HUMAN ISLET SORTING FOR ALPHA, BETA, AND ACINAR CELLS
Klaus H. Kaestner Lab, Suzanne Shapira
Abstract
Fluorescence-activated cell sorting (FACS) relies on cell-type specific properties to isolate individual populations from a heterogenous sample. This protocol for human islet sorting allows for separation of α-cells, β-cells, and acinar cells from donor pancreata for downstream analyses, such as genomic assays, electrophysiology, and single-cell calcium imaging.
Before start
Make sure water bath is on and to temp (37°C).1. Make sure centrifuge is available (for 50mL conicals)
- For Qiagen DNA/RNA AllPrep kit, Prep RLT Plus Buffer
Steps
SET-UP
1. Thaw trypsin in water bath (0.05%, at least 6mL)
2. Thaw FBS
3. Label sorting tubes for samples
a. Sample
b. Aqua live/Dead Only
4. Label TWO sets of collection tubes for each cell type and put 500µL 1XPBS in each tube
a. Alpha
b. Beta
c. Acinar
5. Prepare 2% FBS (50ml 1XPBS + 1ml FBS), keep on ice
Note:
Keep 2% FBS, 1XPBS, trypsin and FBS on ice
PROCEDURE
1. Incubate islets.
2. Combine all islets into TWO 50mL conical tubes.
3. Centrifuge 4min, 1200rpm, RT.
4. Take off supernatant and use supernatant to rinse residual islets out of flask. Spin again, 4min, 1200rpm, RT.
5. Add 3mL 0.05% trypsin to each 50ml tube, pipette up and down ( NOTE: re-suspend in 1ml trypsin using P1000 and then add additional 2mL ).
6. Incubate at 37°C (water bath) for 9min. Pipette up and down every 3min. (t = 7min, 4min, 1min, 0min).
7. Remove tubes from water bath and add 1ml 100% FBS into tube to inhibit trypsin activity.
8. To remove undispersed material, passage contents of two 50mL conical tubes through ONE strainer into ONE 50mL conical in the following order. ( NOTE: use p1000, tip is on strainer, pressure, swirl. tips).
a. Add 1mL 100% FBS to the empty 50mL conical tube 1 and passage contents of tube 1 through the strainer.
b. Add 1mL 100% FBS to the second empty 50mL conical tube and passage contents of tubes through the strainer.
c. Swirl tube, and if clumps visible vortex quickly (2 sec).
9. Going forward, cells remain in ONE conical tube. Spins occur at RT.
10. Centrifuge: 4min, 1200rpm. Take off supernatant. Re-suspend in 25ml 1XPBS. Vortex quickly, then centrifuge 4min, 1200rpm. Take off supernatant.
11. Re-suspend in 1-2ml 2% FBS.
12. Count cells (10µL cells, 10µL trypan blue, 10µL into either side of a Countess chamber. Or make dilution as needed).
13. Adjust volume so cells are at a concentration of 5x106 cells/ml in 2% FBS.
14. For Aqua Live/Dead Only negative control: Remove 100,000 cells (20µL ) and put in FACS tube labeled Aqua. Add 480µL 2%FBS for final volume of 500µL . Store on ice until step 20.
15. Add ALL FOUR primary antibodies to cells (see below for detailed information; different batches of antibody may require optimization)-
a. **HICO-4F9** (HPi1): 1:100
b. **HIC3-2D12** (HPa3): 1:50
c. **HIC1-1C10** (HPx2): 1:500
d. **NPTDase:** 1:270 (TBD by each lot)
16. Incubate for 30 min on ice. Swirl tube every 10 min.
17. Centrifuge: 4 min, 1200rpm. Remove supernatant.
18. Wash by re-suspending in 25mL PBS, centrifuge 4 min, 1200rpm. Re-suspend in 2% FBS to bring concentration back to 5x106 cells/ml.
