A Localized Aldara (5% Imiquimod)–Induced Psoriasiform Dermatitis Model in Mice Using Finn Chambers
Szabina Horváth, Szabina Horváth, Ágnes Kemény, Ágnes Kemény, Erika Pintér, Erika Pintér, Rolland Gyulai, Rolland Gyulai
Abstract
The expanding number of research studies utilizing the imiquimod-induced psoriasiform dermatitis model attests to the usefulness of this procedure. Advantages of this model include rapid development of the skin response and cost-effectiveness. A major limitation is that application of imiquimod cream over large areas of skin, as well as licking and ingestion of the cream, may lead to severe systemic inflammation, which can cause a general decline in health, splenomegaly, and death. In this protocol, Finn chambers are used to localize the imiquimod cream to a small area of the skin. This results in production of severe and reproducible psoriatic skin reactions with significantly less imiquimod, greatly reducing the possibility of untoward systemic effects. Moreover, having psoriasiform and control skin areas on the same mice decreases inter-animal differences. The protocol can be readily adapted for other skin disease models involving topical application of test agents. This article also details functional measurements performed during assays, including skin thickness, blood perfusion, semiquantitative histopathological evaluation, determination of scaling score to monitor psoriatic symptoms, and collection of spleen and body weight data to identify systemic effects. © 2020 The Authors.
Basic Protocol : Use of Finn chambers to induce psoriasiform skin reactions with imiquimod
Support Protocol 1 : Measurement of double-fold dorsal skin thickness
Support Protocol 2 : Measurement of blood perfusion
Support Protocol 3 : Determination of scaling score
Support Protocol 4 : Semiquantitative histopathological scoring
Support Protocol 5 : Assessment of systemic side effects in response to imiquimod application
INTRODUCTION
Imiquimod (IMQ) is a small nucleotide-like molecule commonly used to induce psoriasis-like dermatitis in animal models (Swindell et al., 2017; van der Fits et al., 2009). Imiquimod activates innate and adaptive immune system signaling pathways via TLR7/9, expressed on plasmacytoid dendritic cells (PDCs) (Hanna, Abadi, & Abbas, 2016). It is likely that other actions of imiquimod—such as NLRP1 inflammasome activation (pyroptosis) (Walter et al., 2013) and direct activation of TRPA1 nonselective cation channels located on immune cells and peripheral nerve endings (Kemény et al., 2018)—also contribute to its proinflammatory effects. The popularity of the imiquimod model is due in large measure to the fact that this substance initiates IL-17, IL-23 cytokine-mediated immunological processes of the skin, thereby mimicking the Th17-induced autoimmune component of the human psoriatic condition. In addition, the imiquimod model is cost-effective and reproducible (Hawkes, Gudjonsson, & Ward, 2016). A major problem encountered with this approach in early studies was the robust systemic inflammation that often resulted from smearing 62.5 mg of 5% imiquimod on the shaved backs of mice. This systemic side effect results in a generalized deterioration of the health of the test animal, including fever, weight loss, splenomegaly (enlarged spleen), and death (Alvarez & Jensen, 2016). Such toxicity severely limits the utility of this approach. Moreover, the need to treat large surface areas required that different groups of animals be used to examine and compare results from imiquimod-treated and control subjects. The Basic Protocol below details a modified and improved method for the imiquimod-induced psoriasiform dermatitis model using Finn chambers to achieve localized application of imiquimod (Horváth et al., 2019). This new procedure decreases the likelihood of systemic side effects and makes possible comparisons between treated and untreated skin in the same subjects, thereby reducing inter-assay variability and cost.
In the mouse model of psoriasis, infiltration is measured by measuring double-fold skin thickness using a micrometer (see Support Protocol 1), erythema (increased blood perfusion) is measured using the LASCA laser Doppler method (see Support Protocol 2), and desquamation is determined using the scaling score (see Support Protocol 3). A semiquantitative histopathological evaluation is performed to determine the epidermal thickness as well as the presence of Munro's microabscesses and dilated capillaries (see Support Protocol 4). Finally, measurement of spleen weight and body weight are recommended for monitoring the presence of systemic effects (see Support Protocol 5).
NOTE : All procedures were conducted according to the 1998/XXVIII Act of the Hungarian Parliament on Animal Protection and Consideration Decree of Scientific Procedures of Animal Experiments (243/1988). All protocols were approved by the Ethics Committee on Animal Research of the University of Pécs, in full accordance to the Ethical Codex of Animal Experiments. All protocols involving live animals must be reviewed and approved by an Institutional Animal Care and Use Committee (IACUC) and must conform to government regulations for the care and use of laboratory animals.
Basic Protocol: USE OF FINN CHAMBERS TO INDUCE PSORIASIFORM SKIN REACTIONS WITH IMIQUIMOD
In this procedure, imiquimod cream is applied to the dorsal skin of mice using small (8-mm-diameter) Finn chambers to induce typical psoriatic symptoms and histopathological alterations while minimizing systemic inflammation. Such localized application of small amounts of imiquimod cream reduces side effects by decreasing the likelihood of ingestion of the cream. In addition, it makes it possible to examine treated and control skin on the same animal, reducing inter-animal variability as well as the number of subjects needed for each experiment. Furthermore, like an occlusive dressing, the chamber may enhance penetration of active compounds. Treatment with active compound for as few as four consecutive days is sufficient to provide evidence of efficacy for the treatment of psoriasiform skin inflammation.
Materials
-
C57BL/6 female mice, 8-10 weeks old, 20-25 g
-
Ketamine/xylazine solution (see recipe)
-
Depilatory cream (Veet)
-
Aldara cream (5% imiquimod; Meda Pharma)
-
Vaseline
-
Small animal laboratory scale
-
1-ml syringes with 27-G needles
-
Electric shaver
-
Rubber-edged spatula
-
Adjustable heating plate (Temperature Controller TMP-5a, Supertech Instruments)
-
8-mm (50-mm2) Finn chambers on Scanpor tape (SmartPractice)
-
Forceps
Day 0: Prepare animals
1.Select age- and sex-matched mice with at least six animals per group.
