Preparation of DNA and RNA Fragments Containing Guanine N2-Thioalkyl Tethers
Gang Wang, Gang Wang, Barbara L. Gaffney, Barbara L. Gaffney, Roger A. Jones, Roger A. Jones, Xiaorong Hou, Xiaorong Hou
Abstract
This article describes procedures for preparation of deoxyguanosine and guanosine derivatives in which the guanine N 2 contains a thiopropyl tether, protected as a tert -butyl disulfide. After incorporation into a DNA or RNA fragment, this tether allows site-specific cross-linking to a thiol of a protein or another nucleic acid. © 2023 The Authors. Current Protocols published by Wiley Periodicals LLC.
Basic Protocol 1 : Preparation of diisopropyl-1-(tert -butylthio)-1,2-hydrazinedicarboxylate (4)
Basic Protocol 2 : Preparation of the 2′-deoxyguanosine N 2-propyl-tert -butyl disulfide phosphoramidite (12)
Basic Protocol 3 : Preparation of the guanosine N 2-propyl-tert -butyl disulfide phosphoramidite (20)
Basic Protocol 4 : Preparation of DNA fragments containing N 2-propyl-tert -butyl disulfide guanine
Alternate Protocol : Preparation of RNA fragments containing N 2-propyl-tert -butyl disulfide guanine
Basic Protocol 5 : Conversion of N 2-propyl-tert -butyl disulfide to the free thiol, disulfide 5-thio-2-nitrobenzoic acid disulfide, or ethylamine disulfide
INTRODUCTION
Site-specific disulfide cross-linking of nucleic acid fragments to themselves or to proteins, largely using procedures developed by Verdine & Norman (2003) and Glick (1998), has become an important tool in chemical biology (Banerjee, Santos, & Verdine, 2006; Cain, Zuiderweg, & Glick, 1995; He & Verdine, 2002; Huang, Chopra, Verdine, & Harrison, 1998; Mishina & He, 2003; Pontarelli et al., 2022; Stasinska, Putaj, & Chmielewski, 2019a,b; Tuske et al., 2004). This article provides procedures for synthesis of cross-linkable DNA and RNA fragments. The cross-linking is accomplished through a guanine N 2-thioalkyl tether that is protected as a stable tert -butyl disulfide during oligonucleotide synthesis. After DNA/RNA synthesis and purification, the tert -butyl disulfide can be converted to a more reactive moiety for cross-linking, such as the free thiol, 5-thio-2-nitrobenzoic acid (TNB) disulfide, or ethylamine disulfide. Basic Protocol 1 describes the preparation of the tert -butyl disulfide–protected thiopropylamine hydrochloride (4) used in both Basic Protocols 2 and 3. Basic Protocol 2 describes the conversion of 2′-deoxyguanosine (5) to the N 2-propyl-tert -butyl disulfide phosphoramidite derivative 10 , which is ready for use in automated DNA synthesis. Basic Protocol 3 describes the conversion of guanosine (11) to the N 2-propyl-tert -butyl disulfide phosphoramidite derivative 18 , which is ready for use in automated RNA synthesis. Basic Protocol 4 and the Alternate Protocol describe preparation of DNA or RNA fragments, respectively, containing an N 2-propyl-tert -butyl disulfide guanine. Basic Protocol 5 gives procedures for converting the N 2-propyl-tert -butyl disulfide to the more reactive ethylamine disulfide, TNB disulfide, or free thiol to allow cross-linking of the DNA or RNA.
CAUTION : Carry out all operations involving organic solvents and reagents in a well-ventilated fume hood with appropriate personal protective equipment (at minimum, safety glasses and gloves).
Basic Protocol 1: PREPARATION OF DIISOPROPYL-1-(tert-BUTYLTHIO)-1,2-HYDRAZINEDICARBOXYLATE (4)
The preparation of 4 is shown in Figure 1.The first step is reaction of 3-chloropropylammonium chloride (1) with sodium thiosulfate, followed by treatment with iodine to give 2 (Doi & Musker, 1985). A falling film distillation apparatus with toluene as the refluxing solvent gives 2 in a yield of ∼80%, which is much better than the yield from standard distillation. The free thioalkylamine (3) is obtained by treatment with dithiothreitol (DTT). Conversion to the tert -butyl disulfide is carried out by reaction with diisopropyl azodicarboxylate and tert -butyl thiol (Virgilio & Ellman, 1994; Wunsch, Moroder, & Romani, 1982).

Materials
-
3-Chloropropylammonium hydrochloride (1 , Aldrich)
-
Sodium thiosulfate pentahydrate (Na2S2O3), reagent grade
-
Methanol (MeOH), reagent grade
-
Iodine
-
6 N aq. NaOH
-
Methylene chloride
-
Toluene
-
Dithiothreitol (DTT; Aldrich)
-
Concentrated HCl
-
2-Methyl-2-propanethiol (tert -butyl thiol)
-
Diisopropyl azodicarboxylate (DIAD, Aldrich)
-
Diethyl ether, anhydrous
-
Nitrogen and argon sources
-
25% (w/v) sodium methoxide in MeOH
-
Triethylamine (TEA), anhydrous
-
Dimethylformamide (DMF), anhydrous, argon degassed
-
6 N HCl
-
Reflux condenser
-
Addition funnel
-
Rotary evaporator with diaphragm pump or water aspirator
-
125-ml separatory funnel
-
Falling film distillation apparatus (Aldrich, Z156604; see Sigma-Aldrich, 2001)
-
Shaker
-
Silica gel column: 40-63 μm in 40 × 150–mm prepackaged cartridges (SciPro, cat. no. CO2001)
Prepare 2
1.Dissolve 1 (2.8 g, 21.5 mmol) and sodium thiosulfate pentahydrate (3.44 g, 21.8 mmol, 1.01 eq.) in 80 ml MeOH (50% aq.) and heat overnight under reflux.
2.Add a solution of iodine (2.7 g, 10.6 mmol, 0.49 eq.) in 50 ml MeOH slowly (over 10 hr) through an addition funnel.
3.Concentrate the reaction mixture using a rotary evaporator.
4.Dissolve the residue in 15 ml of 6 N NaOH and extract three times with ∼15-ml portions of methylene chloride.
5.Concentrate the methylene chloride layers to an oil using a rotary evaporator.
6.Repeat the above procedure a total of five times.
7.Combine the five batches of crude material (2) and purify using a falling film distillation apparatus with toluene as the refluxing solvent.
8.Collect 2 as a clear liquid (7.75 g, 43 mmol, 80%) and characterize.
Prepare 3
9.Add DTT (2.15 g, 13.9 mmol, 1.3 eq.) to a portion of 2 (1.95 g, 10.8 mmol) dissolved in 10 ml water.
10.Adjust the pH to 6 using conc. HCl (∼1.7 ml).
11.Shake the reaction mixture at room temperature for 2 days.
12.Concentrate to an oil using a rotary evaporator and use the product (3) without purification.
Prepare 4
13.Dissolve 2-methyl-2-propanethiol (4.5 ml, 40 mmol) and DIAD (8.0 ml, 40.6 mmol, 1.0 eq.) in 100 ml anhydrous ethyl ether under N2.
14.Add 0.2 ml (25%) sodium methoxide in MeOH.
15.Monitor disappearance of the orange color, which should fade after 5 min.
16.Concentrate to an oil using a rotary evaporator and use without purification.
17.Dissolve all of the material from step 16 in 5 ml a mixture of anhydrous TEA and 50 ml argon-degassed DMF.
18.Dissolve all of compound 3 from step 12 in 50 ml argon-degassed DMF.
19.Add all of 3 (from step 18) dropwise to the solution in step 17 and stir the reaction for 1 day under N2 atmosphere.
20.Add 25 ml DMF and continue stirring overnight under N2.
21.Add 1.0 ml TEA, filter the mixture, and concentrate to an oil.
22.Precipitate the crude product from a diethyl ether solution by acidification with 6 N HCl and isolate by filtration.
23.Purify the precipitate by silica gel column chromatography using a gradient of MeOH in methylene chloride from 0% to 10% to afford 3 g of 4 (14 mmol, 64%). Characterize by 1H NMR.
