Whole-mouse clearing and imaging at the cellular level with vDISCO
Fabian F. Voigt, Fritjof Helmchen, Jo A. Van Ginderachter, Hongcheng Mai, Shan Zhao, Ruiyao Cai, Ali Ertürk, Zeynep Ilgin Kolabas, Chenchen Pan, Doris Kaltenecker, Muge Molbay, Tzu-lun Ohn, Cécile Vincke, Mihail I. Todorov
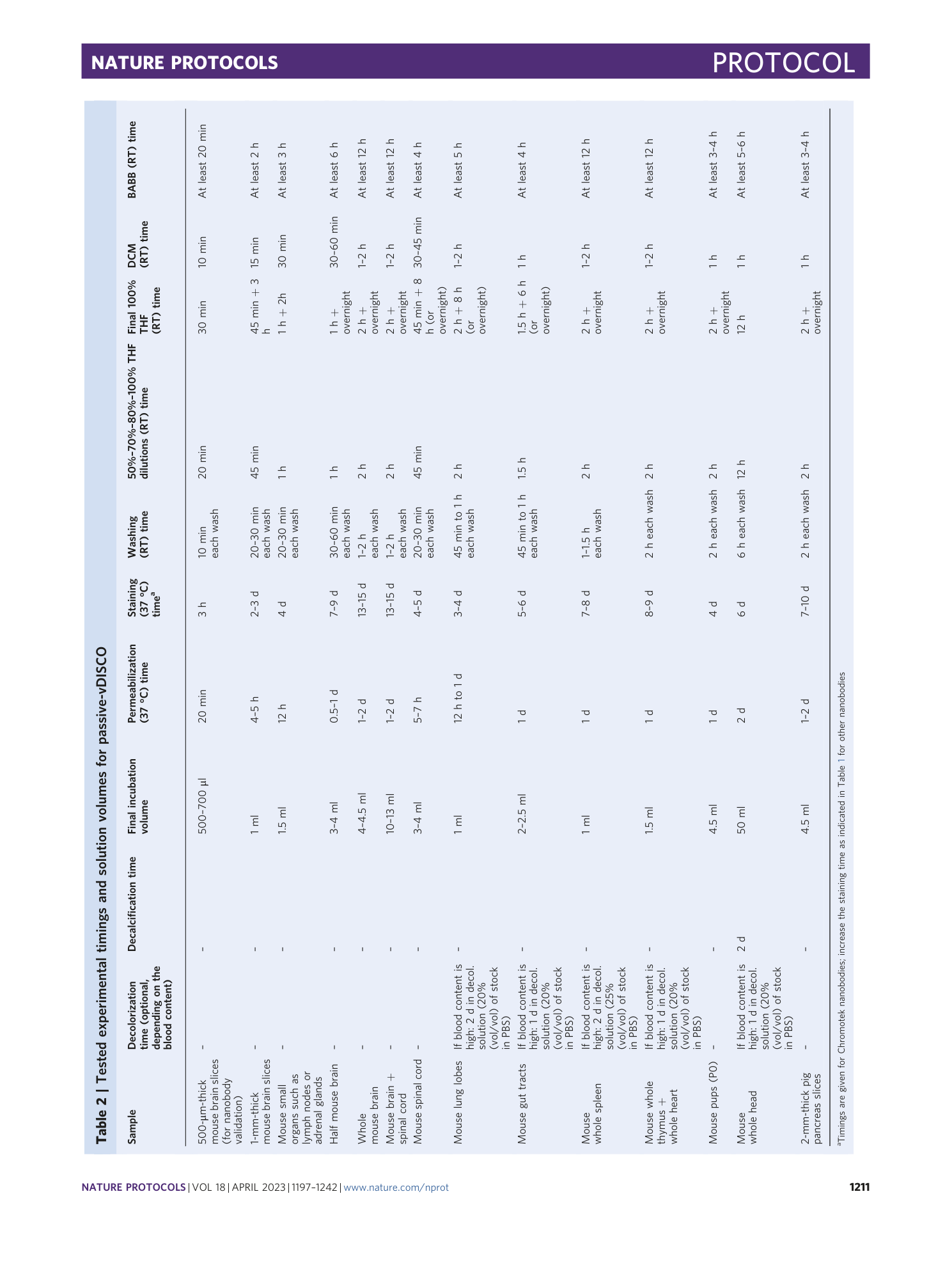
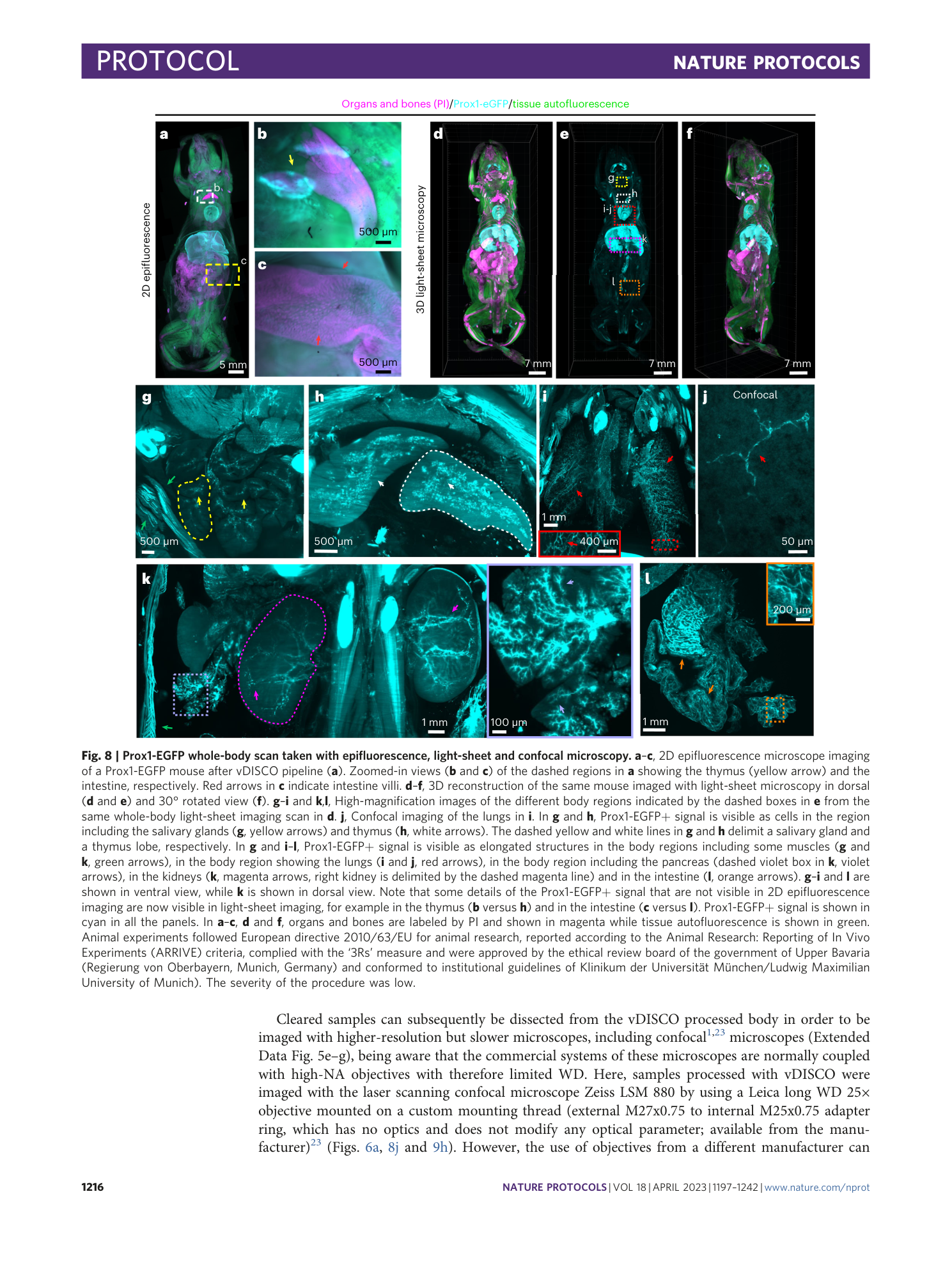