2.Measure body weight and calculate the amount of ketamine/xylazine solution needed for anesthesia.
3.Anesthetize by i.p. injection using a 1-ml syringe with a 27-G needle. Make sure that animals are completely anesthetized (e.g., by tail pinch).
4.Shave an ∼3 × 4–cm area on the dorsal skin using an electric shaver.
5.Remove any remaining hairs with depilatory cream. Remove cream with a rubber-edged spatula, being careful to avoid even microscopic skin lacerations and abrasions. Rinse the denuded area with wet cotton pads.
6.Place anesthetized animals on a 37°C heating plate to maintain constant body temperature during recovery, then return to their home cages.
Days 1-4: Induce psoriatic reaction
7.Measure body weight and anesthetize animal as before.
8.Place animals on a 37°C heating plate to maintain body temperature, then evaluate baseline dorsal skin thickness, blood perfusion, and scaling (see Support Protocols 1-3).
9.Cut out two individual circular Finn chambers per animal from the Scanpore tape, ensuring that there is sufficient adhesive surface to apply firmly to the skin.
10.Fill one chamber with 25 mg Aldara cream and the other with 25 mg Vaseline.
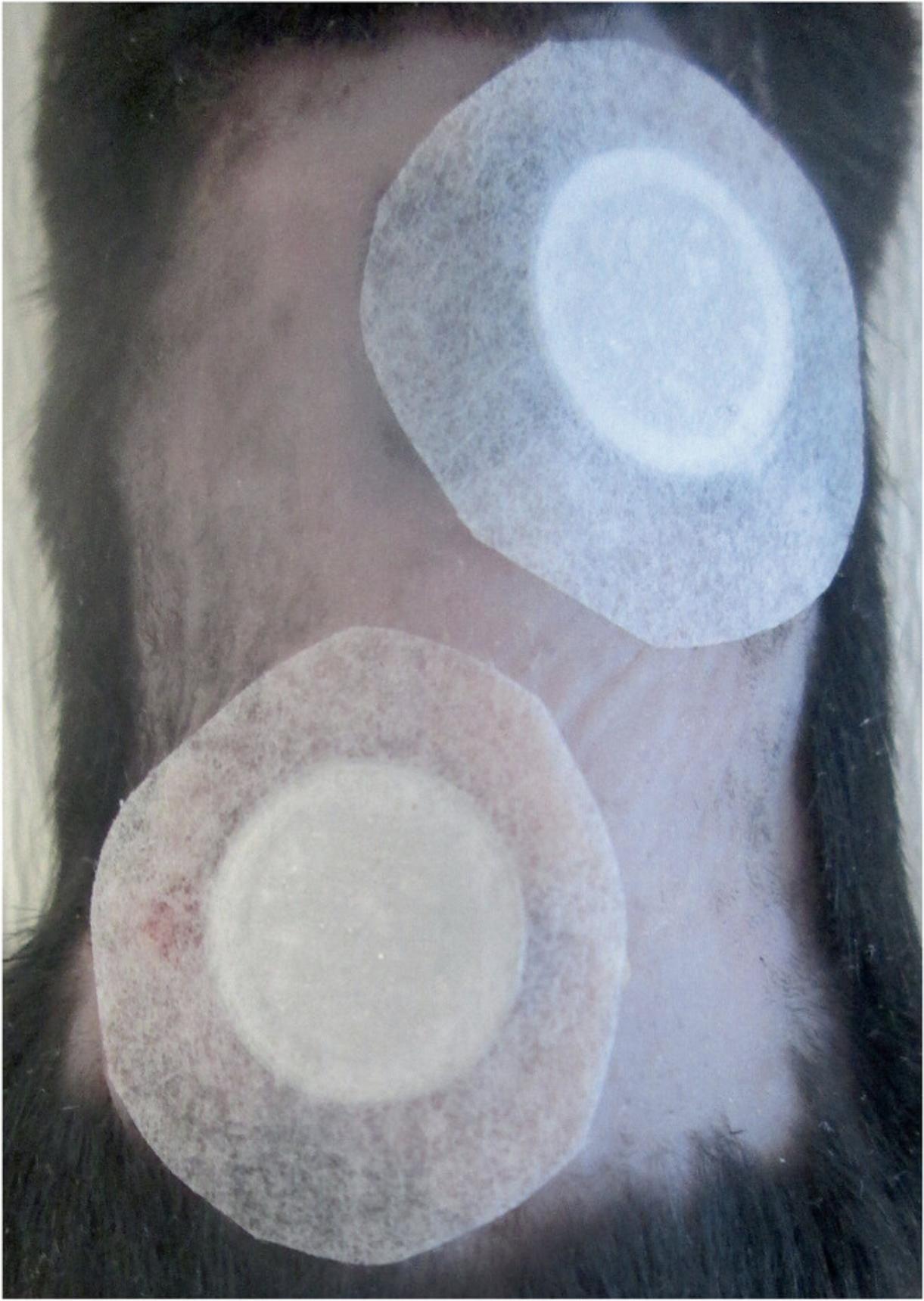
11.Place Finn chambers on two discrete spots on the dorsal skin at least 2 cm apart (Fig. 2).
12.Return mice to their cages and house normally until the next treatment.
13.After 24 hr, use forceps to gently remove the Finn chambers from the dorsal skin.
14.Over the next three days (Days 2, 3, 4), repeat measurements and treatment (steps 7-13), placing fresh Finn chambers on the same locations as on Day 1.
Day 5: Perform functional measurements and collect tissue samples
15.On Day 5, repeat measurements in steps 7-8, then sacrifice animals, collect skin samples for histology (see Support Protocol 4), and measure spleen weight (see Support Protocol 5).