Basic Protocol 2: PREPARATION OF THE 2′-DEOXYGUANOSINE N2-PROPYL-tert-BUTYL DISULFIDE PHOSPHORAMIDITE (12)
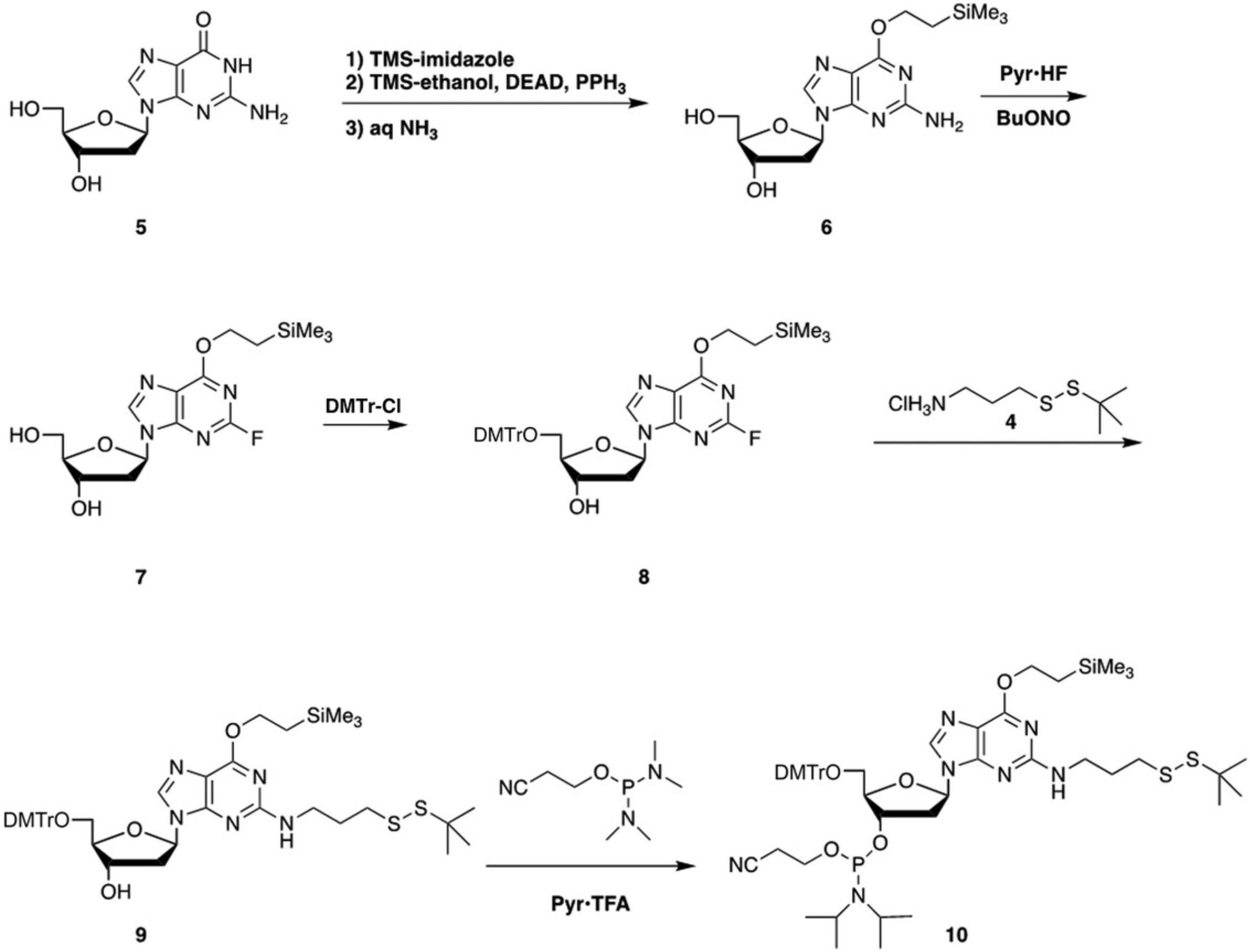
Conversion of 2′-deoxyguanosine (5) to the N 2-propyl-tert -butyl disulfide phosphoramidite derivative 10 is carried out by the series of reactions shown in Figure 2.The first step is protection of the guanine O 6 position by Mitsunobu alkylation with trimethylsilylethanol (DeCorte et al., 1996). The Mitsunobu alkylation is carried out after transient protection of the hydroxyls (Gao, Gaffney, Haden, & Jones, 1986). Thus, treatment of 5 with trimethylsilylimidazole is followed by reaction with trimethylsilylethanol, diethyl azodicarboxylate, and triphenylphosphine. The trimethylsilyl (TMS) groups are cleaved with aqueous dilute NH3 and silica gel column chromatography gives 6 in 88% yield from 5. The next step is nonaqueous diazotization using tert -butyl nitrite and pyridine·HF (Robins & Uznanski, 1981) to give 7 in 85% yield. Protection of the 5′ hydroxyl by reaction with 4,4′-dimethoxytrityl chloride gives 8 in 67% yield. The DMTr group also serves as a lipophilic handle that helps with isolation of 9. Displacement of the 2-fluoro group of 8 is carried out by reaction with 3-aminopropyl-tert -butyl disulfide (4) for 3 days at 60°C to give 9 in 81% yield. Conversion to the phosphoramidite 10 is carried out using 2-cyanoethyl (N,N,N ′,N ′-tetraisopropyl)phosphordiamidite and pyridinium trifluoroacetate (Sanghvi, Guo, Pfundheller, & Converso, 2000) in 61% yield.
Materials
-
2′-Deoxyguanosine monohydrate (5 ; Aldrich)
-
1,4-Dioxane
-
Trimethylsilylimidazole (Aldrich)
-
Nitrogen and argon sources
-
Triphenylphosphine (PPh3)
-
Trimethylsilylethanol (Aldrich)
-
Diethyl azodicarboxylate (DEAD; Aldrich)
-
Concentrated aqueous ammonia (NH3)
-
Methylene chloride
-
Methanol (MeOH), reagent grade
-
XTerra MS or Atlantis μC18 reversed-phase columns
-
0.1 M triethylammonium acetate (TEAA), pH 6.8
-
Acetonitrile (CH3CN; ACN)
-
Phosphorus pentoxide (P2O5)
-
Pyridine, anhydrous
-
Toluene, anhydrous
-
70% pyridine·HF (Aldrich)
-
Dry ice/acetone bath
-
tert -Butyl nitrite (Aldrich)
-
Potassium carbonate (K2CO3)
-
Ethyl acetate (EtOAc)
-
Triethylamine (TEA)
-
4,4′-Dimethoxytrityl chloride (DMTr·Cl; Aldrich)
-
5% (w/v) sodium bicarbonate (NaHCO3)
-
Diethyl ether
-
Diisopropyl-1-(tert -butylthio)-1,2-hydrazinedicarboxylate (4 ; see Basic Protocol 1)
-
1 M NaOH
-
2-Cyanoethyl (N,N,N ′,N ′-tetraisopropyl)phosphordiamidite (Cambridge Major)
-
Pyridinium trifluoroacetate (Aldrich)
-
Hexane
-
Syringes
-
Rotary evaporator with diaphragm pump or water aspirator
-
Silica gel column: 40-63 μm in 40 × 150–mm prepackaged cartridges (SciPro, cat. no. CO2001)
-
Reversed-phase HPLC system with ESI-MS
-
250-ml separatory funnel
Prepare 6
1.Dry 5 (1.43 g, 5.0 mmol) by concentrating three times with 50-ml portions of 1,4-dioxane, then suspend in 70 ml 1,4-dioxane.
2.Add trimethylsilylimidazole (2.0 ml, 13.6 mmol, 2.7 eq.) by syringe to the stirred suspension under N2. Stir for 30 min.
3.Add triphenylphosphine (6.55 g, 25.0 mmol, 5.0 eq.), trimethylsilylethanol (3.6 ml, 22.5 mmol, 5.0 eq.), and DEAD (3.97 ml, 25.2 mmol, 5.0 eq.) and stir for 1 hr.
4.Add a mixture of 20 ml conc. aq. NH3 and 35 ml water and stir overnight.
5.Add water (∼200 ml) and extract the solution three times with ∼100-ml portions of methylene chloride.
6.Concentrate the combined methylene chloride layers to an oil using a rotary evaporator.
7.Purify the residue by silica gel column chromatography using a gradient of 0%-10% MeOH in methylene chloride.
8.Monitor fractions by HPLC using XTerra MS or Atlantis μC18 columns (or similar) with a gradient of 2%-80% 0.1 M TEAA, pH 6.8, in CH3CN.
9.Combine fractions containing pure product, concentrate to a foam, and dry in a vacuum desiccator over P2O5 to give 1.62 g of 6 (4.41 mmol, 88% from 5).
10.Characterize by ESI-MS in negative mode.
Prepare 7
11.Add 6 (0.74 g, 2 mmol) to a solution of 10 ml anhydrous pyridine, 5 ml anhydrous toluene, and 11 ml of 70% pyridine·HF (423 mmol, 211 eq.) at –30°C (a dry ice/acetone bath) under argon.