vDISCO
whole-mouse clearing
cellular imaging
nanobody-based immunolabeling
fluorescent protein stabilization

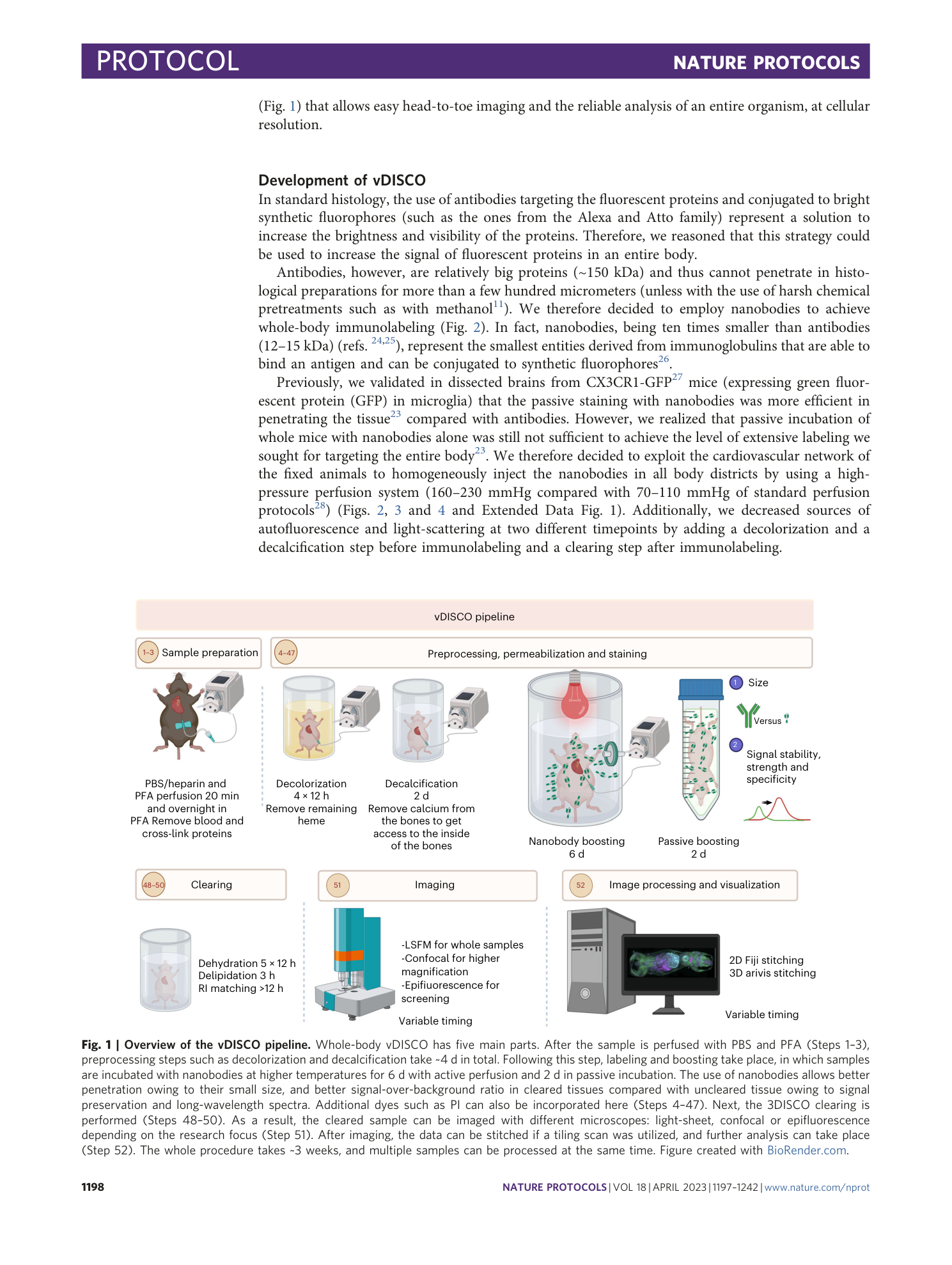
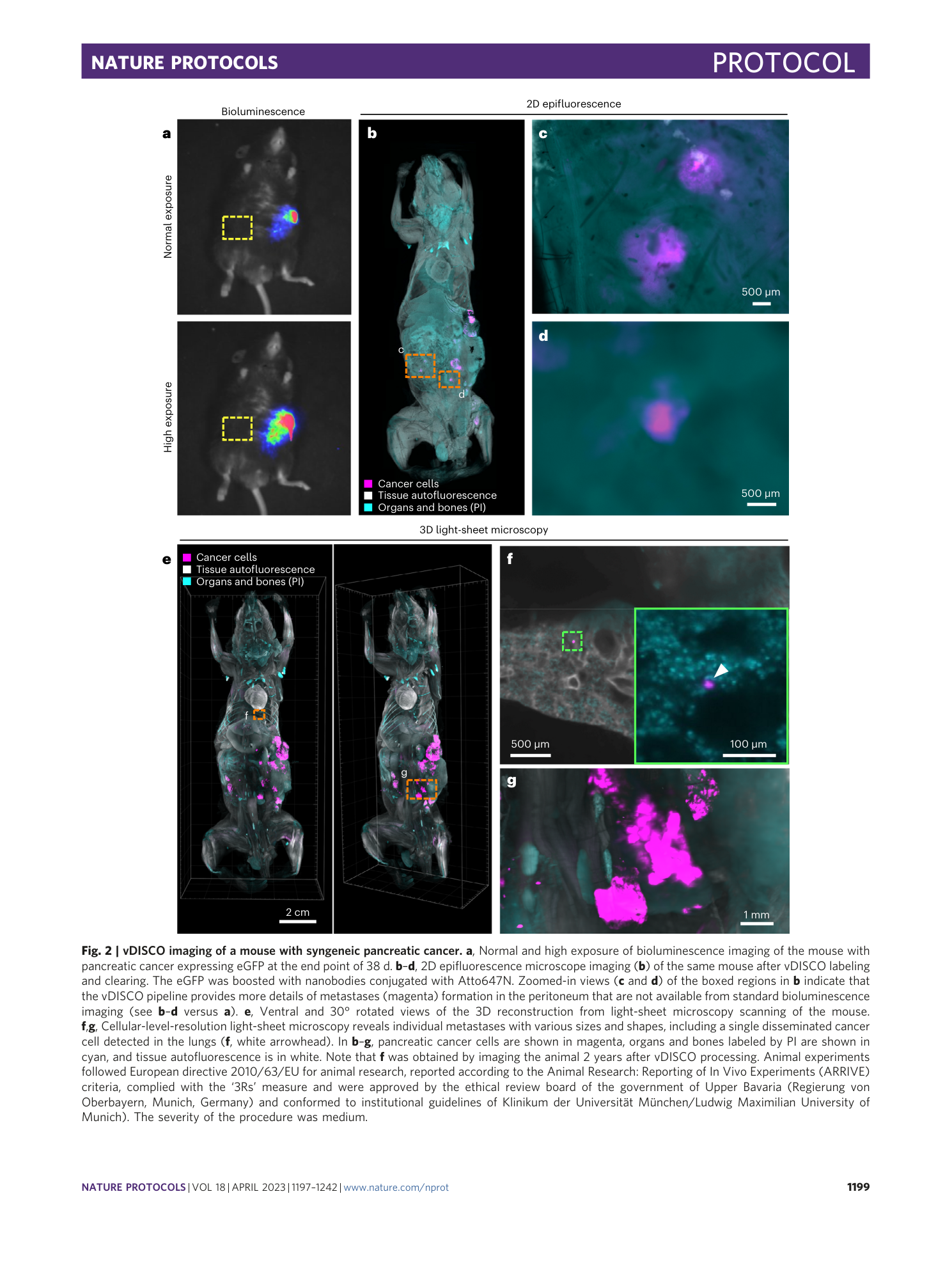
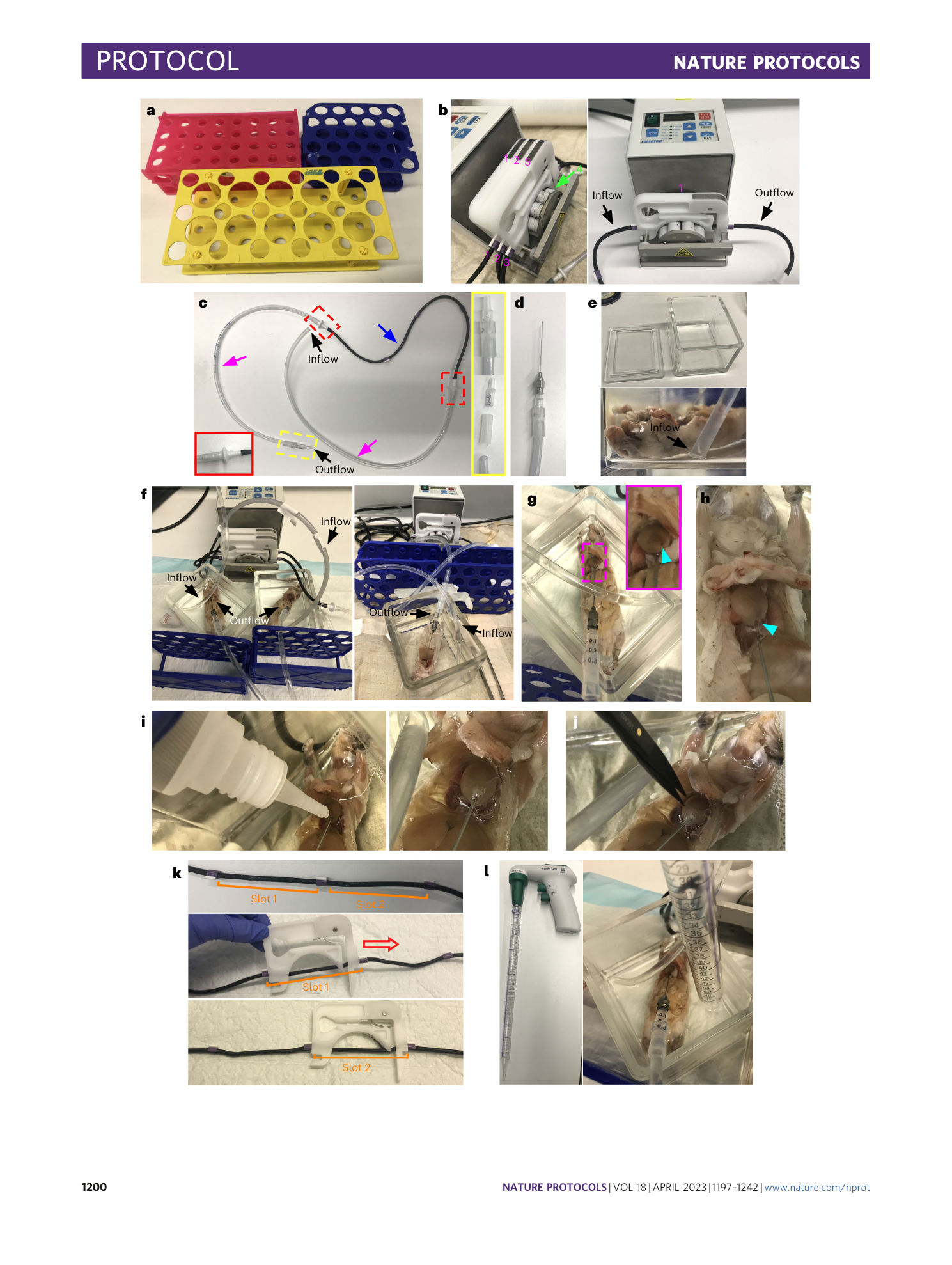


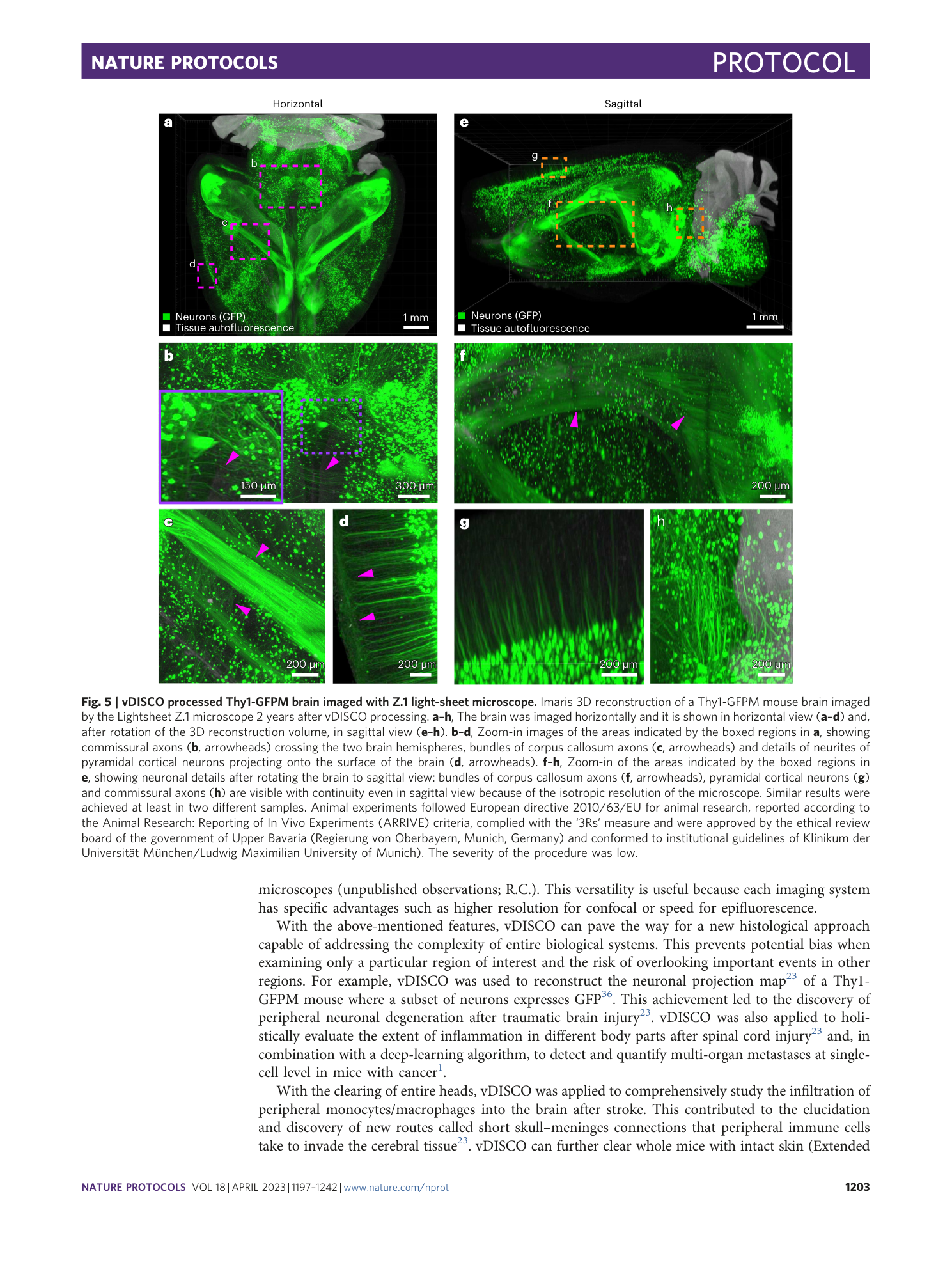


































Extended
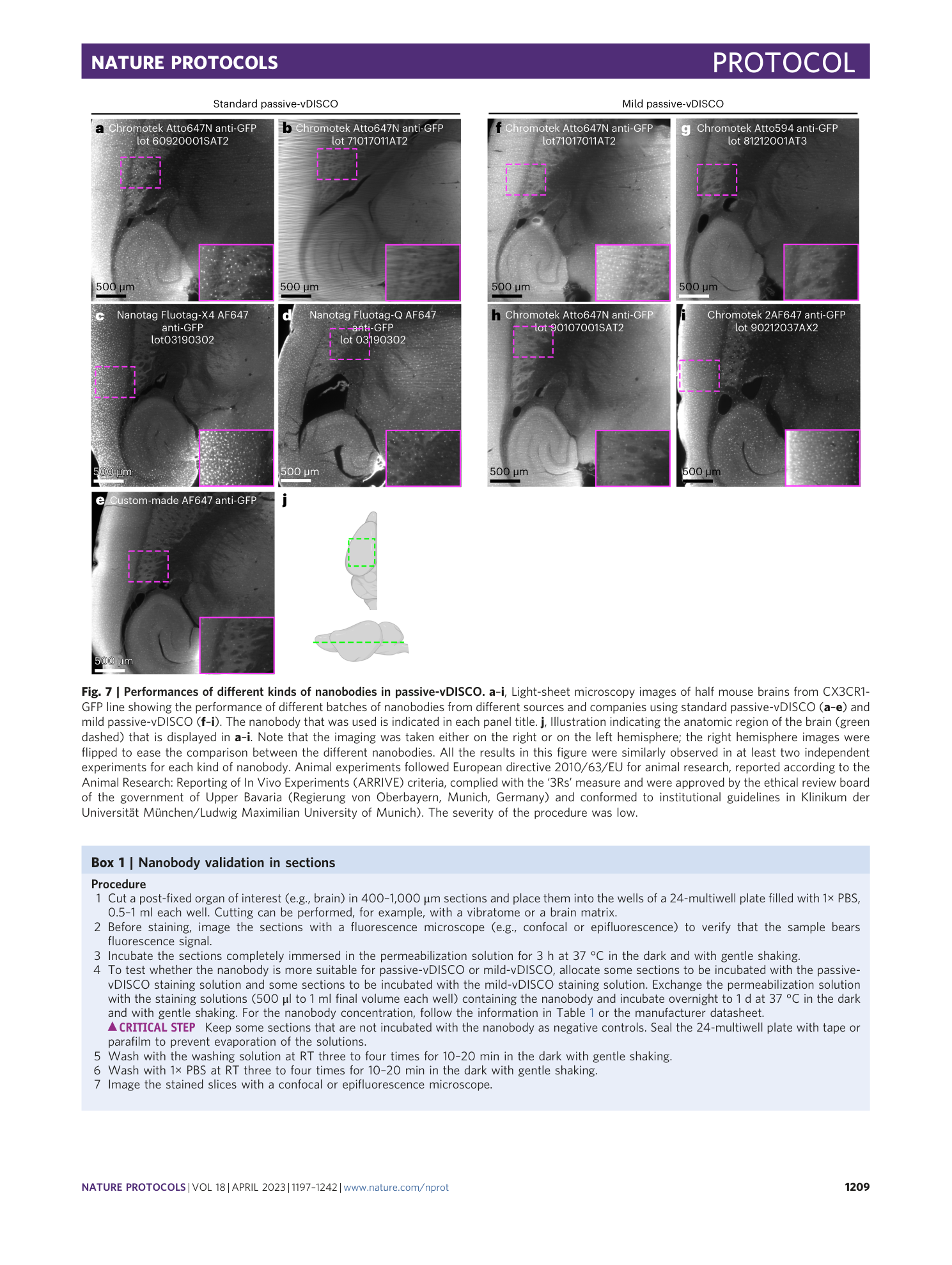