Support Protocol 1: MEASUREMENT OF DOUBLE-FOLD DORSAL SKIN THICKNESS
A typical symptom of imiquimod-induced psoriasiform dermatitis in mice is an increase in epidermal thickness. Dorsal skin thickness should be monitored daily after anesthesia but before treatment by measuring the treated skin areas in vivo with an engineer's micrometer.
Materials
- Mice enrolled in Finn chamber experiment (Days 1-5; see Basic Protocol)
- 70% (v/v) ethanol
- Engineer's micrometer (Moore and Wright, Sheffield, England)
1.With the anesthetized animal placed on a heating pad, pinch the dorsal skin in a rostral-caudal direction above the spine (Fig. 3A).
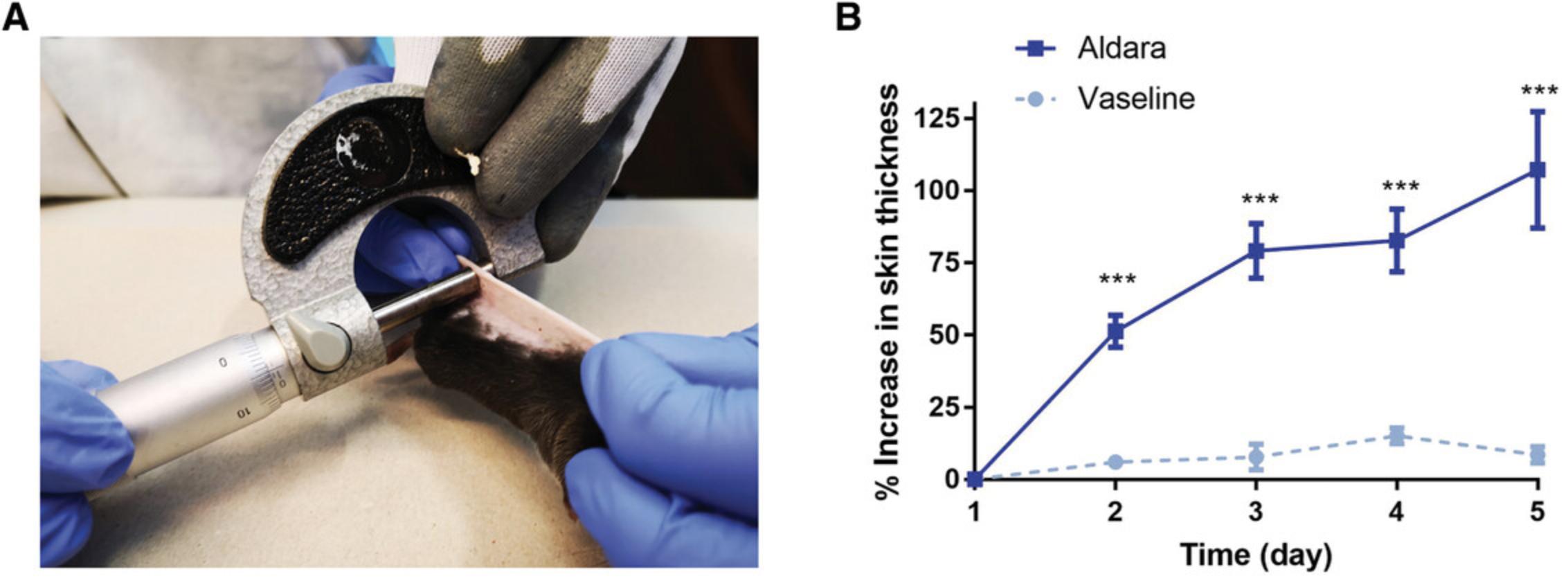
2.Place the treated area of the double-folded mouse skin between the anvil and spindle face of the micrometer. Perform thickness measurement and record.
3.Thoroughly wipe the micrometer with 70% ethanol after each measurement to avoid cross-contamination of imiquimod- and Vaseline-treated areas.
4.Repeat each day of the 5-day experiment.
5.Express data as percent increase in skin thickness compared with Day 1 baseline values (Fig. 3B).
Support Protocol 2: MEASUREMENT OF BLOOD PERFUSION
Increased blood perfusion (erythema) is a hallmark of inflammatory skin conditions. Here, mean perfusion of the treated skin area is quantified non-invasively using Laser speckle contrast analysis (LASCA) imaging. Blood perfusion should be measured after anesthesia but before treatment on each day of the 5-day experiment.
When measuring blood perfusion, anesthesia must be sufficiently stable to avoid involuntary movements, which could affect the accuracy of the measurement. Additionally, body temperature must be maintained, as fluctuations in this parameter can have a significant influence on peripheral microcirculation.
Materials
- Mice enrolled in Finn chamber experiment (Days 1-5; see Basic Protocol)
- Laser speckle contrast analysis laser Doppler (PeriCam PSI, Perimed)
- PimSoft software (Perimed, Sweden)
1.With the anesthetized animal placed on a heating pad, define a 3 × 4–cm area in the data acquisition software adjusted to the dorsal skin of mouse.
2.Select regions of interest (ROI) according to the treated areas on the dorsal skin (Fig. 4A).