12.Add tert -butyl nitrite (0.8 ml, 6.7 mmol, 3.4 eq.) dropwise by syringe over 10 min. Stir for 2 hr at –30°C.
13.Quench excess HF by slowly pouring the reaction mixture into a stirred solution of 30 g K2CO3 in 50 ml water in an ice bath.
14.Extract the mixture with three 100-ml portions of EtOAc.
15.Concentrate the combined organic layers to dryness using a rotary evaporator.
16.Purify the residue by silica gel column chromatography using a gradient of 0%-10% MeOH in methylene chloride. Monitor fractions by HPLC as in step 8.
17.Combine fractions containing pure product, concentrate to a foam, and dry in a vacuum desiccator over P2O5 to give 0.63 g of 7 (1.7 mmol, 85%).
18.Characterize by ESI-MS in negative mode.
Prepare 8
19.Dry 7 (0.75 g, 2.0 mmol) by concentrating three times with 10-ml portions of anhydrous pyridine, then dissolve in 30 ml anhydrous pyridine.
20.Add DMTr·Cl (0.87 g, 2.56 mmol, 1.3 eq.) to the stirred solution under N2, then stir for 3 hr.
21.Add mixture to 100 ml aq. 5% NaHCO3 and extract three times with 50-ml portions of diethyl ether.
22.Concentrate the organic layers to dryness using a rotary evaporator.
23.Purify the residue by silica gel column chromatography using a gradient of 0%-5% MeOH in methylene chloride (containing 0.5% pyridine). Monitor fractions by HPLC as in step 8.
24.Combine fractions containing pure product, concentrate to a foam, and dry in a vacuum desiccator over P2O5 to give 0.91 g of 8 (1.35 mmol, 67%).
25.Characterize 8 by ESI-MS in negative mode.
Prepare 9
26.Dissolve 4 (0.691 g, 3.20 mmol, 4.0 eq.) in 10 ml of 1 M NaOH.
27.Extract three times with 30-ml portions of methylene chloride.
28.Concentrate the combined organic layers to an oil using a rotary evaporator.
29.Dissolve the residue in a mixture of 25 ml anhydrous pyridine and 0.2 ml TEA.
30.Add 8 (0.54 g, 0.80 mmol) dissolved in 20 ml pyridine to the stirred solution under N2 and stir at 60°C for 3 days
31.Concentrate the mixture to an oil using a rotary evaporator.
32.Purify the residue by silica gel column chromatography using a gradient of 25%-60% EtOAc in hexane (containing 0.5% pyridine). Monitor fractions by HPLC as in step 8.
33.Combine fractions containing pure product, concentrate to a foam, and dry in a vacuum desiccator over P2O5 to afford 0.54 g of 9 (0.65 mmol, 81%).
34.Characterize by ESI-MS in negative mode.
Prepare 10
35.Add 2-cyanoethyl (N,N,N ′,N ′-tetraisopropyl)phosphordiamidite (0.36 ml, 1.13 mmol, 1.6 eq.) by dry syringe to 9 (0.58 g, 0.70 mmol) dissolved in 10 ml methylene chloride under argon or nitrogen.
36.Cool the solution in an ice bath and then add pyridinium trifluoroacetate (0.24 g, 1.24 mmol, 1.8 eq.) dissolved in 15 ml methylene chloride by dry syringe. Monitor the reaction by HPLC as in step 8.
37.Concentrate the solution to dryness using a rotary evaporator.
38.Purify the residue by silica gel column chromatography using a gradient of 15%-60% EtOAc (containing 0.5% pyridine) in hexane. Monitor fractions by HPLC.
39.Combine fractions containing pure product, concentrate to a foam, and dry in a vacuum desiccator over P2O5 to afford 0.44 g of 10 (0.43 mmol, 61%).
40.Characterize by ESI-MS in positive mode.
Basic Protocol 3: PREPARATION OF THE GUANOSINE N2-PROPYL-tert-BUTYL DISULFIDE PHOSPHORAMIDITE (20)
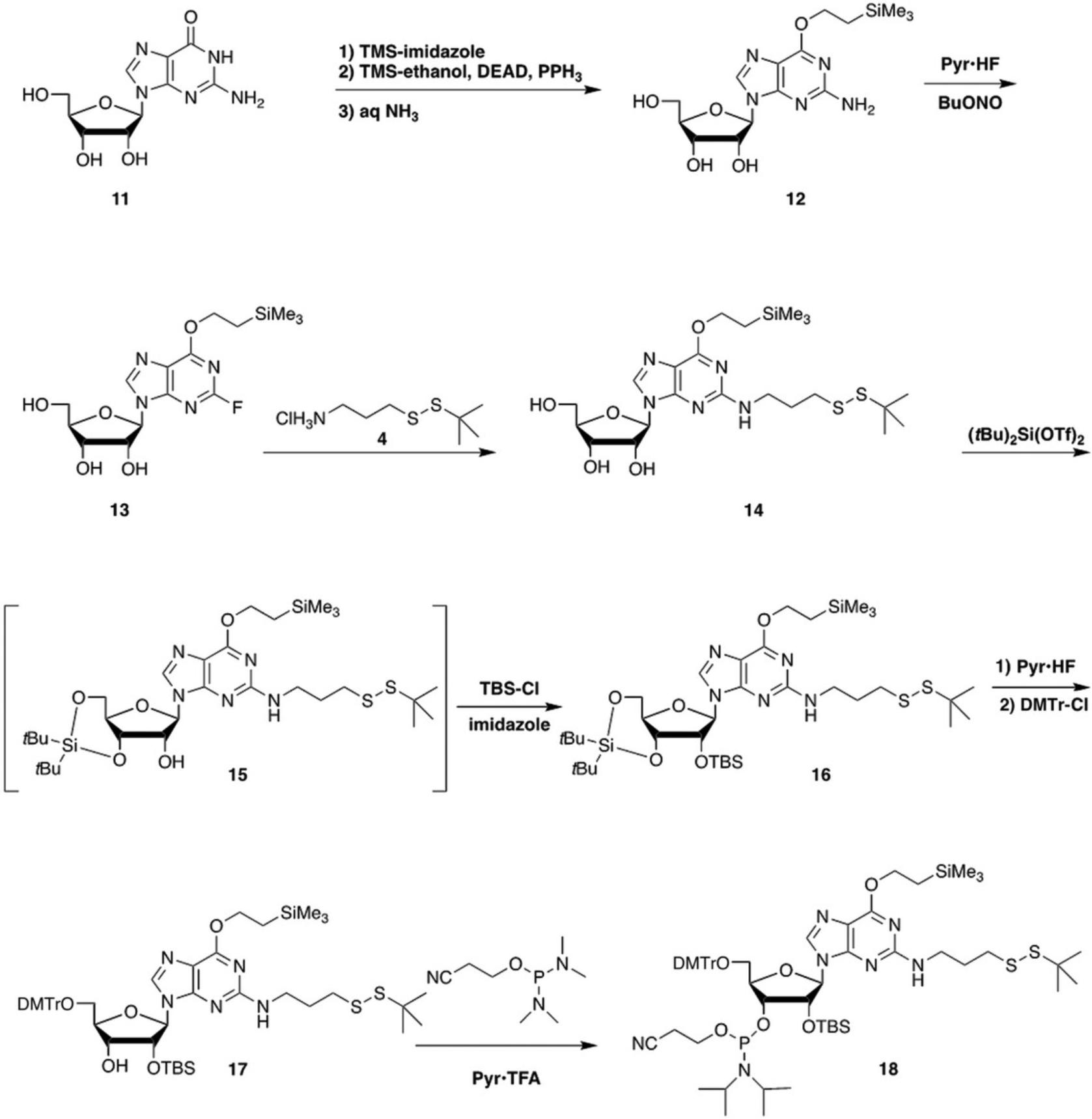
The conversion of guanosine (11) to the N 2-propyl-tert -butyl disulfide phosphoramidite derivative 18 is carried out by the series of reactions shown in Figure 3.The initial reactions to get to the 2-fluoro-O 6-trimethylsilylethyl derivative (13) are the same as the first steps in Basic Protocol 2.The next steps differ in that displacement of the 2-fluoro must be done before introduction of the 2′-O -tert -butyldimethylsilyl (TBS) group, to avoid cleavage of the TBS by the released fluoride. The displacement gives 14 in 92% yield. Protection of the 2′ hydroxyl is carried out after protection of the 3′ and 5′ hydroxyls as a di-tert -butylsilylene (Serebryany & Beigelman, 2002). Cleavage of the di-tert -butylsilylene followed by dimethoxytritylation gives 17 in 85% yield from 14. Conversion to the phosphoramidite 18 is carried out using 2-cyanoethyl (N,N,N ′,N ′-tetraisopropyl)phosphordiamidite and pyridinium trifluoroacetate (Sanghvi et al., 2000) in 80% yield.
