A novel laboratory method to simulate climatic stress with successful application to experiments with medically relevant ticks
Sang Hyo Kim, Caleb Nielebeck, Lauren Dedmon, Mark Pangilinan, Jahred Quan, William Ota, Javier D. Monzón
Abstract
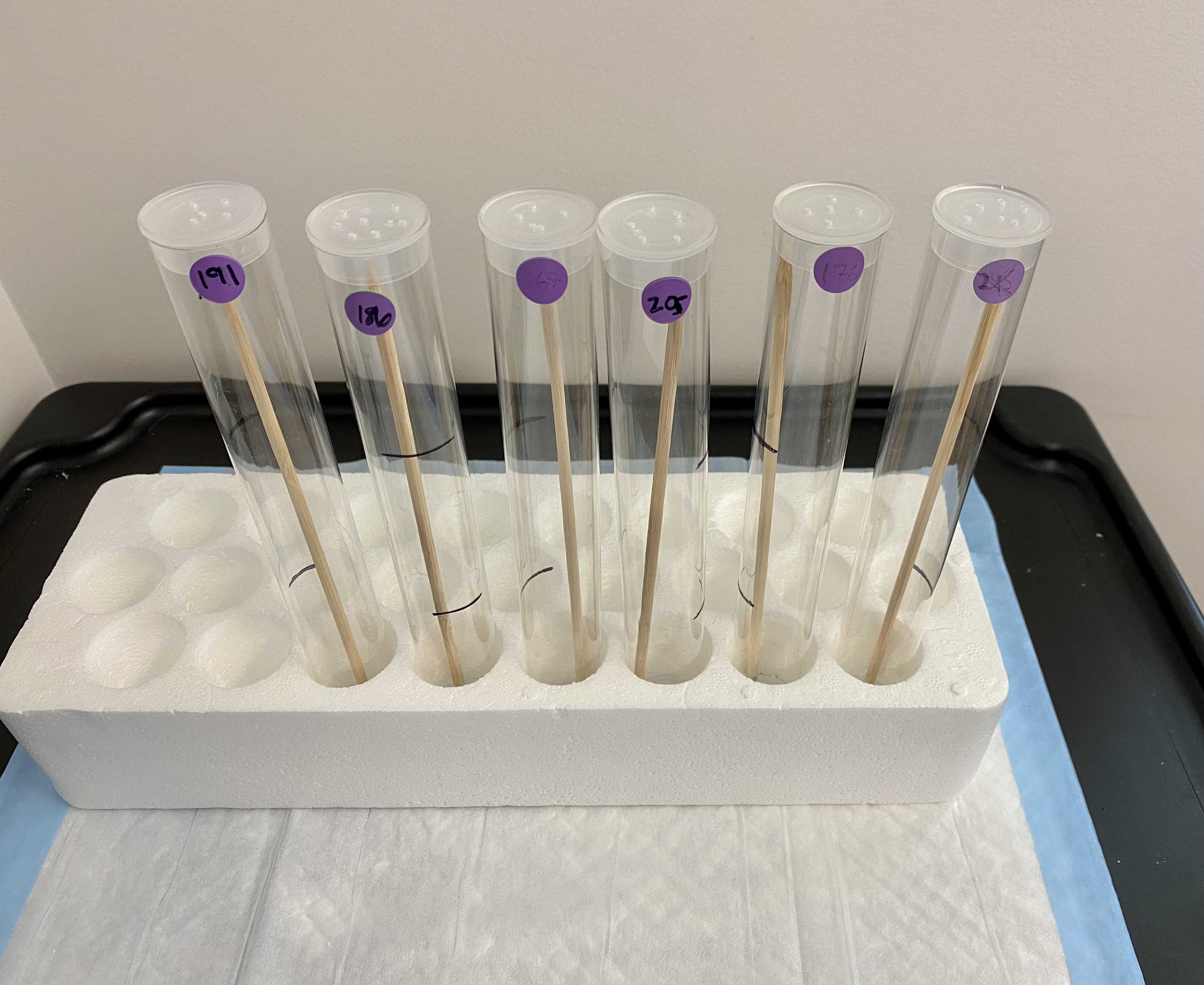
This protocol details a novel method to isolate individual ticks and manipulate their environment. We successfully used this method to investigate how humidity affects survival and host-seeking (questing) behavior of three species of ticks: the lone star tick (Amblyomma americanum), American dog tick (Dermacentor variabilis), and black-legged tick (Ixodes scapularis). We placed 72 adult females of each species into individual plastic tubes and separated them into three experimental relative humidity (RH) treatments representing distinct climates: 32% RH, 58% RH, and 84% RH. For 30 days we assessed the survival and questing behavior of each tick.