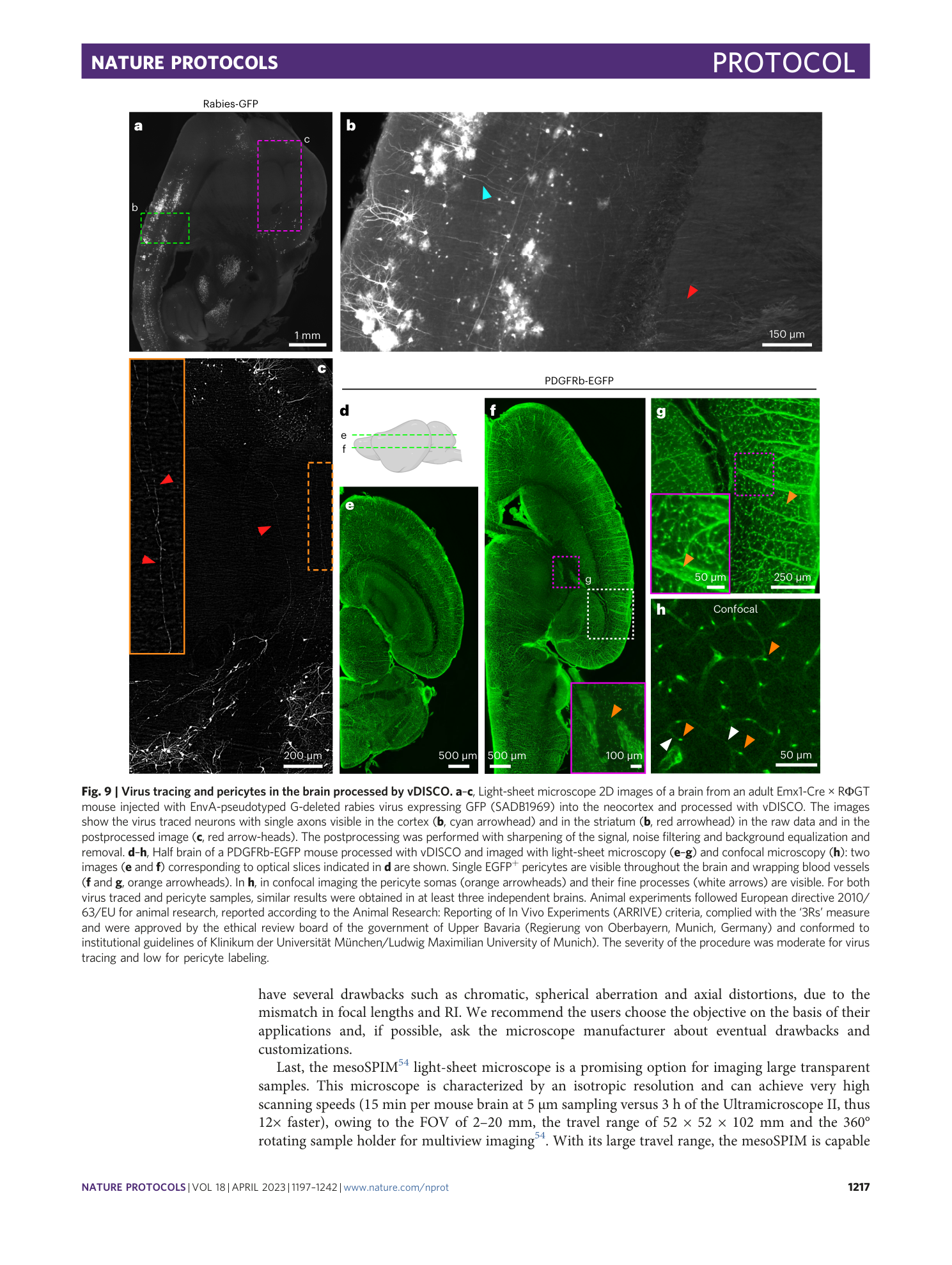
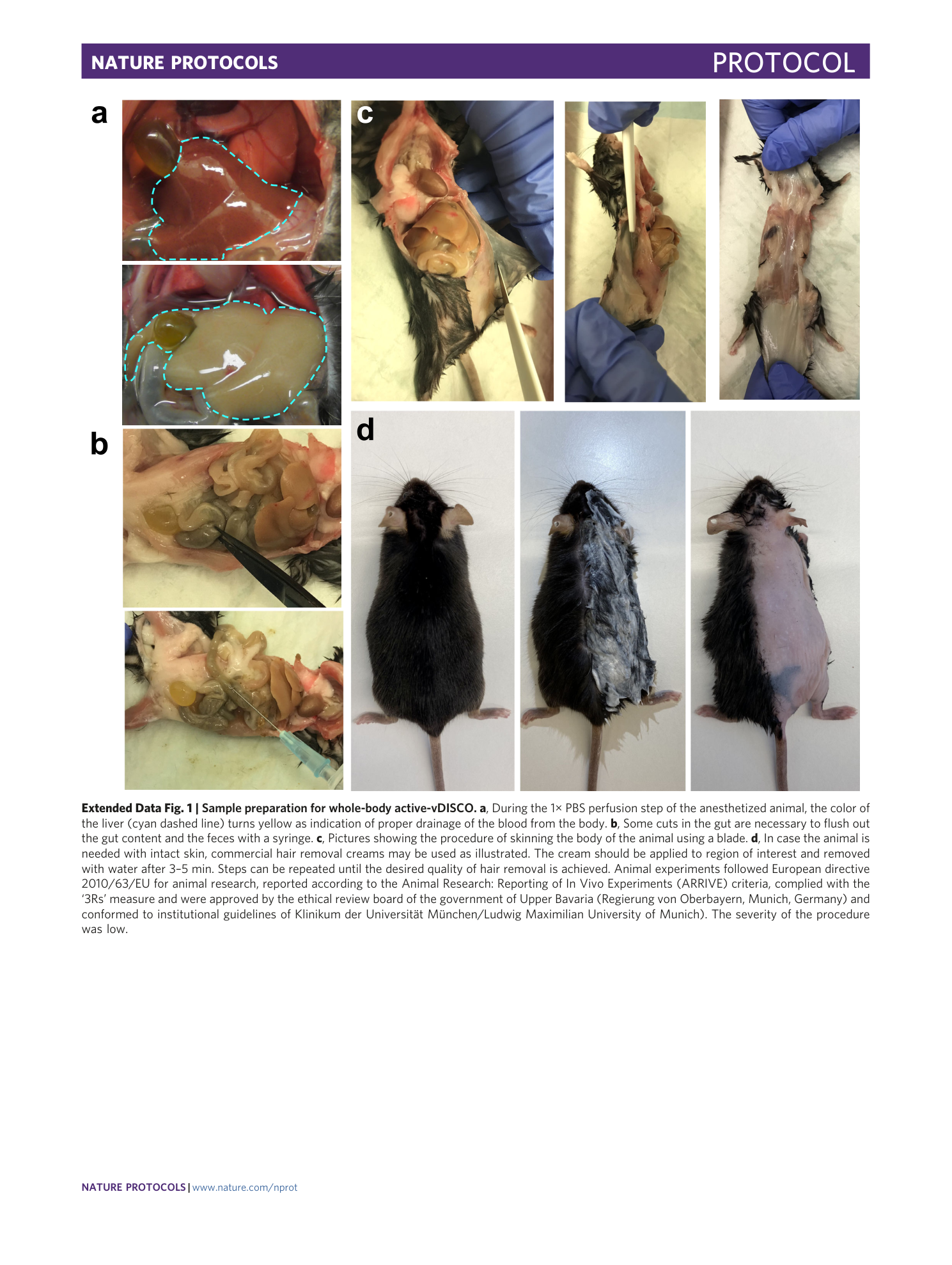
Extended Data Fig. 1 Sample preparation for whole-body active-vDISCO.
a , During the 1× PBS perfusion step of the anesthetized animal, the color of the liver (cyan dashed line) turns yellow as indication of proper drainage of the blood from the body. b , Some cuts in the