Materials
-
Guanosine monohydrate (11 ; Aldrich)
-
1,4-Dioxane
-
Trimethylsilylimidazole (Aldrich)
-
Nitrogen or argon source
-
Triphenylphosphine (Aldrich)
-
Trimethylsilylethanol (Aldrich)
-
Diethyl azodicarboxylate (DEAD; Aldrich)
-
Concentrated aqueous ammonia (NH3)
-
Methylene chloride
-
Ethyl acetate (EtOAc)
-
Pyridine, anhydrous
-
Toluene, anhydrous
-
70% pyridine·HF (Aldrich)
-
Dry ice/acetone bath
-
tert -Butyl nitrite (Aldrich)
-
Potassium carbonate (K2CO3)
-
Methanol (MeOH)
-
Triethylamine (TEA)
-
Phosphorus pentoxide (P2O5)
-
Diisopropyl-1-(tert -butylthio)-1,2-hydrazinedicarboxylate (4 ; see Basic Protocol 1)
-
1 M NaOH
-
Dimethylformamide (DMF), anhydrous, argon degassed
-
Di-tert -butylsilyl ditriflate (Aldrich)
-
Imidazole
-
tert -Butyldimethylchlorosilane (Aldrich)
-
Saturated aq. sodium bicarbonate (NaHCO3)
-
4,4′-Dimethoxytrityl chloride (DMTr·Cl; Aldrich)
-
Acetone, HPLC grade
-
2-Cyanoethyl (N,N,N ′,N ′-tetraisopropyl)phosphordiamidite (Aldrich, Fisher)
-
Pyridinium trifluoroacetate (Aldrich)
-
Hexane
-
Syringes
-
Rotary evaporator with diaphragm pump or water aspirator
-
Filter for vacuum filtration
-
Silica gel column: 40-63 μm in 40 × 150–mm prepackaged cartridges (SciPro, cat. no. CO2001)
-
250-ml separatory funnel
Prepare 12
1.Dry 11 (1.42 g, 4.7 mmol) by concentrating three times with 50-ml portions of 1,4-dioxane, then suspend in 70 ml 1,4-dioxane.
2.Add trimethylsilylimidazole (2.2 ml, 15 mmol, 3.2 eq.) by syringe to the stirred suspension under N2. Stir for 1 hr.
3.Add triphenylphosphine (6.5 g, 25 mmol, 5.3 eq.), trimethylsilylethanol (7.2 ml, 50 mmol, 10.6 eq.), and DEAD (4.9 ml, 31 mmol, 6.6 eq.) and stir for 2 hr.
4.Add a mixture of 20 ml conc. aq. NH3 and 35 ml water and stir overnight.
5.Add water (∼200 ml) and extract the solution three times with ∼100-ml portions of methylene chloride.
6.Concentrate the combined methylene chloride layers to dryness using a rotary evaporator.
7.Dissolve the residue in 20 ml of 1,4-dioxane, concentrate to ∼15 ml, and then allow to stand overnight to crystallize triphenylphosphine oxide.
8.Filter to remove the triphenylphosphine oxide, concentrate the filtrate, and dissolve the residue in 20 ml of 9:1 EtOAc/methylene chloride.
9.Filter to obtain 1.65 g of 12 (4.30 mmol, 91%).
10.Characterize by ESI-MS in negative mode.
Prepare 13
11.Add 12 (0.69 g, 1.8 mmol) to a solution of 8 ml anhydrous pyridine, 3 ml anhydrous toluene, and 11 ml 70% pyridine·HF (423 mmol, 235 eq.) at –30°C (a dry ice/acetone bath) under argon.
12.Add tert -butyl nitrite (0.70 ml, 5.9 mmol, 3.3 eq.) dropwise by syringe over 10 min and then stir for 2 hr at –30°C.
13.Quench excess HF by slowly pouring the reaction mixture into a stirred solution of 30 g K2CO3 in 50 ml water in an ice bath.
14.Extract the mixture with three 100-ml portions of EtOAc.
15.Concentrate the combined organic layers to dryness using a rotary evaporator.
16.Purify the residue by silica gel column chromatography using a gradient of 0%-10% MeOH in methylene chloride (with 0.5% TEA).
17.Monitor fractions by HPLC as described (see Basic Protocol 2, step 8).
18.Combine fractions containing pure product, concentrate to a white solid using a rotary evaporator, and dry in a vacuum desiccator over P2O5 to give 0.610 g of 13 (1.58 mmol, 88%).
19.Characterize by ESI-MS in negative mode.
Prepare 14
20.Dissolve 4 (0.86 g, 4.0 mmol, 2.0 eq.) in 10 ml of 1 M NaOH.
21.Extract with three 30-ml portions of methylene chloride.
22.Concentrate the combined organic layers to an oil using a rotary evaporator.
23.Dissolve the residue in a mixture of 20 ml anhydrous pyridine and 1 ml TEA.
24.Add 13 (0.77 g, 2.0 mmol) dissolved in 20 ml pyridine to the stirred solution under N2 and stir at 60°C for 3 days.
25.Concentrate the mixture to an oil.
26.Purify the residue by silica gel column chromatography using a gradient of 0%- 8% MeOH (containing 0.5% pyridine) in methylene chloride. Monitor fractions by HPLC (see Basic Protocol 2, step 8).
27.Combine fractions containing pure product, concentrate to a foam, and dry in a vacuum desiccator over P2O5 to afford 1.01 g of 14 (1.85 mmol, 92%).
28.Characterize by ESI-MS in negative mode.
Prepare 15 and 16
29.Dissolve 14 in 30 ml anhydrous DMF.
30.While stirring at 0°C, add di-tert -butylsilyl ditriflate (0.72 ml, 2.0 mmol, 1.0 eq.) dropwise over 5 min to give 15. Stir at 0°C for 30 min.
31.Add imidazole (0.68 g, 10 mmol, 5.0 eq.) and stir for 5 min at 0°C and then for 25 min at room temperature.
32.Add tert -butyldimethylchlorosilane (0.362 g, 2.40 mmol, 1.2 eq.) and heat to 60°C for 2 hr.
33.Concentrate 16 to an oil using a rotary evaporator and use in the next reaction without purification.
34.Characterize by ESI-MS in positive mode.
Prepare 17
35.Dissolve 16 in 10 ml methylene chloride and cool in an ice bath.
36.Slowly add a cold solution of pyridine·HF (1 ml, 38.5 mmol, 19.2 eq.) dissolved in 8 ml cold pyridine and stir in the ice bath for 1 hr.
37.Add ∼50 ml saturated aq. NaHCO3 and separate the layers in a separatory funnel. Wash the methylene chloride layer with a second portion of saturated aq. NaHCO3.
38.Concentrate the organic layer to an oil and dry by concentrating from pyridine three times.
39.Dissolve the oil in 20 ml anhydrous pyridine.
40.Add DMTr·Cl (0.745 g, 2.2 mmol, 1.1 eq.) at 0°C under N2 and stir overnight.
41.Pour into ∼50 ml saturated aq. NaHCO3 and extract three times with ∼50-ml portions of methylene chloride.
42.Concentrate the combined organic layers to an oil using a rotary evaporator.
43.Purify the residue by silica gel column chromatography using a gradient of 0%-5% acetone in methylene chloride (containing 0.5% pyridine). Monitor fractions by HPLC (see Basic Protocol 2, step 8).
44.Combine fractions containing pure product, concentrate to a foam, and dry in a vacuum desiccator over P2O5 to give 1.63 g of 17 (1.7 mmol, 85% from 14).
45.Characterize by ESI-MS in negative mode.
Prepare 18
46.Add 2-cyanoethyl (N,N,N ′,N ′-tetraisopropyl)phosphordiamidite (0.32 ml, 1.0 mmol, 2.0 eq.) by dry syringe to 17 (0.48 g, 0.50 mmol) dissolved in 10 ml methylene chloride under argon or nitrogen.
47.Cool the solution in an ice bath and then add pyridinium trifluoroacetate (0.20 g, 1.0 mmol, 2.0 eq.) dissolved in 15 ml methylene chloride by dry syringe. Monitor the reaction by HPLC (see Basic Protocol 2, step 8).
48.Concentrate the solution to dryness using a rotary evaporator.
49.Purify the residue by silica gel column chromatography using a gradient of 10%-50% EtOAc in hexane (containing 0.5% pyridine). Monitor fractions by HPLC.
50.Combine fractions containing pure product, concentrate to a foam, and dry in a vacuum desiccator over P2O5 to afford 0.47 g of 18 (0.40 mmol, 80%).
51.Characterize by ESI-MS in positive mode.
