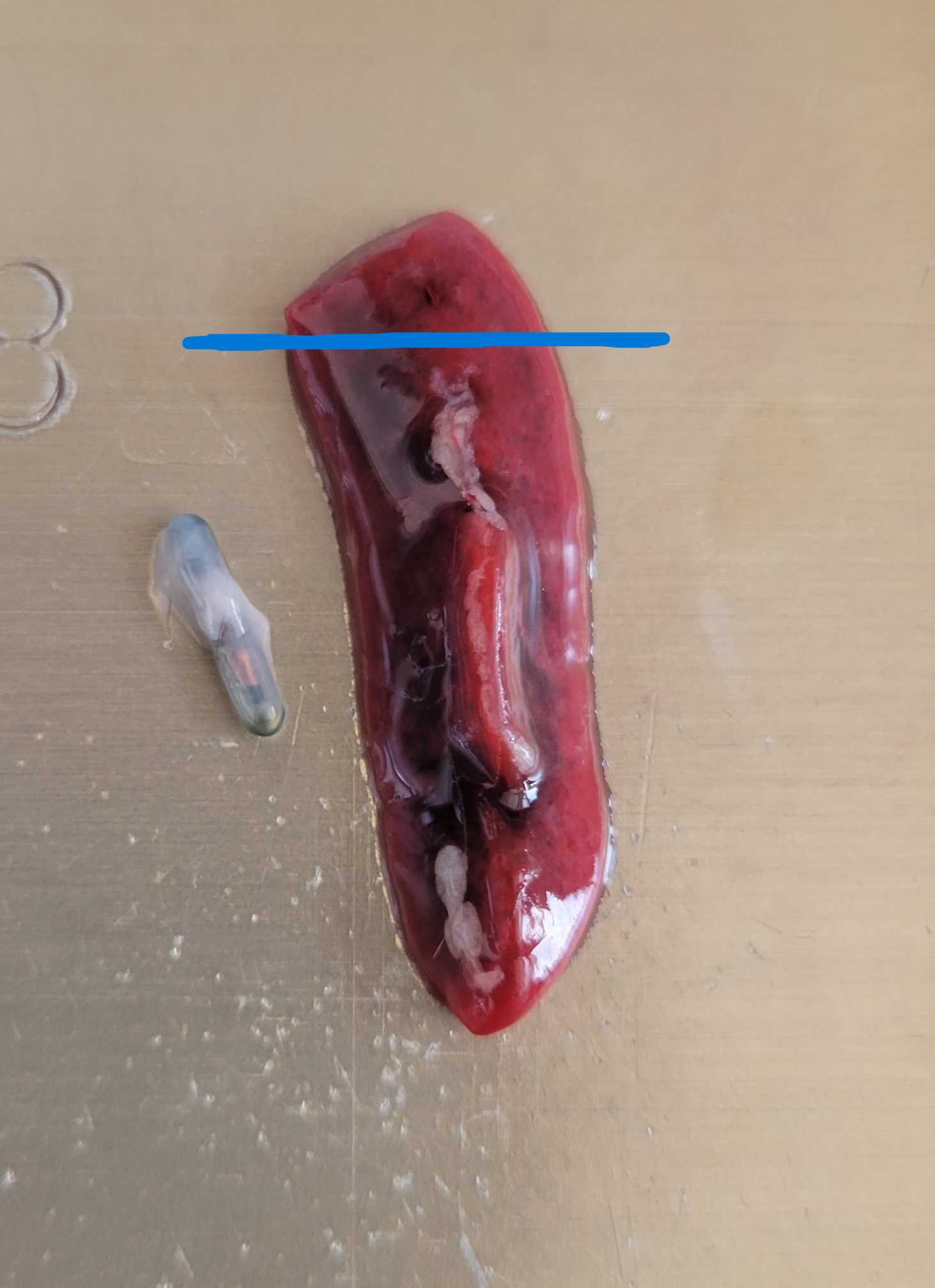
- Good "healthy" spleens should be intact/firm and have a vibrant deep red color.
- Jot down in the comments section of the corresponding well if the spleen looks particularly bad
Return tissue to tube and onto the tube rack on dry ice.
- Ensure you maintain the order in which you scanned the samples. This will be important for keeping records of the location of the sample in the database.
- It is important that the tissue is immediately transferred onto dry ice when finished cutting.
Spray and wipe cutting block, razor and tweezers with 70% EtOH and Kimwipes
- I recommend using a 100mL waste beaker to catch ethanol waste when spraying down the tweezers and razor
Wipe gloved hands with RNAse Zap Wipes
Repeat steps 13-16 until all 16 samples are completed
Logging Processed Samples
Label new processed sample box (use cryo freezer box 2'', 89 slots)
- Palmer lab boxes are labelled as such: ( PI Name) processed Box ## , Date, Initials of Lab Tech, Project
Place processed samples in labelled box while maintaining the order in which you scanned the samples
Enter storage sample box name in column G and sample position in column H in the barcode processing excel sheet
Store processed samples in -80C Freezer
Database Entry
Create new extraction database on google sheets. Copy from the template below. Create another sheet for each new plate.
- Column A - Enter DNA plate code (this is the name that you had written on the deepwell plate) ex. John03
- Columns D and E - Copy and Paste Transponder ID's and Barcodes from the barcode processing excel sheet.
- Column K - Copy and Paste the Origin from the barcode processing excel sheet
- Column M - Copy and Paste the Tissue Type from the barcode processing excel sheet
- Column N and O - Copy and Paste the Storage Box of Sample and Position in Box
- Column Q - Copy and Paste any comments that you have put in the barcode processing excel sheet
- Column P - Enter freezer location of the processed sample box location
Lysis and DNA Isolation
Continue to EPmotion DNA Extraction Protocol if you have an EPmotion.
It is safe to store processed sample plate at -80°C if you plan to continue a few days later.