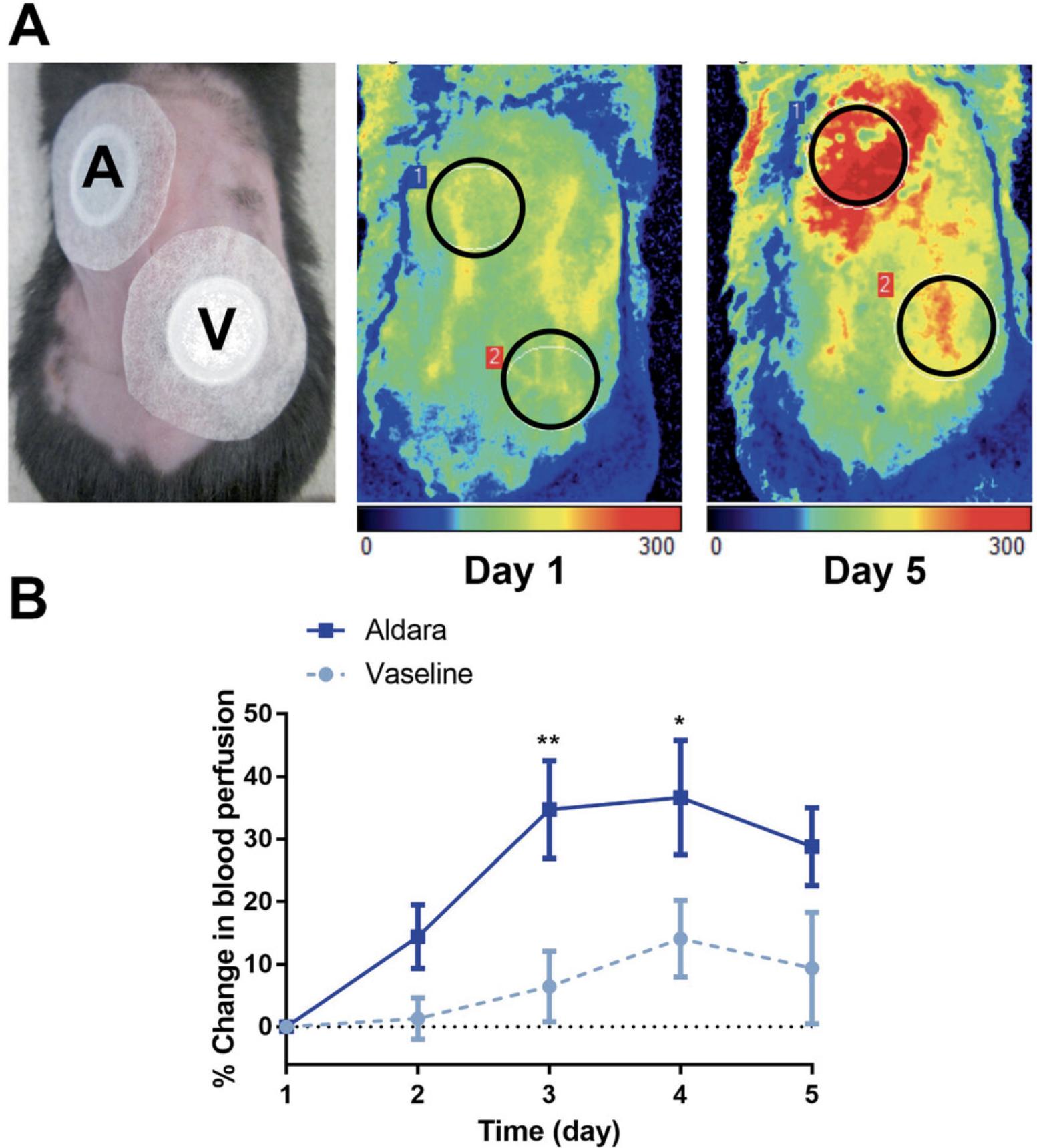
3.Measure the mean perfusion of the two ROIs for 2 min each.
4.Repeat each day of the 5-day experiment.
5.Express data as percent change in blood perfusion relative to Day 1 baseline values (Fig. 4B).
Support Protocol 3: DETERMINATION OF SCALING SCORE
Scaling should be monitored daily after anesthesia but before treatment. The calculation of the scaling score is based on Luo, Wu, Zhao, Liu, & Wang (2016).
Materials
- Mice enrolled in Finn chamber experiment (Days 1-5; see Basic Protocol)
- Digital camera (e.g., Sony Cybershot DSCW170)
- Camera stand (e.g., Manfrotto 290)
1.With the anesthetized animal on a heating pad, use a digital camera on a camera stand to take individual photographs of each animal from a standard distance (determined by the focal distance of the camera).
2.Have three trained dermatologists who are blinded for the study score each of the photographic images. Assign scores from 0-4, where 0 = no scaling, 1 = slight scaling, 2 = moderate scaling, 3 = marked scaling, and 4 = maximal scaling (Fig. 5A).
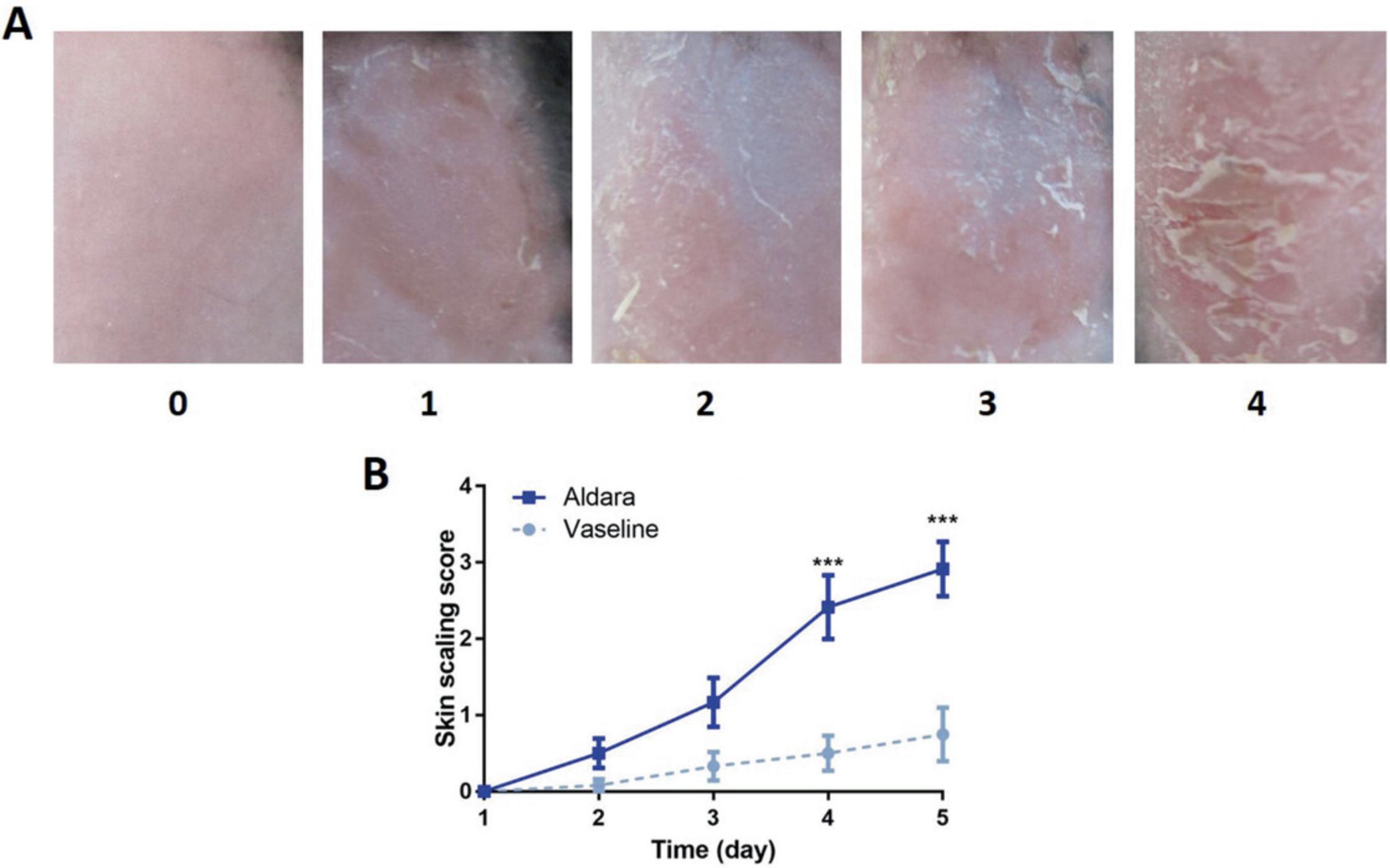
3.Plot average score versus day of treatment (Fig. 5B).
Support Protocol 4: SEMIQUANTITATIVE HISTOPATHOLOGICAL SCORING
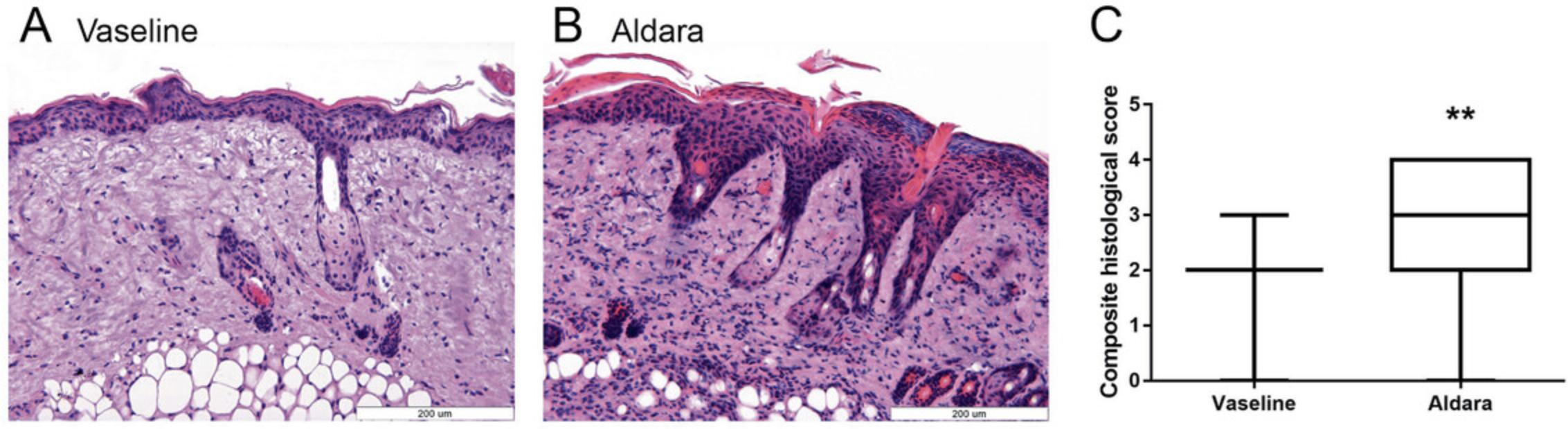