gut are necessary to flush out the gut content and the feces with a syringe. c , Pictures showing the procedure of skinning the body of the animal using a blade. d , In case the animal is needed with intact skin, commercial hair removal creams may be used as illustrated. The cream should be applied to region of interest and removed with water after 3–5 min. Steps can be repeated until the desired quality of hair removal is achieved. Animal experiments followed European directive 2010/63/EU for animal research, reported according to the Animal Research: Reporting of In Vivo Experiments (ARRIVE) criteria, complied with the ‘3Rs’ measure and were approved by the ethical review board of the government of Upper Bavaria (Regierung von Oberbayern, Munich, Germany) and conformed to institutional guidelines of Klinikum der Universität München/Ludwig Maximilian University of Munich). The severity of the procedure was low.
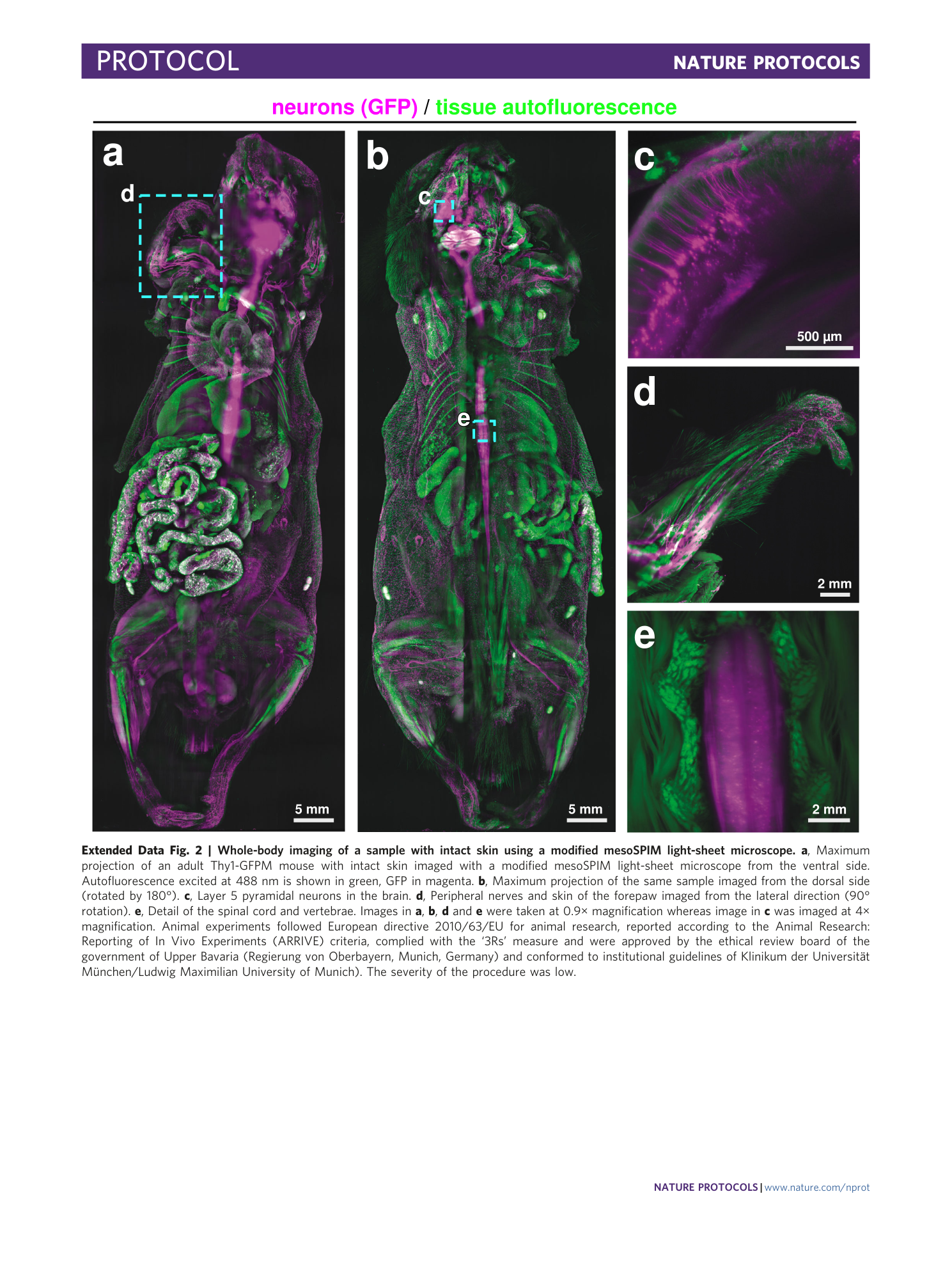
Extended Data Fig. 2 Whole-body imaging of a sample with intact skin using a modified mesoSPIM light-sheet microscope.
