Recombinant αS purification Protocol
Jane Balster
Abstract
This protocol details the recombinant αS purification.
Attachments
Steps
Culture Growth and Induction
Day 1: Inoculate 5mL of LB/Amp [100: 1µL of 100 Amp stock (freezer) for every 1mL of culture] with 1 colony from LB/Amp plate and put in a shaker at 37°C w/250rpm .
- Modification:
- Inoculate
10mLof LB/Amp with 1 colony (allows more rapid growth).
- If no viable plate:
- Take a stab from glycerol stock (
-80°CFreezer) and grow . - Streak LB/Amp agar plate with culture.
Day 2: Inoculate 500mL of LB/Amp (100: 1µL of 100 stock for every 1mL of culture) with 5mL of culture and put in shaker at 37°C until O.D = 0.5 – 0.6 (~1.5h -3h 0m 0s)
- Modification:
- Inoculate
1000mLof LB/Amp with10mLof culture and put in shaker. - Nano-drop:
- Use
2µLdrop. - Use LB or LB/Amp as a blank.
- Formula to determine the time it will take the culture to hit an OD of 0.5 (assuming 30 min doubling time):
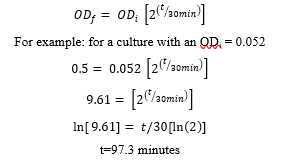
Induce with IPTG ( : of ) for every of culture) for 4h- at . 400: 6.4µL of 0.5Molarity (M)) for every 10mLof culture) for 4h-6h 0m 0s at 37°C.
Spin down culture at 4600x g,4°C in 500mL buckets (max volume 350mL) in JLA-10.5 rotor (blue rotor, standing centrifuge).
Day 3 : Resuspend in 50mLof lysis buffer. Add 1 tablet of protease inhibitor cocktail (NOT MINI) and 50µL of 500PMSF (500 stock in floor -20°C; Final conc. 500; incubate at 37°C without shaking for 35min-0h 40m 0s.
Lysis Buffer:
| A | B |
|---|---|
| NaOH | 40 mM |
| Tris pH 8.0 | 20 mM |
| EDTA | 1 mM |
| Triton X-100 | 0.1% |
Add 500µL of 1Molarity (M) MgCl2, 500µL of 1Molarity (M) CaCl2, and 20µL of DNase from Roche (200µL total) and incubate at 37°C with 250rpm shaking for1h 0m 0shour.
Add 1mL 0.25Molarity (M)EDTA, mix well, and remove cellular debris by centrifugation at 16900x g (JA 25.50 rotor).
Add 0.116g of ammonium sulfate per mL of supernatant and stir at 4°C for 1h 0m 0s. Centrifuge at 20000x g,0h 0m 0s for 0h 30m 0s, use new tubes. (~5.8g; Toss pellet).
Add 0.244g of ammonium sulfate per mL of supernatant and stir at 4°C for 1h 0m 0s (or 1h 0m 0s). Centrifuge at20000x g,0h 0m 0s for 0h 30m 0s, use new tubes. (~12.9g; **Keep pellet).
- Quality Control Checkpoint: Pellet should be at bottom of tube. Excessive proteolysis is indicated by floating pellet or significant smearing of pellet along the side of the tube.
Day 4: Resolubilize in 25mLs Buffer A and add PMSF to 1. Stir in refrigerator for ~0h 30m 0s.
Buffer A:
| A | B |
|---|---|
| Thris pH 8.0 | 25 mM |
| NaCl | 20 mM |
| EDTA | 1 mM |
Dialyze against 1L of Buffer A with 0.5 PMSF .
Day 5: Filter through 0.22 filter, Milex, Millipore.
LPLC: Run over Anion Exchange column HiTrap Q FF (program: SC IExAlphasS, Buffer A, filtered Buffer B), aS will start to come off at ~20% Buffer B). Keep samples at 4°C. SEE DETAILED QFF PROTOCOL
Buffer B (filtered):
| A | B |
|---|---|
| Tris pH 8.0 | 25 mM |
| NaCl | 1 M |
| EDTA | 1 mM |
Concentrate αS positive fractions with Amicon Ultracel-3KD MWCO, Millipore, and exchange into filtered Buffer C. Perform during Day 6 equilibration.
- Spin the samples at
4500x g,0h 0m 0sfor ~0h 20m 0still final sample volume <=1mL(lower volume better e.g.750µL).
Buffer C (filtered):
| A | B |
|---|---|
| Tris pH 8 | 25 mM |
| NaCl | 1 M |
| EDTA | 1 mM |
Day 6: LPLC: Run over size exclusion column in Buffer C. aS will start to come off at ~80mL. SEE DETAILED SEC PROTOCOL – Freeze tubes until Conc./BCA.
Concentrate aS positive fractions with Amicon Ultracel-3KD MWCO, Millipore, and exchange into sterile PBS.
- BCA samples and aliquot at desired concentration/volume (recommended at
5). - Freeze aliquots at
-80°C