An important characteristic of imiquimod-induced psoriasiform dermatitis is its ability to recapitulate the structural changes associated with human psoriatic skin. This includes an increase in epidermal thickness, the appearance of Munro's microabscesses, and tortured dilated capillaries. Semiquantitative scoring of these parameters on hematoxylin-eosin stained tissue is used to determine the severity of psoriasiform skin inflammation.
Materials
-
Mice enrolled in Finn chamber (Day 5; see Basic Protocol)
-
6% formalin
-
Hematoxylin-eosin solution
-
Forceps and scissors
-
Microscope slides
-
Light microscope with 20× objective
-
AnalySIS software (Olympus) or similar
-
Additional reagents and equipment for standard methods of sectioning fixed tissue and staining with hematoxylin-eosin
1.Sacrifice mice by cervical dislocation.
2.Excise the 8-mm treated full-thickness skin tissue using sterile forceps and scissors.
3.Fix in 6% formalin for 48 hr.
4.Prepare three 5-µm sections from the center of the treated area on microscope slides and stain with hematoxylin-eosin according to standard procedures.
5.Count the total number of Munro's microabscesses throughout the entire section.
6.Determine the number of dilated capillaries present in the dermal-epidermal border of the entire section.
7.Measure the diameter of the epidermal layer of three separate sites in the field of view using the arbitrary distance function of AnalySIS software. Repeat for up to three fields of view on each tissue section. Average the collected data.