a , Maximum projection of an adult Thy1-GFPM mouse with intact skin imaged with a modified mesoSPIM light-sheet microscope from the ventral side. Autofluorescence excited at 488 nm is shown in green, GFP in magenta. b , Maximum projection of the same sample imaged from the dorsal side (rotated by 180°). c , Layer 5 pyramidal neurons in the brain. d , Peripheral nerves and skin of the forepaw imaged from the lateral direction (90° rotation). e , Detail of the spinal cord and vertebrae. Images in a , b , d and e were taken at 0.9× magnification whereas image in c was imaged at 4× magnification. Animal experiments followed European directive 2010/63/EU for animal research, reported according to the Animal Research: Reporting of In Vivo Experiments (ARRIVE) criteria, complied with the ‘3Rs’ measure and were approved by the ethical review board of the government of Upper Bavaria (Regierung von Oberbayern, Munich, Germany) and conformed to institutional guidelines of Klinikum der Universität München/Ludwig Maximilian University of Munich). The severity of the procedure was low.
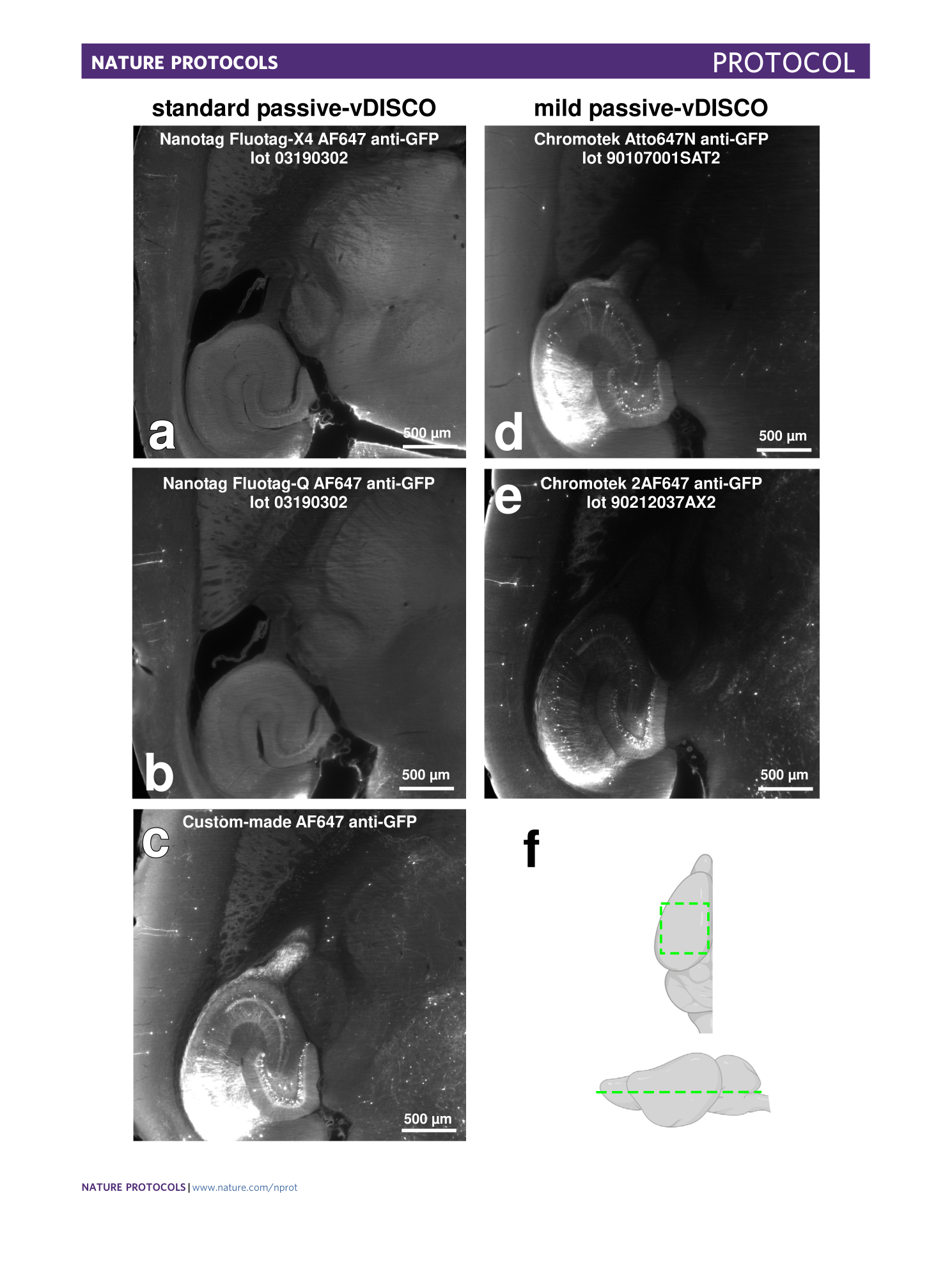
Extended Data Fig. 3 Performances of different kinds of nanobodies in passive-vDISCO for Thy1-GFPM line.
a – e , Light-sheet microscopy images of half mouse brains from Thy1-GFPM lines showing the performances of different batches of nanobodies from different sources and companies using standard passive-vDISCO ( a – c ) and mild passive-vDISCO ( d and e ). The used nanobody is indicated in the panel title. f , Illustration indicating the anatomic region of the brain (green dashed) that was displayed in a – e . Note that the imaging was taken either on the right or on the left hemisphere; right hemisphere images were flipped to ease the comparison between the different nanobodies. All the results in this figure were similarly observed in at least two independent experiments for each kind of nanobody. Animal experiments followed European directive 2010/63/EU for animal research, reported according to the Animal Research: Reporting of In Vivo Experiments (ARRIVE) criteria, complied with the ‘3Rs’ measure and were approved by the ethical review board of the government of Upper Bavaria (Regierung von Oberbayern, Munich, Germany) and conformed to institutional guidelines of Klinikum der Universität München/Ludwig Maximilian University of Munich). The severity of the procedure was low.