Basic Protocol 4: PREPARATION OF DNA FRAGMENTS CONTAINING N2-PROPYL-tert-BUTYL DISULFIDE GUANINE
The phosphoramidite 10 (synthesized as in Basic Protocol 2) can be incorporated into DNA fragments using standard automated phosphoramidite chemistry with coupling yields similar to those for commercial amidites, provided that 10 is pure. Coupling yields are strongly dependent on purity of phosphoramidites. The preparation of high-purity phosphoramidites requires that both the precursor-protected nucleoside and the phosphitylating reagent itself be of high purity. In addition, the final chromatographic purification must be done quickly to avoid decomposing the phosphoramidite. The procedures below assume standard automated synthesis with the trityl-on option. These are the procedures that we generally use and have used for isolating DNA fragments with the thioalkyl tether. Procedures for isolating RNA fragments with the thioalkyl tether synthesized using phosphoramidite 18 require additional steps, which are provided in the Alternate Protocol.
Materials
-
Phosphoramidite 10 (see Basic Protocol 2)
-
Aqueous ammonia (NH3), concentrated and 10%
-
Triethylamine (TEA) dried over CaH2
-
Acetonitrile (CH3CN; ACN)
-
0.1 M triethylammonium acetate (TEAA)
-
0.6 M acetic acid, HPLC grade
-
Ammonium bicarbonate (NH4HCO3), solid
-
0.1 M NH4HCO3, pH 6.8-7.0
-
AG 50W-X2 ion-exchange resin (Bio-Rad)
-
DNA/RNA synthesizer
-
15- and 50-ml conical centrifuge tubes
-
Sintered-glass funnel, medium porosity
-
SpeedVac evaporator
-
Reversed-phase (RP) HPLC system with ESI-MS
-
Semi-preparative RP-HPLC system
-
Anion-exchange HPLC system
-
UV/Vis spectrophotometer
-
Lyophilizer
-
Syringe filters, 4.5 mm, 0.45-μm pore size
-
3-ml syringes
-
Silica gel column: 40-63 μm in 40 × 150–mm prepackaged cartridges (SciPro, cat. no. CO2001)
-
Glass column
-
Additional reagents and equipment for DNA synthesis (see Current Protocols article Beaucage & Caruthers, 2001) and HPLC purification of oligonucleotides (see Current Protocols article Sinha & Jung, 2015)
Deprotect base and isolate crude DNA
1.Incorporate phosphoramidite 10 into DNA using trityl-on synthesis (see Beaucage & Caruthers, 2001).
2.Transfer the support from the synthesis cartridge to a 50-ml centrifuge tube and add ∼5 ml conc. aq. NH3. Seal carefully and allow to sit for 2 days at room temperature without opening.
3.Filter the mixture in a medium-porosity sintered glass funnel and divide the filtrate between two new centrifuge tubes. Wash the support with ∼5 ml of 10% aq. NH3 in small portions and add the washes to the centrifuge tubes.
4.Add one to two drops of TEA to each tube, balance the tubes on a pan balance, and concentrate on a SpeedVac evaporator for 1-2 hr to remove most of the NH3.
5.Save a small sample to analyze by both RP-HPLC (LC-MS; usually using a gradient of 2%-40% ACN with 0.1 M TEAA) and anion-exchange HPLC. Check the mass spectrum across the main peak and across the failure sequences. Measure the OD260 in a UV spectrometer and estimate the number of μmol of material present.
6.Freeze and lyophilize.
Carry out trityl-on reversed-phase purification
7.Dissolve the sample in ∼3 ml of 0.1 M TEAA and filter it through a 4.5-mm, 0.45-μm pore size filter disk into a new 15-ml tube.
8.Using a new 3-ml syringe, draw up the filtered sample, displace the air, and load into the injector loop of the HPLC system.
9.Rinse the original tube with ∼1.5 ml fresh TEAA and filter through the same filter into the same tube, draw into the syringe, and load into the injector. Repeat this procedure two more times.
10.Run the separation using a gradient of 2%-40% ACN with 0.1 M TEAA. Manually move the collection line from tube to tube. Use a large fraction size when nothing is coming out at the beginning and end, but quite small volumes when you get close to your product.
11.Check the relevant fractions by both RP and anion-exchange HPLC. Combine into up to four fractions, as appropriate.
12.Dilute fractions at least 50% with water and measure the OD260.
13.Freeze and lyophilize.
Detritylate the nucleic acid
14.Dissolve each fraction in 5-10 ml of 0.6 M acetic acid. Check that the pH is <3.5 using an appropriate pH electrode. If necessary, add more acid.
15.Neutralize with a few drops of conc. aq. NH3 for DNA or a small amount of solid NH4HCO3 for RNA.
16.Check each fraction by RP-LC-MS (2% to 40% to be sure of complete detritylation, 2% to 20% to see better resolution) and by anion-exchange HPLC.
17.Freeze and lyophilize.
Carry out trityl-off reversed-phase purification
18.Purify the more pure fraction as described above using a gradient of 2%-20% ACN with 0.1 M TEAA.
19.Check fractions by RP-LC-MS and anion-exchange HPLC. Combine as appropriate and repeat purification if necessary to obtain pure material.
20.Purify the less pure fraction (combined with less pure fractions from above).
21.Check fractions, combine, and repeat as appropriate.
22.Combine all pure fractions and measure OD260 values.
23.Freeze and lyophilize.
Desalt purified nucleic acid
These steps remove excess TEAA and exchange all triethylammonium ions for NH4 ions.
24.Follow the above procedure in general, but use 0.1 M NH4HCO3, pH 6.8-7.0, for the buffer. If necessary, adjust the pH with CO2.
25.Run a semi-prep RP-HPLC column using a gradient of 2%-30% ACN with 0.1 M NH4HCO3.
26.Check fractions and combine.
27.Measure OD260, reserving an amount equivalent to 5-10 OD units for mass spectrometric analysis (for which the NH4 form is best).
28.Freeze and lyophilize.
Carry out sodium exchange
These steps use a cation-exchange resin, AG 50W-X2, to convert each phosphate to the sodium salt. Unlike ammonium salts, which can lose ammonia on lyophilization, sodium salts are completely stable to lyophilization.
29.Use an amount of resin that has up to 10 eq. sodium for each phosphate (determined from the OD measurements) in an appropriate glass column with a stopcock connected to a UV detector.
30.Dissolve the sample in ∼2 ml water and load it onto a glass column, being careful to touch the pipet only to the sides near the resin. Open the stopcock so that the sample drains into the resin bed.
31.Rinse the original container with 1 ml water and transfer it to the column, carefully washing down the first 0.5 in. (1.3 cm) or so. Allow it to drain to the resin bed. Continue with additional rinses the same way, each time rinsing the column from a bit higher up.
32.Continue until the peak on the chart recorder is back to a horizontal line.
33.Check fractions by HPLC, combine, and measure OD600.
34.Freeze and lyophilize.
Characterize purified product
35.Analyze the NH4 form of the sample by infusing it into the LC-MS to ensure that the mass is correct.
Alternate Protocol: PREPARATION OF RNA FRAGMENTS CONTAINING N2-PROPYL-tert-BUTYL DISULFIDE GUANINE
The phosphoramidite 18 can be incorporated into RNA fragments using standard automated phosphoramidite chemistry essentially as described in Basic Protocol 4, but requires additional steps. Below, after the crude base-deprotected RNA is isolated, the desilylation procedure is carried out using a mixture of TEA·HF, NMP, and TEA dried over CaH2. This is followed by quenching the reaction and precipitating the RNA using isopropoxytrimethylsilane as a fluoride scavenger. After these steps, the rest of the procedure (beginning with trityl-on purification) proceeds as in Basic Protocol 4.All cautions about yields and purity relating to DNA fragments apply to RNA fragments as well.
NOTE : RNA is sensitive to degradation from commonly occurring RNase enzymes. Wear fresh nitrile gloves, change them frequently, and use sterile supplies. Keep tubes containing RNA covered as much as possible, and avoid breathing, talking, sneezing, etc., over any open tubes.
Additional Materials (also see Basic Protocol 4)
-
Phosphoramidite 18 (Basic Protocol 3)
-
40% (w/v) aqueous methylamine
-
50% (v/v) ethanol (EtOH)
-
Triethylamine·HF (TEA·HF; Aldrich)
-
Methyl-2-pyrrolidinone (NMP; Aldrich)
-
Isopropoxytrimethylsilane (Aldrich)
-
Ethyl ether, anhydrous
-
65°C water bath
-
SpeedVac evaporator
-
1.5-ml centrifuge tubes
-
Large rubber septum
-
Vent needle connected to bubbler
-
Tabletop centrifuge
-
Spatula cleaned with nitric acid and rinsed well
-
Additional reagents and equipment for RNA synthesis (see Current Protocols article Wincott, 2001)
Deprotect base and isolate crude RNA
1.Incorporate phosphoramidite 18 using trityl-on synthesis (see Wincott, 2001).