8.Set the lowest and highest data values for each measured parameter (number of Munro's microabscesses, number of dilated capillaries and thickness of epidermal layer) as the low and high reference points for that parameter, then divide the difference between them into five equal ranges. Assign each range a point value from 0 to 4, with the lowest range being 0 and highest being 4.Plot each data set separately or calculate a composite score by averaging the three different scores (Munro's, capillaries, thickness) derived from the same experimental setup (i.e., same treatment, same exposure time, etc.).
Support Protocol 5: ASSESSMENT OF SYSTEMIC SIDE EFFECTS IN RESPONSE TO IMIQUIMOD APPLICATION
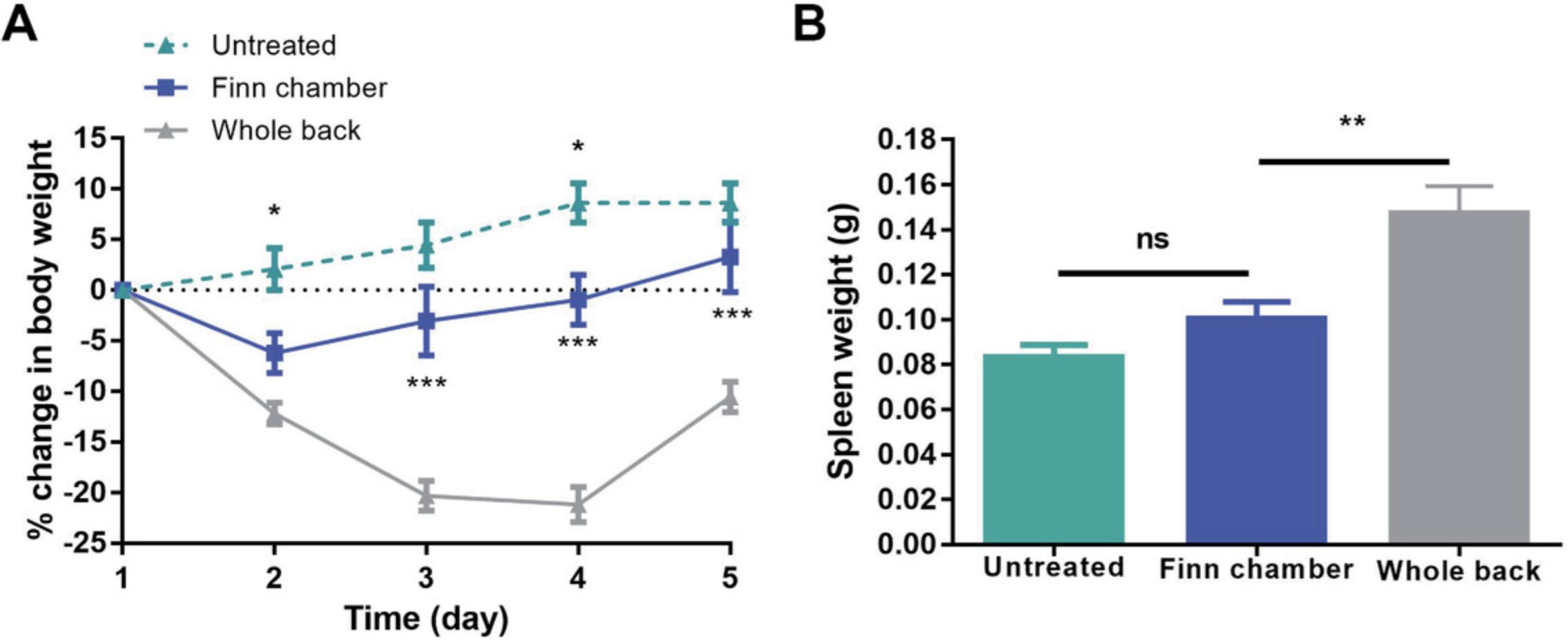
One of the major problems with the original imiquimod model was the appearance of systemic side effects including splenomegaly, loss of body weight, and increased serum concentrations of inflammatory cytokines, complicating the interpretation of the findings. This problem is largely overcome by using Finn chambers for localized application, which significantly minimizes the amount of irritant applied to the skin. Described below are ways to measure body weight and splenomegaly to assess the presence of imiquimod side effects.
Materials
- Mice enrolled in Finn chamber experiment (Day 5; see Basic Protocol)
- Sterile forceps and scissors
- Analytical balance
1.Using measurements from the Basic Protocol, plot the percent change in body weight on all days relative to Day 1 (Fig. 7A).
2.Sacrifice mice by cervical dislocation.
3.Open the peritoneum with a ventral midline incision using sterile forceps and scissors.
4.Remove and immediately weigh the spleen on an analytical balance (Fig. 7B).
REAGENTS AND SOLUTIONS
Ketamine/xylazine solution
- 1200 µl 50 mg/ml ketamine (Richter Gedeon)
- 150 µl 20 mg/ml xylazine (Lavet Pharmaceuticals)
- 4650 µl physiological saline
- Store up to 1 week at 4°C
Final concentrations are 10 mg/ml ketamine and 0.5 mg/ml xylazine. An intraperitoneal injection of 100 μl solution per 10 g body weight will give the desired doses of 100 mg/kg ketamine and 5 mg/kg xylazine.
COMMENTARY
Background Information
In the original imiquimod-induced psoriasiform model, 62.5-80 mg of imiquimod or Vaseline control were smeared on the shaved whole back skin of the mouse (Kim et al., 2014; van der Fits et al., 2009). Often this quantity of imiquimod elicited severe systemic effects, as evidenced by a general decline in health, weight loss of 20-25%, fever, and death (Alvarez & Jensen, 2016). To minimize this problem, we modified the procedure to identify the optimal dose of imiquimod and application method for inducing a consistent skin reaction. Given their design, Finn chambers were found to be ideal for administering the minimal amount of cream, greatly reducing the occurrence of systemic side effects. Moreover, Finn chambers improved the penetration of active test substances into the skin through an occlusive effect, while minimizing scratching and licking of the creams by experimental animals or their littermates.
Critical Parameters and Troubleshooting
Imiquimod-induced psoriasiform dermatitis results is induced by topical application of imiquimod cream. For proper penetration of the active ingredient and measurement of relevant symptoms, it is imperative that the fur be completely removed with an electric shaver and depilatory cream. As these procedures may cause minor skin injuries, care must be taken in to avoid such damage, and time should be allowed for the tissue to heal before undertaking experiments. Typically, this is addressed by waiting at least one day after fur removal before initiating a study. For optimal results, it is important to place Finn chambers in the same locations each day of the experiment.
To obtain consistent and reliable results using Doppler measurement of blood perfusion, deep anesthesia of mice is necessary, as any involuntary movement can influence the assessment. It is also crucial to maintain animal body temperature using a 37°C heating pad, as fluctuations in body temperature can influence blood perfusion.
For troubleshooting guidance, see Table 1.
| Problem | Possible cause | Solution |
|---|---|---|
| Finn chambers detach from skin within a short time | Size of adhesive surface is too small | Cut larger adhesive surface around chambers |
| Symptoms of inflammation observed on Vaseline-treated skin | Finn chambers were not placed on the same skin area each day of the experiment | Ensure that the same areas are treated each day |
| Signs of scratching on dorsal skin after shaving | Irritation from depilatory cream | Use odorant-free or “sensitive-skin” depilatory cream |
| Microinjuries on dorsal skin after shaving | Use of coarse spatula for removing depilatory cream | Use a soft, rubber-edged spatula |
Understanding Results
The extent of the skin reaction to imiquimod differs among mouse strains, with females being most sensitive in all cases (Alvarez & Jensen, 2016; Swindell et al., 2017). It is also possible that the extent of imiquimod-induced psoriasiform skin inflammation is dependent on the general health of the animal (e.g., body weight), which might explain variations in response to the irritant (Yu et al., 2019, 2020). Given these factors, all experiments should be repeated at least three times to obtain the most robust and reliable findings.
Time Considerations
A single experiment requires 6 days to complete. This includes 1 day for preliminary preparation (measurement of body weight, depilation), 4 days for imiquimod and Vaseline treatments and assessment of in vivo parameters, and 1 final day to complete in vivo measurements and obtain tissue samples for subsequent analysis.
Imiquimod and Vaseline treatments on the dorsal skin of mice using Finn chambers require 15 min per six mice, as does the double-fold skin thickness measurement. Blood perfusion testing requires ∼25 min per six mice, with body weight measurements taking about 6 min per six mice. Collection of skin and spleen samples and measurement of spleen weights require 30 min per six mice. Processing excised skin samples for histological analysis takes 7 days, and histopathological scoring of hematoxylin-eosin slides takes ∼15 min per sample.
Acknowledgments
This work was supported by NKFIH K_128210; PEPSYS-GINOP-2.3.2-15-2016-00050, OTKA-NN-114458; EFOP 3.6.2-16-2017-00009 Establishing and Internationalizing the Thematic Network for Clinical Research. KÁ was supported by János Bolyai Research Scholarships of the Hungarian Academy of Sciences and ÚNKP-19-4 New National Excellence Program of the Ministry for Innovation and Technology.
Literature Cited
- Alvarez, P., & Jensen, L. E. (2016). Imiquimod treatment causes systemic disease in mice resembling generalized pustular psoriasis in an IL-1 and IL-36 dependent manner. Mediators of Inflammation , 2016, 5–8. doi: 10.1155/2016/6756138.
- Hanna, E., Abadi, R., & Abbas, O. (2016). Imiquimod in dermatology: An overview. International Journal of Dermatology , 55, 831–844. doi: 10.1111/ijd.13235.
- Hawkes, J. E., Gudjonsson, J. E., & Ward, N. L. (2016). The snowballing literature on imiquimod-induced skin inflammation in mice : A critical appraisal. Journal of Investigative Dermatology , 137(3), 546–549. doi: 10.1016/j.jid.2016.10.024.
- Horváth, S., Komlódi, R., Perkecz, A., Pintér, E., Gyulai, R., & Kemény, Á (2019). Methodological refinement of Aldara-induced psoriasiform dermatitis model in mice. Scientific Reports , 9, 3685. doi: 10.1038/s41598-019-39903-x.
- Kemény, Á., Kodji, X., Horváth, S., Komlódi, R., Szőke, É., Sándor, Z., … Gyulai, R. (2018). TRPA1 acts in a protective manner in imiquimod-induced psoriasiform dermatitis in mice. Journal of Investigative Dermatology , 138(8), 1774–1784. doi: 10.1016/j.jid.2018.02.040.
- Kim, H. R., Lee, A., Choi, E. J., Hong, M. P., Kie, J. H., Lim, W., … Seoh, J. Y. (2014). Reactive oxygen species prevent imiquimod-induced psoriatic dermatitis through enhancing regulatory T cell function. PLoS One , 9(3), e91146. doi: 10.1371/journal.pone.0091146.
- Luo, D.-Q., Wu, H.-H., Zhao, Y.-K., Liu, J.-H., & Wang, F. (2016). Different imiquimod creams resulting in differential effects for imiquimod-induced psoriatic mouse models. Experimental Biology and Medicine , 241, 1733–1738. doi: 10.1177/1535370216647183.
- Swindell, W. R., Michaels, K. A., Sutter, A. J., Diaconu, D., Fritz, Y., Xing, X., … Ward, N. L. (2017). Imiquimod has strain-dependent effects in mice and does not uniquely model human psoriasis. Genome Medicine , 9, 24. doi: 10.1186/s13073-017-0415-3.
- van der Fits, L., Mourits, S., Voerman, J. S. A., Kant, M., Boon, L., Laman, J. D., … Lubberts, E. (2009). Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. Journal of Immunology , 182, 5836–5845. doi: 10.4049/jimmunol.0802999.
