NEDC matrix application for metabolite imaging using MALDI-MSI
Antonia Fecke, Karl W Smith, Siva Swapna Kasarla, Philipp Baeuml, Prasad Phapale
Abstract
Matrix-assisted laser desorption/ionization mass spectrometry (MALDI-MS) has emerged as a analytical technique more than 30 years ago. Originally developed as a method to analyze large biomolecules like proteins or peptides, MALDI-MS has over the years found a new application in the field of biological tissue imaging (MALDI-MSI).
MALDI-MS has been utilized as an imaging tool for biological tissues, giving insight into the spatial distribution of molecules within complex tissue samples. Metabolomics research, which focuses on the study of metabolites present in a biological system, can benefit from this technology.
Small molecules such as metabolites or lipids can be analyzed under the aspect of spatial distribution across tissue sections. This enables the investigation of metabolic pathways, biomarkers or the correlation of metabolic changes with specific tissue regions, uncovering potential links between metabolite variations and physiological or pathological conditions.
Despite its potential, untargeted metabolomics using MALDI-MSI still faces several limitations. One major challenge is the diversity of matrices and protocols which are used to enhance ionization efficiency and the lack of information on metabolome coverage within different types of methods. One matrix of interest is NEDC (N-(1-napthyl)ethylendiamine dihydrochloride), which has gained recognition for its efficiency to visualize a broad range of metabolites.
This protocol provides a guide on how to prepare fresh frozen tissue samples for MALDI-MSI of metabolites using the NEDC matrix. It covers cryosectioning and preparation of tissue samples and provides a method for NEDC matrix application using the Sunchrom Sprayer.
Steps
Sample preparation and cryosectioning
Remove fresh frozen tissue sample stored at -80°C and place in cryostat at -20°C (kidney)/ -17°C (liver)/ -22°C (brain)
Mount the sample on chuck by pipetting small droplets of water and placing the sample on top until the water is frozen
Make sections of 12 µm thickness
Collect the sections on an ITO slide (for MALDI-MSI) or a glass slide (for H&E staining) and thaw mount the tissue onto the slide byy warming the slide with the back of your hand
Perform serial sectioning and xcollect the samples alternately on ITO and glass slides to allow for localzation and orientation of each ion image generated in MALDI-MSI to its histological counterpart
Place the slides in a slide holder and store at -80°C
Matrix application
Dry the sample in a vaccuum dessiccator at -0.1 MPa until dry (20-25 min)
Prepare N-(1-naphtyl)ethylenediamine hydrochloride (NEDC) at a concentration of 7 mg/mL in MeOH:ACN:H2O (70:25:5)
Turn on sprayer, pump (35 psi) and the connected computer
Take 250 µL syringe, fill it with MeOH and rinse the lines of the sprayer manually
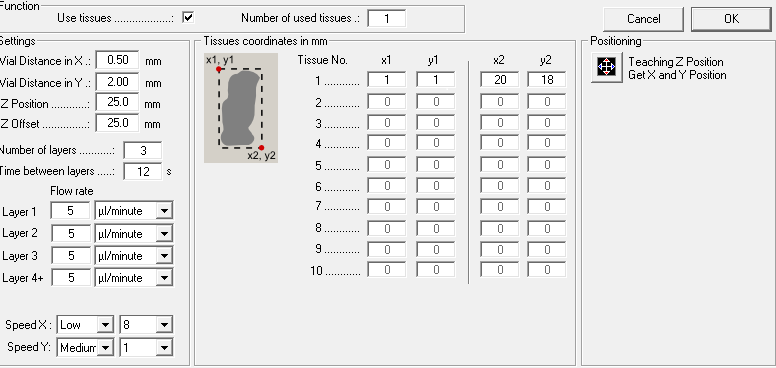
Open the Suncollect software and enter the sprayer settings and coordinates of your tissue (based on the size of your sample)
Sprayer setting:
| A | B |
|---|---|
| Vial Distance in X | 0.50 mm |
| Vial distance in Y | 2.00 mm |
| Z Position | 35.00 mm |
| Z Offset | 25.00 mm |
| Speed X | Low, 8 |
| Speed Y | Medium 1 |
Method parameters for the SunChrom sprayer
Fill the syringe with the prepared NEDC (solution (overfill), remove airbubbles and connect the syringe to the dispenser
Manually press the syringe until you have removed remaining airbubbles in the lines (the spray will stop shortly if there's an airbubble)
Place your tissue
In the suncollect software select your syringe size and set it to filled
Sprayer method
| A | B |
|---|---|
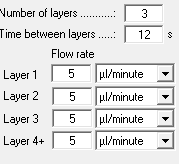
| Layer 1-3 | 5 µL/mL |
| Layer 4-6 | 10 µL/mL |
| Layer 7-9 | 15 µL/mL |
| Layer 10-21 | 20 µL/mL |
Flowrate for the different layers of the NEDC method
Start the spraying method. While spraying, watch the syringe and refill inbetween the layers if empty. While refilling, also set the syringe status to filled again in the software
After spraying, remove the tissue
Clean you sample holder and fill the syringe again with MeOH to manually clean the lines
Consumables
| A | B |
|---|---|
| Cryostat | Cm 1860, Leica, Nußloch, Germany |
| Blades | DB80 LX Low Profile Microtome Blades, Leica, Nußloch, Germany |
| ITO-slides | Part No. CG-90IN-S115, Delta Technologies, Loveland, USA |
| Glass slides | Micro Slides 2948-75X25, Corning Incorporated, NY, USA |
| N-(1-naphtyl)ethylenediamine hydrochloride | CAS: 1465-25-4, PCode: 1003246811, Merck, St Louis, USA |
| Sprayer | SunChrom Micro Fraction Collector MALDI Spotter Suncollect |
| Sprayer software | SunCollect Version 1.7.43 |
Used consumables and instruments