2.Transfer the support from the synthesis cartridge to a 50-ml centrifuge tube and add ∼5 ml of 40% methylamine using a sterile pipet. Tighten the cap securely, wrap with Parafilm, and heat the tube for 10 min in a 65°C water bath. Remove and let cool to room temperature.
3.Filter the mixture through a sintered-glass funnel by transferring with a sterile pipet. Rinse the tube and support with ∼10 ml of 50% EtOH in portions.
4.Transfer the solution to a new 50-ml centrifuge tube. Rinse the original tube and support with another 10 ml of 50% EtOH and add to the new centrifuge tube. Repeat once more with ∼5 ml of 50% EtOH. Make sure the final volume is not more than ∼30 ml.
5.Use a pan balance to prepare another tube for balancing that has the same mass of 50% ethanol.
6.Dry completely using a SpeedVac.
Desilylate crude RNA
7.Prepare the required amount of TEA·HF based on the number of μmol measured for the crude material. Prepare the other reagents based on the amount of TEA·HF.
- Use 125 eq. TEA·HF (mol. wt., 161; density, 0.989) per TBS group
- Use 1.5 volumes NMP (mol. wt., 99; density, 1.028) per volume TEA·HF
- Use 0.75 volume TEA (mol. wt., 101; density, 0.726) per volume TEA·HF
- Use 2 eq. isopropoxytrimethylsilane (mol. wt., 132; density, 0.745) per eq. TEA·HF
8.Dissolve the RNA in NMP in a centrifuge tube. Vortex as necessary to get it into solution.
9.Add the TEA (filtered if necessary to remove CaH2).
10.Add the TEA·HF.
11.Close the tube, seal with Parafilm, and heat at 65°C for 2 hr.
12.Cool the tube and open carefully. Replace the cap with a large septum and insert a vent needle connected to a bubbler.
13.Heat the tube in a 65°C water bath and slowly add the scavenger (isopropoxytrimethylsilane) while swirling the tube.
14.Adjust the rate of addition so that the evolution is not too vigorous.
15.Continue heating at 65°C until bubbling stops, which means all of the fluoride has been consumed by the scavenger (typically ∼40 min).
16.Cool the tube and add 15 ml anhydrous ethyl ether to complete precipitation of the RNA.
17.Centrifuge for ∼5 min in a tabletop centrifuge.
18.Decant the supernatant and wash twice by adding 10 ml ethyl ether, vortexing, centrifuging again, and decanting the supernatant.
19.Remove the residual ether using a SpeedVac evaporator for ∼10 min.
20.Remove a sample using a spatula (cleaned with nitric acid and rinsed well) and dissolve in a small vial of 0.1 M TEAA.
21.Check by RP-LC-MS and anion-exchange HPLC (Sinha & Jung, 2015).
22.Proceed as described for DNA (see Basic Protocol 4, step 7), being careful to observe the usual precautions associated with preventing RNA degradation.
Basic Protocol 5: CONVERSION OF N2-PROPYL-tert-BUTYL DISULFIDE TO THE FREE THIOL, 5-THIO-2-NITROBENZOIC ACID DISULFIDE, OR ETHYLAMINE DISULFIDE
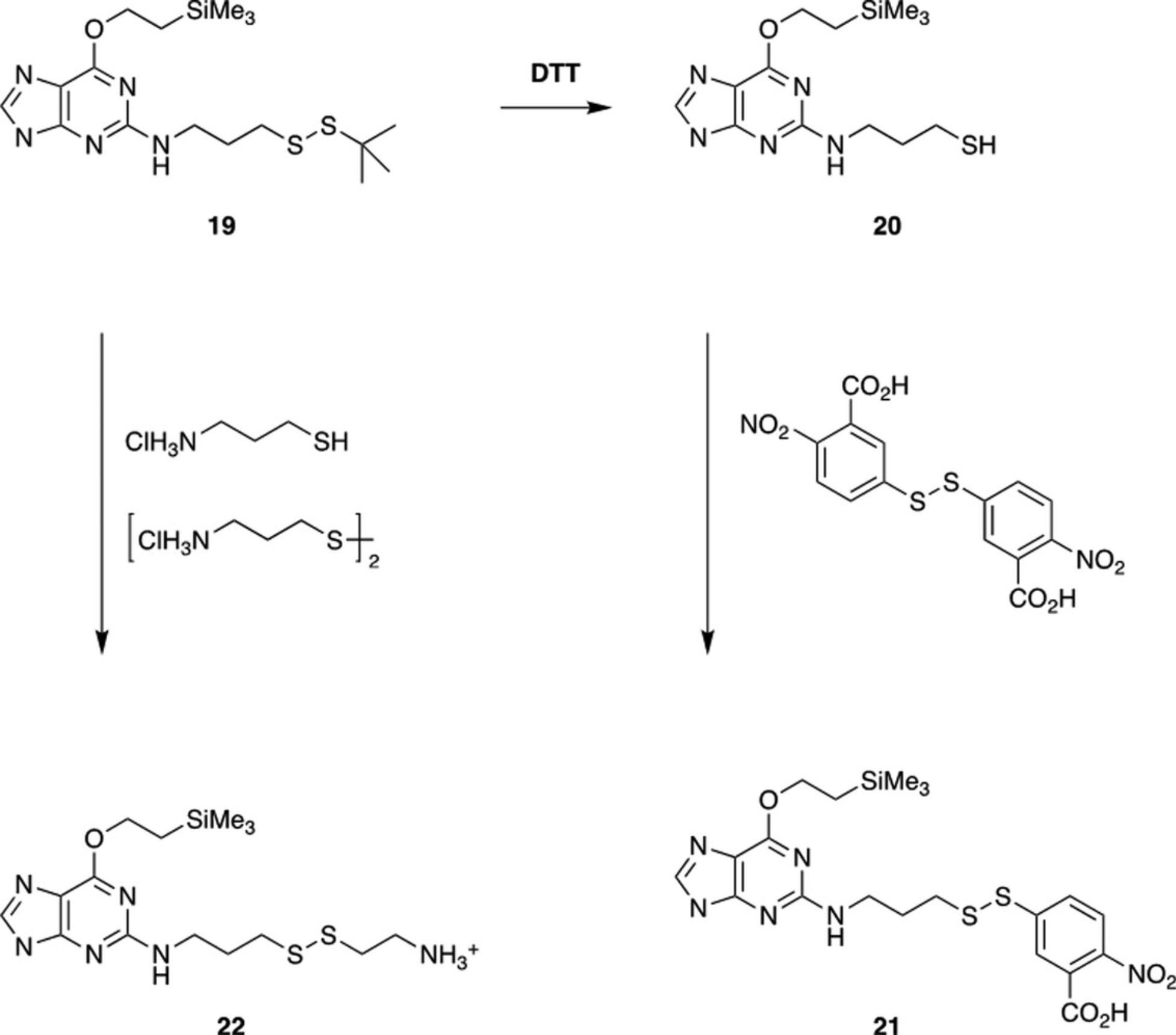
The tert -butyl thiol protecting group is necessarily unreactive and must be removed to allow cross-linking. Conversion of purified DNA or RNA fragments (19) to more reactive species is carried out as shown in Figure 4.Treatment with DTT gives the free thiol (20), which can be used itself for cross-linking or converted to the nitrobenzoic acid disulfide (21) by treatment with 5,5′-dithiobis(2-nitrobenzoic acid). Alternatively, reaction of 19 with a mixture of cysteamine hydrochloride and cystamine dihydrochloride gives the ethylamine disulfide (22). The yields of pure products range from 50% to 80%.
Materials
- Dithiothreitol (DTT; Aldrich)
- Concentrated aqueous ammonia (NH3)
- Purified DNA/RNA (19 ; see Basic Protocol 4 or Alternate Protocol)
- 5,5′-Dithiobis(2-nitrobenzoic acid) (Aldrich)
- 0.1 M potassium phosphate buffer, pH 8.0
- Cysteamine hydrochloride (2-aminoethanethiol hydrochloride; Aldrich)
- Cystamine dihydrochloride (2,2′-diaminodiethyl disulfide dihydrochloride; Aldrich)
- 40°C water bath
Prepare DNA/RNA thiols (20)
1.Dissolve DTT (∼200 eq.) in 2 ml water and adjust pH to 8 using conc. aq. NH3.
2.Add purified DNA/RNA (19).
3.Heat at 40°C for 3 hr.