Before start
We acquired adult female ticks from the Oklahoma State University Tick Rearing Facility and experimented with one temperature range and three relative humidities. This protocol can be modified for different species, life stages, temperatures, humidities, and other small organisms.
Steps
Set up
Place six tubes in each airtight container along with a humidity pack, labelling each container
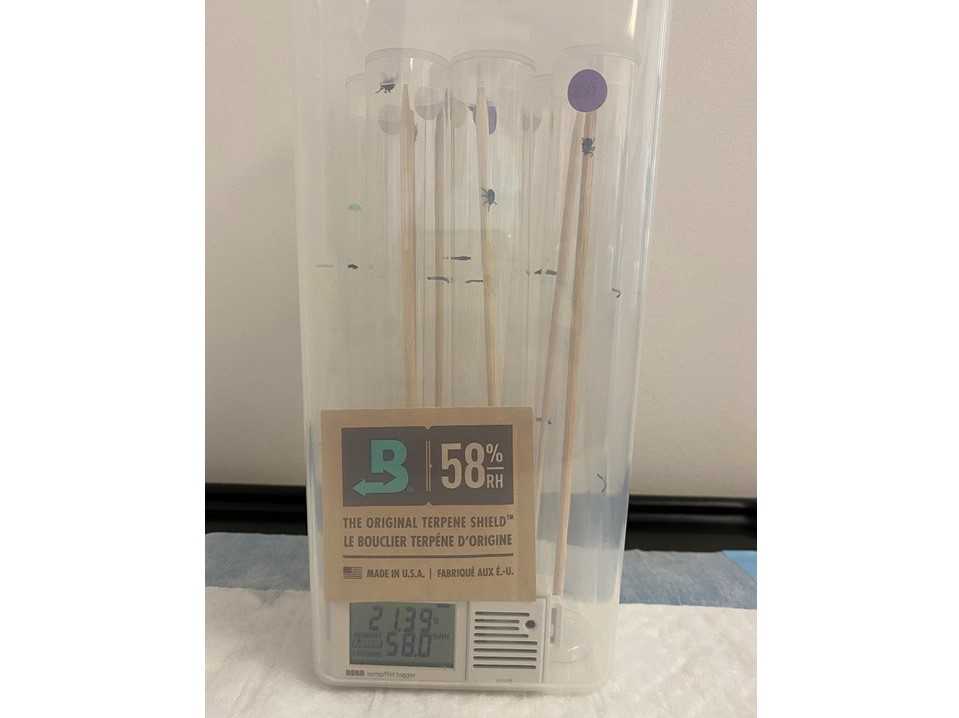
Confirm the humidity in one container of each RH level with the data logger
Program the climate chamber
To cycle between 20°C to 30°C, the temperature increments should be as follows:
- 3:00 -
25°C - 6:00 -
27.5°C - 9:00 -
30°C - 12:00 -
27.5°C - 15:00 -
25°C - 18:00 -
22.5°C - 21:00 -
20°C - 24:00 -
22.5°C
To create a 12:12 light:dark photoperiod, lighting increments should be as follows:
- 9:00 - Lights on
- 21:00 - Lights off
Data collection
Place all of the airtight containers, filled with ticks and humidity packs, into the climate chamber and start the program
Each day thereafter, during the 9:00 to 12:00 or 30°C increment, assess each tick for survivorship and questing behavior (see guidelines for qualifications of survivorship and questing)
Periodically move the data logger to a new bin to confirm that no unexpected changes to the climate inside the containers has occurred
Repeat steps 4 and 5 for 30 days or until all ticks have died