19. Prepare Aqua reagents: Add 50µLComponent B to 1 vial of Component A. Combined Aqua reagents (good for 2 weeks after constitution); LIVE/DEAD™ Fixable Aqua Dead Cell Stain Kit , for 405 nm excitation, Invitrogen: L34957
20. Add ALL THREE secondary antibodies at 1:200 and Aqua reagents. See below for secondary antibodies information.
21. Use 1µL aqua reagents to each 1mLof cells.
a. Add `0.5µL` aqua reagents to the Aqua Live/Dead Only tube.
b. Add appropriate amount (µl) of aqua reagents to the sample.
22. Incubate for 30 min at 4°C , with tubes wrapped in foil. Mix (swirl) every 10 minutes.
23. Centrifuge: 4 min, 1200 rpm. Take off supernatant. Wash: re-suspend in 25mL PBS, centrifuge 4 min, 1200rpm. Re-suspend in 2% FBS buffer to 5x106cells/ml 6cells/ml. Filter through strainer (attached to the blue tube) to transfer to FACS tube.
24. Centrifuge Aqua Live/Dead Only cells 2 minutes at 2K g, was in 500µL1XPBS, spin again, resuspend in 500µL 1X PBS. Wash Aqua-only with 500µL . Resuspend in 500µL 2% FBS.
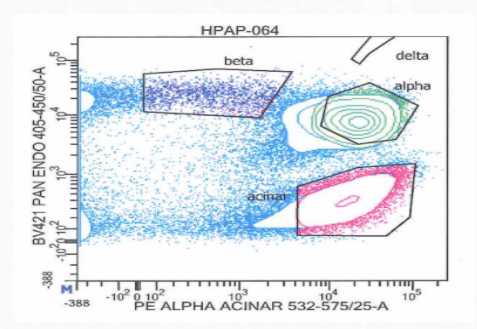
After sort
1. 100,000 alpha, beta, and acinar cells aliquoted for ATACseq
2. 20,000 alpha and beta cells plated for single-cell calcium imaging
3. 20,000 alpha and beta cells plated for electrophysiology
4. 250,000 to 500,000 cells for use in Qiagen DNA/RNA AllPrep kit: for >500,000 cells, use DNA/RNA Universal AllPrep kit; for <500,000 cells, use Qiagen DNA/RNA ALLPrep Micro kit.
a. Centrifuge cells, then carefully remove all supernatant by aspiration.
b. Loosen pellet by flicking and add RLT Plus buffer (prepared withBeta-mercaptoethanol)
- <5 x 106cells,
350µL - 5 x 106– 1 x 107cells,
600µL
5. Pipet the lysate directly into a QIAshredder spin column and centrifuge for 2 min at maximum speed.
6. Continue with AllPrep protocol, or snap freeze and store at -80°C for future use.
Ab Information
1. Primary antibodies
a. HICO-4F9 (HPi1) is a mouse IgG1 1that labels all human islet cells (to slightly varying degrees; beta cells are a little bit brighter than the others). Invitrogen MA5-16126. (RRID: AB_11157008)
b. HIC3-2D12 (HPa3) is a mouse IgM that differentially labels endocrine subtypes. Alpha, Gamma, and Epsilon cells are brightly labeled, Delta cells are moderately labeled, and Beta cells are dim-to-negative. This antibody also dimly labels duct cells, but these can be easily distinguished by their HIC1-2B4 negativity. Grompe lab at OHSU.
c. HIC1-1C10 (HPx2) is a mouse IgM that labels acinar cells. Novus Biologicals NBP1-18952.
d. NPTDase is a mouse IgG2b 2b that labels beta and delta cells. Powers lab at Vanderbilt U
2. Secondary antibodies
a. Brilliant Violet 421™ anti-mouse IgG1: Biolegend 406615 (RRID: AB_2562233)
b. R-Phycoerythrin AffiniPure F(ab')₂ Fragment Goat Anti-Mouse IgM, μ Chain: Jackson ImmunoResearch 115-116-075 (RRID: AB_2338628)
c. Rat anti-Mouse IgG2b, FITC, eBioscience™: Invitrogen 11-4220-82 (RRID: AB_2572495)