4.Purify, desalt, and characterize as described for DNA and RNA fragments 19 (see Basic Protocol 4 or Alternate Protocol).
Prepare DNA/RNA DNB disulfides (21)
5.Dissolve 5,5′-dithiobis(2-nitrobenzoic acid) (∼1000-2000 eq.) in 200 ml of 0.1 M potassium phosphate, pH 8.0.
6.Add purified DNA/RNA thiol (20).
7.Maintain 30 min at room temperature.
8.Purify, desalt, and characterize as described above for DNA and RNA fragments 19 (see Basic Protocol 4 or Alternate Protocol).
Prepare DNA/RNA ethylamine disulfides (22)
9.Dissolve cysteamine hydrochloride (∼1000-10,000 eq.) and 0.028 g cystamine dihydrochloride (∼200-1000 eq.) in 1 ml water and adjust pH to 8 using conc. aq. NH3.
10.Add purified DNA/RNA 19.
11.Heat at 40°C for 2 hr.
12.Purify, desalt, and characterize as described above for DNA and RNA fragments 19 (see Basic Protocol 4 or Alternate Protocol).
COMMENTARY
Background Information
Nucleic acid thioalkyl tethers have been introduced by Verdine using a post-synthetic displacement strategy, the “convertible nucleoside” method, that is applicable to the guanine N 2 (Allerson, Chen, & Verdine, 1997; MacMillan & Verdine, 1991) and by Glick using pyrimidines already containing a tether (Glick, 1991; Goodwin & Glick, 1993, 1994). The strategy we developed, like the Glick method, uses a nucleoside already containing the thioalkyl group, protected as a tert -butyl disulfide. Although the tert -butyl disulfide has the stability needed for a protecting group, this stability makes it too unreactive for cross-linking. One option, as reported by Glick, is to convert it to the thiol using DTT; air oxidation is then needed for cross-linking. Alternatively, we have converted the tert -butyl disulfide to two more reactive disulfides: the thioethylamine disulfide (Ferentz et al., 1993; Huang et al., 1998) and the still more reactive 5-thio-2-nitrobenzoic acid (TNB) disulfide (Gao, Butler, & Bushman, 2001; Wolfe, Ferentz, Grantcharova, Churchill, & Verdine, 1995). The thioethylamine disulfide is likely to be the most useful, with moderate reactivity that gives good selectivity. Moreover, it can be prepared in one step from the tert -butyl disulfide using a mixture of cysteamine hydrochloride (2-aminoethanethiol hydrochloride) and cystamine dihydrochloride (2,2′-diaminodiethyl disulfide dihydrochloride). Although not described here, the tert -butyl thiol protecting group can be exchanged to the thioethylamine while the DNA/RNA is still attached to the support. In this case, acetyl-protected cytidine (N 4-acetylcytidine) must be used for DNA/RNA synthesis to prevent partial displacement of the N 4-benzoyl with cysteamine.
Protection of the guanine O 6 is necessary for the fluorination reaction to proceed in good yield. For this purpose, we used a Mitsunobu alkylation with transient protection of the hydroxyls (Gao et al., 1986) to introduce the trimethylsilylethyl (TSE) group (DeCorte et al., 1996). Because of its acid lability, the TSE group is cleaved at the first detritylation step during oligonucleotide synthesis, but it is stable up to that point. It is introduced in ∼90% yield, and the subsequent nonaqueous diazotization using tert -butyl nitrite and pyridine·HF (Robins & Uznanski, 1981) proceeds in ∼85% yield.
After the fluorination step, the 2′-deoxyribonucleoside and ribonucleoside syntheses diverge. In the former case, introduction of the dimethoxytrityl group provides a convenient lipophilic handle for workup after displacement of the 2-fluoro group with 3-aminopropyl-tert -butyl disulfide. In the latter case, the sequence must be reversed to avoid loss of the tert -butyldimethylsilyl group by the released fluoride. Thus, displacement with 3-aminopropyl-tert -butyl disulfide in 92% yield is followed by temporary protection of the 3′ and 5′ hydroxyls as a di-tert -butylsilylene (Serebryany & Beigelman, 2002), introduction of the TBS, and, finally, dimethoxytritylation, in 85% yield.
Preparation of the thiopropylamine disulfide is straightforward (Doi & Musker, 1985), but does benefit from using a falling film distillation apparatus for isolation of the product (Sigma-Aldrich, 2001). Falling film distillation minimizes heating of the mixture being distilled, thus greatly reducing decomposition.
Isolation of the synthetic RNA fragments is done by precipitation of the crude RNA after desilylation. Precipitation is done by treatment of the reaction mixture with isopropoxytrimethylsilane, which primarily serves to scavenge fluoride ions, but also helps to precipitate the crude RNA (Song & Jones, 1999). In this way, the crude RNA is quickly separated from the large excess of fluoride used in the desilylation. Purification of both the DNA and RNA fragments is done by semi-preparative reversed-phase HPLC, both before and after detritylation. We also use anion-exchange HPLC to check fractions, and occasionally use semi-preparative anion-exchange HPLC for purification, but have not done so with any of the fragments containing the thiopropyl tether. A variety of procedures have been used for detritylation after the trityl-on purification. We showed some time ago that when there is a 3′ phosphate group, the 5′ dimethoxytrityl group can be cleaved by only mildly acidic conditions (Gaffney & Jones, 1989). At a pH near 3.5, the dimethoxytrityl group is cleaved in 20-30 min. This is slow enough to follow conveniently by HPLC, and the small amount of acetic acid used is readily neutralized with aqueous ammonia and removed by lyophilization. In general, we prefer lyophilization to concentration on a SpeedVac, although the latter works well for removal of volatiles.
Using the procedures detailed above we have made a DNA 19-mer, a DNA 20-mer, and an RNA 23-mer (Hou, Wang, Gaffney, & Jones, 2009), each with one guanine residue containing a tert -butyl-disulfide-protected thiopropyl tether at the N 2. These molecules are in use in a variety of cross-linking experiments, with some results published recently (Das et al., 2009).
Critical Parameters and Troubleshooting
The purity of all solvents and reagents is critical to obtaining high yields and pure compounds. Where specified, solvents must be anhydrous. Running reactions under an inert atmosphere, either nitrogen or argon, is good laboratory practice. The syntheses described above require understanding of and experience with common organic synthetic procedures. It is important that intermediates be pure for subsequent steps, even if the yield is lower as a result of purification. While in principle it is possible to monitor all of the steps by TLC, we have used only HPLC because it offers much higher resolution. Moreover, an ESI mass spectrometer detector provides valuable information throughout these syntheses and is highly recommended but is not absolutely necessary. Several steps use pyridine·HF and TEA·HF, which are hazardous and must be handled with care. All reagents should be used in accordance with these procedures and all applicable safety instructions from the supplier.
Anticipated Results
An experienced synthetic chemist should be able to obtain yields comparable to those reported if the procedures are followed carefully. Less skilled researchers may require several attempts to master the reactions, but should eventually succeed, provided the procedures are followed exactly.
Time Considerations
The preparation of the 2′-deoxyphosphoramidite 10 should take ∼1 week, whereas that of the ribophosphoramidite 18 will take almost 2 weeks. The time for DNA or RNA synthesis varies significantly with scale, and in any case will be much less than the time needed for purification, which can easily take a week, depending on the number of purifications. Lyophilizations normally take 1 to 2 days. If LC-MS capability is available, characterization can be completed in minutes. Conversion to more reactive disulfides takes about a day. In total, preparation of a DNA or RNA fragment with an active thiopropyl tether will take 2-4 weeks.
Acknowledgment
This work was supported by NIH grant GM66671.
Author Contributions
Xiaorong Hou : Investigation; Gang Wang : Investigation; Barbara L. Gaffney : Project administration, writing (review & editing); Roger A. Jones : Project administration, writing (review & editing).