Extended Data Fig. 4 Strategy to make spinal cord straight for passive-vDISCO.
a , Required materials: a plastic Pasteur pipette and some fine needles. The cyan arrowheads indicate the cutting points. b , The plastic Pasteur pipette is then longitudinally cut in half. c , Positioning of the needles to constrain the brain with the spinal cord inside one of the halves of the pipette. d , The whole setting is put into a container such as a 50 ml tube for passive-vDISCO protocol. Animal experiments followed European directive 2010/63/EU for animal research, reported according to the Animal Research: Reporting of In Vivo Experiments (ARRIVE) criteria, complied with the ‘3Rs’ measure and were approved by the ethical review board of the government of Upper Bavaria (Regierung von Oberbayern, Munich, Germany) and conformed to institutional guidelines of Klinikum der Universität München/Ludwig Maximilian University of Munich). The severity of the procedure was low.
Extended Data Fig. 5 Mounting of cleared samples for epifluorescence imaging and inverted confocal imaging.
a – d , Mounting of different samples for AxioZoom epifluorescence imaging: different glass containers used to mount cleared organs (red dashed circle) and slices (magenta boxes) for AxioZoom epifluorescence imaging ( a ); epifluorescence imaging of dissected organs (red arrowhead) and slices (magenta arrowhead) with the AxioZoom microscope ( b and c ); epifluorescence imaging of the whole body with the AxioZoom microscope ( d ). e – g , Mounting of different samples for inverted confocal microscope imaging: a slice ( e ) and a whole brain ( f ) are placed onto a glass-bottom dish, then the dish with the lid is positioned onto the stage of the microscope ( g ). Animal experiments followed European directive 2010/63/EU for animal research, reported according to the Animal Research: Reporting of In Vivo Experiments (ARRIVE) criteria, complied with the ‘3Rs’ measure and were approved by the ethical review board of the government of Upper Bavaria (Regierung von Oberbayern, Munich, Germany) and conformed to institutional guidelines of Klinikum der Universität München/Ludwig Maximilian University of Munich). The severity of the procedure was low.
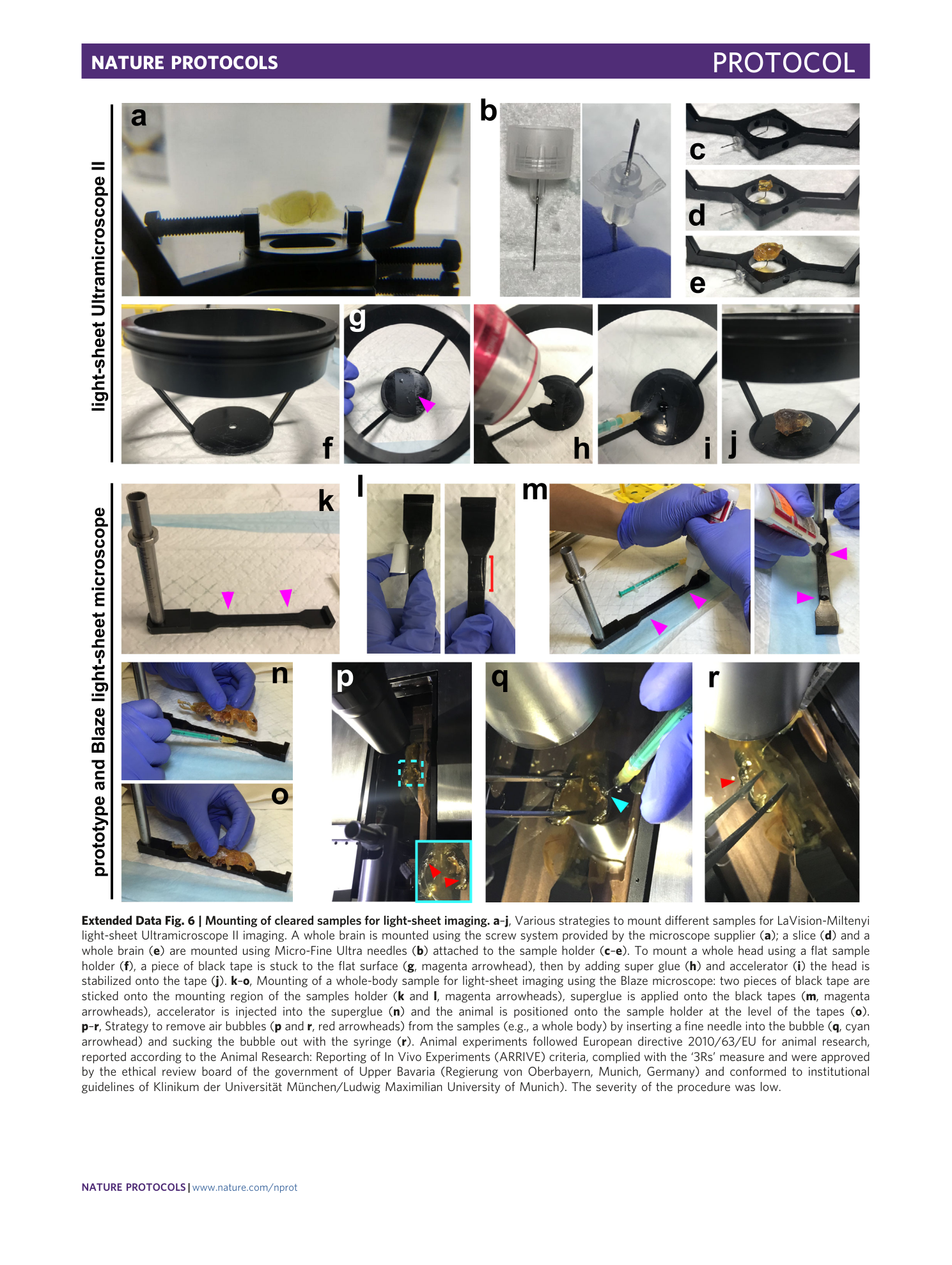
Extended Data Fig. 6 Mounting of cleared samples for light-sheet imaging.
a – j , Various strategies to mount different samples for LaVision-Miltenyi light-sheet Ultramicroscope II imaging. A whole brain is mounted using the screw system provided by the microscope supplier ( a ); a slice ( d ) and a whole brain ( e ) are mounted using Micro-Fine Ultra needles ( b ) attached to the sample holder ( c – e ). To mount a whole head using a flat sample holder ( f ), a piece of black tape is stuck to the flat surface ( g , magenta arrowhead), then by adding super glue ( h ) and accelerator ( i ) the head is stabilized onto the tape ( j ). k – o , