Yale Murine TMC - Frozen Tissue Sectioning Protocol for Spatial Transcriptomics (DBiTSeq) and Immunofluorescence
Rong Fan, Yun-Hee Youm, Yufeng Liu, Vishwa Deep Dixit, Bo Tao
Abstract
Protocol for frozen tissue sectioning for use in DBiTSeq and immunofluorescence staining
Steps
Tissue Embedding
Embed tissue according to this protocol: dx.doi.org/10.17504/protocols.io.kqdg3x8keg25/v1
Cryosectioning
Spray the workplace and tools (blades, tweezers, brushes, etc) with RNAseZap to decontaminate the environment. Spray 100% ethanol onto the cryostat to remove any residue of RNAseZap or reagent residue. Set the cryostat to -20°C. Prepare dry ice in a foam box. Place the empty clean slide box in the dry ice.
Remove tissue blocks from the -80°C freezer/dry ice and allow them to warm in cyrostat to sectioning temperature (-20°C) for 20 minutes. Locate the region of interest in the tissue block.
Trim the block if needed. Collect the defect tissue slides on a regular glass slide to test for RIN number. Usually, 3-4 sections of a 1 cm x 1 cm tissue on a glass slide is good for a RIN test.
Adjust cyrostat thickness to 7-10 μm. For spatial transcriptomes, 7-10 μm is the best thickness, but generally 8-13 μm works as well.
One section per slide needs to be put onto the center of the Ultra clean poly-L-lysine glass slide (see picture below). The glass slides are at room temperature. Label the bottom side of the slide for sample name with a marker. Make sure the top side of the slide is free of dust or any labeling. Use Dustoff gas to blow off the surface.
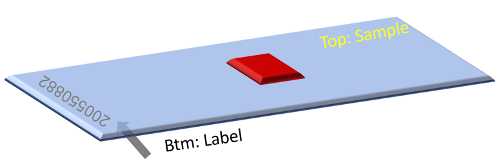
Once the tissue slice is picked up onto the glass slide, allow 30 seconds to 1 min at room temperature before transferring to a slide box in dry ice.
Save the slide box in a sealed plastic bag at -80° C before use. The bag helps prevent water condensation on the slides.
For slides used for H&E, thaw them at room temperature for 10 min, then dry them with compressed air for at least 5 min. Make sure no moisture is observed on the slide. Dip the slides in 4% paraformaldehyde at room temperature for 15 min (alternative: 30 min in pre-chilled methanol at -20° C). Wash with deionized water 3 times. Air blow dry the slides or leave them at room temperature overnight before doing H&E staining.