Conflict of Interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Literature Cited
- Allerson, C. R., Chen, S. L., & Verdine, G. L. (1997). A chemical method for site-specific modification of RNA: The convertible nucleoside approach. Journal of the American Chemical Society , 119, 7423–7433. doi: 10.1021/ja962858n
- Banerjee, A., Santos, W. L., & Verdine, G. L. (2006). Structure of a DNA glycosylase searching for lesions. Science , 311, 1153–1157. doi: 10.1126/science.1120288
- Beaucage, S. L., & Caruthers, M. H. (2001). Synthetic strategies and parameters involved in the synthesis of oligodeoxyribonucleotides according to the phosphoramidite method. Current Protocols in Nucleic Acid Chemistry , 00, 3.3.1–3.3.20. doi: 10.1002/0471142700.nc0303s00
- Cain, R. J., Zuiderweg, E. R. P., & Glick, G. D. (1995). Solution structure of a DNA hairpin and its disulfide cross-linked analog. Nucleic Acids Research , 23, 2153–2160. doi: 10.1093/nar/23.12.2153
- Das, K., Bandwar, R. P., White, K. L., Feng, J. Y., Sarafianos, S. G., Tuske, S., … Arnold, E. (2009). Structural basis for the role of the K65R mutation in HIV-1 reverse transcriptase polymerization, excision antagonism, and tenofovir resistance. Journal of Biological Chemistry , 284, 35092–35100. doi: 10.1074/jbc.M109.022525
- DeCorte, B. L., Tsarouhtsis, D., Kuchimanchi, S., Cooper, M. D., Horton, P., Harris, C. M., & Harris, T. M. (1996). Improved strategies for postoligomerization synthesis of oligodeoxynucleotides bearing structurally defined adducts at the N2 position of deoxyguanosine. Chemical Research in Toxicology , 9, 630–637. doi: 10.1021/tx9501795
- Doi, J. T., & Musker, W. K. (1985). A general mechanism for the oxidative cleavage of amine disulfides and cystine in aqueous iodine: Isolation of cyclic sulfinamides. Journal of Organic Chemistry , 50, 1–4. doi: 10.1021/jo00201a001
- Ferentz, A. E., Keating, T. A., Irimura, T., Osawa, T., Ogawa, H., & Matsumoto, I. (1993). Synthesis and characterization of disulfide cross-linked oligonucleotides. Journal of the American Chemical Society , 115, 9006–9014. doi: 10.1021/ja00073a016
- Gaffney, B. L., & Jones, R. A. (1989). Thermodynamic comparison of the base pairs formed by the carcinogenic lesion O 6-methylguanine with reference both to Watson-Crick pairs and to mismatched pairs. Biochemistry , 28, 5881–5889. doi: 10.1021/bi00440a026
- Gao, K., Butler, S. L., & Bushman, F. (2001). Human immunodeficiency virus type 1 integrase: Arrangement of protein domains in active cDNA complexes. EMBO Journal , 20, 3565–3576. doi: 10.1093/emboj/20.13.3565
- Gao, X., Gaffney, B. L., Haden, S., & Jones, R. A. (1986). Transient protection. 2. One-flask synthesis of 6-O -[(4-nitrophenyl)ethyl]-2′-deoxyguanosine nucleosides. Journal of Organic Chemistry , 51, 755–758. doi: 10.1021/jo00355a038
- Glick, G. D. (1991). Synthesis of a conformationally restricted DNA hairpin. Journal of Organic Chemistry , 56, 6746–6747. doi: 10.1021/jo00024a009
- Glick, G. D. (1998). Design, synthesis, and analysis of conformationally constrained nucleic acids. Biopolymers , 48, 83–96. doi: 10.1002/(SICI)1097-0282(1998)48:1<83::AID-BIP8>3.0.CO;2-E
- Goodwin, J. T., & Glick, G. D. (1993). Incorporation of alkylthio chains at C-5 of deoxyuridine. Tetrahedron Letters , 34, 5549–5552. doi: 10.1016/S0040-4039(00)73878-0
- Goodwin, J. T., & Glick, G. D. (1994). Synthesis of a disulfide stabilized RNA hairpin. Tetrahedron Letters , 35, 1647–1650. doi: 10.1016/0040-4039(94)88309-2
- He, C., & Verdine, G. L. (2002). Trapping distinct structural states of a protein/DNA interaction through disulfide crosslinking. Chemistry & Biology, 9, 1297–1303.
- Hou, X., Wang, G., Gaffney, B. L., & Jones, R. A. (2009). Synthesis of guanosine and deoxyguanosine phosphoramidites with cross-linkable thioalkyl tethers for direct incorporation into RNA and DNA. Nucleosides, Nucleotides, and Nucleic Acids , 28, 1076–1094. doi: 10.1080/15257770903368385
- Huang, H. R., Chopra, R., Verdine, G. L., & Harrison, S. C. (1998). Structure of a covalently trapped catalytic complex of HIV-1 reverse transcriptase: Implications for drug resistance. Science , 282, 1669–1675. doi: 10.1126/science.282.5394.1669
- MacMillan, A. M., & Verdine, G. L. (1991). Engineering tethered DNA molecules by the convertible nucleoside approach. Tetrahedron , 47, 2603–2616. doi: 10.1016/S0040-4020(01)81793-2
- Mishina, Y., & He, C. (2003). Probing the structure and function of the Escherichia coli DNA alkylation repair AlkB protein through chemical cross-linking. Journal of the American Chemical Society , 125, 8730–8731. doi: 10.1021/ja034636c
- Pontarelli, A., Liu, J. T., Movasat, H., Ménard, S., Oh, J. K., & Wilds, C. J. (2022). Synthesis of a convertible linker containing a disulfide group for oligonucleotide functionalization. Organic Letters , 24(30), 5579–5583. doi: 10.1021/acs.orglett.2c02149
- Robins, M. J., & Uznanski, B. (1981). Nucleic acid related compounds. 34. Non-aqueous diazotization with tert -butyl nitrite: Introduction of fluorine, chlorine, and bromine at C-2 of purine nucleosides. Canadian Journal of Chemistry , 59, 2608–2611. doi: 10.1139/v81-375
- Sanghvi, Y. S., Guo, Z., Pfundheller, H. M., & Converso, A. (2000). Improved process for the preparation of nucleosidic phosphoramidites using a safer and cheaper activator. Organic Process Research & Development, 4, 175–181. doi: 10.1021/op990086k
- Serebryany, V., & Beigelman, L. (2002). An efficient preparation of protected ribonucleosides for phosphoramidite RNA synthesis. Tetrahedron Letters , 43, 1983–1985. doi: 10.1016/S0040-4039(02)00181-8
- Sigma-Aldrich. (2001). Aldrich falling film distillation heads. Aldrichchimica Acta , 34, 44.
- Sinha, N. D., & Jung, K. E. (2015). Analysis and purification of synthetic nucleic acids using HPLC. Current Protocols in Nucleic Acid Chemistry , 61, 10.5.1–10.5.39. doi: 10.1002/0471142700.nc1005s61
- Song, Q., & Jones, R. A. (1999). Use of silyl ethers as fluoride scavengers in RNA synthesis. Tetrahedron Letters , 40, 4653–4654. doi: 10.1016/S0040-4039(99)00826-6
- Stasinska, A., Putaj, P., & Chmielewski, M. K. (2019a). Disulfide bridge as a linker in nucleic acids’ bioconjugation. Part I: An overview of synthetic strategies. Bioorganic Chemistry , 92, 103223. doi: 10.1016/j.bioorg.2019.103223
- Stasinska, A., Putaj, P., & Chmielewski, M. K. (2019b). Disulfide bridge as a linker in nucleic acids’ bioconjugation. Part II: A summary of practical applications. Bioorganic Chemistry , 95, 103518. doi: 10.1016/j.bioorg.2019.103518
- Tuske, S., Sarafianos, S. G., Clark, A. D. Jr., Ding, J., Naeger, L. K., White, K. L., … Arnold, E. (2004). Structures of HIV-1 RT-DNA complexes before and after incorporation of the anti-AIDS drug tenofovir. Nature Structural & Molecular Biology, 11, 469–474. doi: 10.1038/nsmb760
- Verdine, G. L., & Norman, D. P. G. (2003). Covalent trapping of protein-DNA complexes. Annual Review of Biochemistry , 72, 337–366. doi: 10.1146/annurev.biochem.72.121801.161447
- Virgilio, A. A., & Ellman, J. A. (1994). Simultaneous solid-phase synthesis of β-turn mimetics incorporating side-chain functionality. Journal of the American Chemical Society , 116, 11580–11581. doi: 10.1021/ja00104a053
- Wincott, F. E. (2001). Strategies for oligoribonucleotide synthesis according to the phosphoramidite method. Current Protocols in Nucleic Acid Chemistry , 00, 3.5.1–3.5.12. doi: 10.1002/0471142700.nc0305s00
- Wolfe, S. A., Ferentz, A. E., Grantcharova, V., Churchill, M. E., & Verdine, G. L. (1995). Modifying the helical structure of DNA by design: Recruitment of an architecture-specific protein to an enforced DNA bend. Chemistry & Biology, 2, 213–221. doi: 10.1016/1074-5521(95)90271-6
- Wunsch, E., Moroder, L., & Romani, S. (1982). 1-(tert -Butylthio)-1,2-hydrazinedicarboxylic acid derivatives. Hoppe-Seyler's Zeitschrift FüR Physiologische Chemie , 363, 1461–1464. doi: 10.1515/bchm2.1982.363.2.1461