- Walter, A., Schäfer, M., Cecconi, V., Matter, C., Urosevic-Maiwald, M., Belloni, B., … van den Broek, M. (2013). Aldara activates TLR7-independent immune defence. Nature Communications , 4, 1560. doi: 10.1038/ncomms2566.
- Yu, S., Wu, X., Shi, Z., Huynh, M., Jena, P. K., Sheng, L., … Hwang, S. T. (2020). Diet-induced obesity exacerbates imiquimod-mediated psoriasiform dermatitis in anti-PD-1 antibody-treated mice: Implications for patients being treated with checkpoint inhibitors for cancer. Journal of Dermatological Science , 97, 194–200. doi: 10.1016/j.jdermsci.2020.01.011.
- Yu, S., Wu, X., Zhou, Y., Sheng, L., Jena, P. K., Han, D., … Hwang, S. T. (2019). A Western diet, but not a high-fat and low-sugar diet, predisposes mice to enhanced susceptibility to imiquimod-induced psoriasiform dermatitis. Journal of Investigative Dermatology , 139, 1404–1407. doi: 10.1016/j.jid.2018.12.002.
Citing Literature
Number of times cited according to CrossRef: 10
- Laura Micheli, Marzia Vasarri, Donatella Degl’Innocenti, Lorenzo Di Cesare Mannelli, Carla Ghelardini, Antiga Emiliano, Alice Verdelli, Marzia Caproni, Emanuela Barletta, Posidonia oceanica (L.) Delile Is a Promising Marine Source Able to Alleviate Imiquimod-Induced Psoriatic Skin Inflammation, Marine Drugs, 10.3390/md22070300, 22 , 7, (300), (2024).
- Jiseo Song, Hyo Kyeong Kim, Hyunsoo Cho, Suh Jin Yoon, Jihae Lim, Kyunglim Lee, Eun Sook Hwang, TAZ deficiency exacerbates psoriatic pathogenesis by increasing the histamine-releasing factor, Cell & Bioscience, 10.1186/s13578-024-01246-0, 14 , 1, (2024).
- Weijie Hu, Jing Shen, Chenchen Zhou, Zongguang Tai, Quangang Zhu, Zhongjian Chen, Yahui Huang, Chunquan Sheng, Discovery of Janus Kinase and Histone Deacetylase Dual Inhibitors as a New Strategy to Treat Psoriasis, Journal of Medicinal Chemistry, 10.1021/acs.jmedchem.4c01681, (2024).
- Hala M. Gabr, Wael Abo El-Kheir, Phases of translational stem cell research, Stem Cell Therapy, 10.1016/B978-0-12-821569-2.00008-9, (111-119), (2023).
- Simon Straß, Johanna Geiger, Mariella Martorelli, Sophia Geiger, Natascha Cloos, Manuel Keppler, Tina Fischer, Laura Riexinger, Anna Schwamborn, Jamil Guezguez, Nadja Späth, Santiago Cruces, Jan-Hinrich Guse, Thaisa Lucas Sandri, Michael Burnet, Stefan Laufer, Isostearic acid is an active component of imiquimod formulations used to induce psoriaform disease models, Inflammopharmacology, 10.1007/s10787-023-01175-3, 31 , 2, (799-812), (2023).
- Dorottya Kocsis, Hichem Kichou, Katalin Döme, Zsófia Varga-Medveczky, Zsolt Révész, Istvan Antal, Franciska Erdő, Structural and Functional Analysis of Excised Skins and Human Reconstructed Epidermis with Confocal Raman Spectroscopy and in Microfluidic Diffusion Chambers, Pharmaceutics, 10.3390/pharmaceutics14081689, 14 , 8, (1689), (2022).
- Dorottya Kocsis, Szabina Horváth, Ágnes Kemény, Zsófia Varga-Medveczky, Csaba Pongor, Rózsa Molnár, Anna Mihály, Dániel Farkas, Bese Márton Naszlady, András Fülöp, András Horváth, Balázs Rózsa, Erika Pintér, Rolland Gyulai, Franciska Erdő, Drug Delivery through the Psoriatic Epidermal Barrier—A “Skin-On-A-Chip” Permeability Study and Ex Vivo Optical Imaging, International Journal of Molecular Sciences, 10.3390/ijms23084237, 23 , 8, (4237), (2022).
- Dorottya Kocsis, Victoria Klang, Eva‐Maria Schweiger, Zsófia Varga‐Medveczky, Anna Mihály, Csaba Pongor, Zsolt Révész, Zoltán Somogyi, Franciska Erdő, Characterization and ex vivo evaluation of excised skin samples as substitutes for human dermal barrier in pharmaceutical and dermatological studies, Skin Research and Technology, 10.1111/srt.13165, 28 , 5, (664-676), (2022).
- Hyunsoo Cho, Jeong Hwan Je, Jio Kang, Mi Gyeong Jeong, Jiseo Song, Yejin Jeon, Kyunglim Lee, Eun Sook Hwang, Dimeric translationally controlled tumor protein–binding peptide 2 attenuates imiquimod-induced psoriatic inflammation through induction of regulatory T cells, Biomedicine & Pharmacotherapy, 10.1016/j.biopha.2022.113245, 152 , (113245), (2022).
- Tamara Moreno-Sosa, María Belén Sánchez, Elisa Olivia Pietrobon, Juan Manuel Fernandez-Muñoz, Felipe Carlos Martín Zoppino, Flavia Judith Neira, María José Germanó, Diego Esteban Cargnelutti, Alicia Carolina Innocenti, Graciela Alma Jahn, Susana Ruth Valdez, Juan Pablo Mackern-Oberti, Desmoglein-4 Deficiency Exacerbates Psoriasiform Dermatitis in Rats While Psoriasis Patients Displayed a Decreased Gene Expression of DSG4, Frontiers in Immunology, 10.3389/fimmu.2021.625617, 12 , (2021).

