Assessment of PKC-dependent activation of LRRK1 in vitro
Dario Alessi, Asad Malik, Athanasios Karapetsas
Abstract
We describe a non-radioactive assay that we deploy for analysing the kinase activity of recombinant LRRK1 following in vitro activation by Protein kinase C (PKC) isoforms. This assay can also be used to analyse the effect of PKC on LRRK1 immunoprecipitated from cells.
Attachments
Steps
Preparation of lipid vesicles for PKC activation
Clean a disposable glass culture tube by washing. Allow to air-dry.
Clean a disposable glass culture tube by washing with 100% methanol. (1/3)
Clean a disposable glass culture tube by washing with 100% methanol. (2/3)
Clean a disposable glass culture tube by washing with 100% methanol. (3/3)
Pipette 0.5µL of Diacylglycerol (stock concentration is 10mg/mL) and 5µL of Phosphatidylserine (stock concentration is 10mg/mL) into the cleaned and dried glass tube.
Vacuum dry lipids using a SpeedVac system for 0h 10m 0s. This should leave a visible, translucent lipid pellet.
Resuspend lipids in 50µL of 25millimolar (mM) HEPES 7.4, 50millimolar (mM) KCl. Vortex gently until pellet is no longer visible.
Kinase Reaction Step 1: Phosphorylation of LRRK1 by PKC
| A | B |
|---|---|
| HEPES pH 7.5 | 50 mM |
| KCl | 100 mM |
| 2‐Mercaptoethanol | 0.2% (v/v) |
| MgCl2 | 20 mM |
| ATP | 2 mM |
| CaCl2 | 2 mM |
| Phosphatidylserine | 200 μg/ml |
| Diacylglycerol | 20 μg/ml |
For each reaction, add 10µL of the primary “2X master mix” to a clean Eppendorf tube.
Add 5µL of 200nanomolar (nM) LRRK1 wild type protein (final concentration is 50nanomolar (nM)) to each reaction and allow equilibration 30On ice for 0h 5m 0s.
Start the kinase reaction by adding 5µL of 400nanomolar (nM) PKC Alpha protein (final concentration is 100nanomolar (nM)).
After 0h 30m 0s, transfer the Eppendorf tubes from Step 8 30On ice.
Kinase Reaction Step 2: Phosphorylation of Rab7A by PKC-activated LRRK1
Prepare a secondary “master mix” (=Master Mix B) containing
| A | B |
|---|---|
| HEPES pH 7.5 | 25 mM |
| KCl | 50 mM |
| MgCl2 | 10 mM |
| ATP | 1 mM |
| Rab7A | 1 μM |
Start the second step of the kinase reaction by adding 10µL Master Mix B to the Eppendorf tubes from Step 5.
Transferring the Eppendorf tubes to the thermo mixer set at 30°C, 1,000 rpm. Incubate for 0h 45m 0s.
Stop the kinase reaction by adding 10µL of 4× LDS (supplemented with 5% (v/v) 2‐Mercaptoethanol) loading buffer to the reaction mix to a final concentration of 1×.
If using LRRK1 immunoprecipitated from cells, stop the kinase reaction by adding 30µL of 4× LDS loading buffer to the reaction mix to a final concentration of 2×, incubate the mixture at 70°C on a heat block for 0h 10m 0s to elute LRRK1 from the resin, and collect the eluent by centrifugation through a 0.22‐μm‐pore‐size Spinex column.
Incubate the samples for 0h 5m 0s at 70°C on a heat block before proceeding to quantitative immunoblotting analysis.
Analysis of kinase reaction products by quantitative immunoblotting analysis:
The reaction products can be analysed by quantitative immunoblotting analysis (as described in XXXX). Table 1 lists the primary antibodies that we recommend using, which include antibodies to detect Rab7A phosphorylation at Serine-72.
| A | B | C | D | E |
|---|---|---|---|---|
| Antibody Target | Company | Cat. number | Host species | Dilution |
| pS72 Rab7A | Abcam Inc. | MJF-38, Clone 1 | Rabbit | 1 mg/ml |
| Rab7A (Total) | Sigma | R8779 | Mouse | 1.430556 |
| LRRK1 (total) (C-terminus) | MRC-PPU Reagents and Services, University of Dundee | S405C | Sheep | 1 mg/ml |
| PKC Alpha | Abcam Inc. | ab11723 | Mouse | 1.430556 |
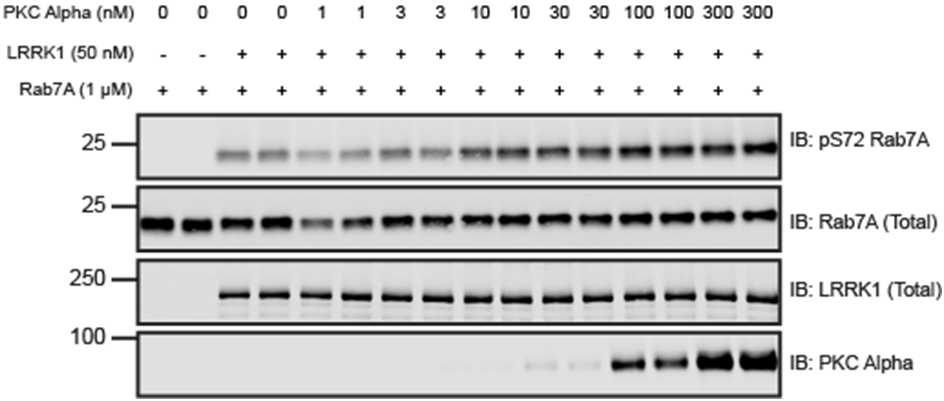
Figure 1: PKC alpha dose-dependent activation of recombinant LRRK1 in vitro. Recombinant LRRK1 wild type [27-2015] was incubated with increasing concentrations of PKC Alpha (1 to 300 nM) at 30°C for 0h 30m 0s with excess Mg-ATP. Reactions were subsequently incubated with 1micromolar (µM) recombinant Rab7A and subjected to a 0h 45m 0s kinase reaction at 30°C in the presence of excess Mg-ATP. Kinase reactions were subjected to immunoblot analysis with the indicated antibodies and the membranes were developed using the Odyssey CLx scan Western Blot imaging system.

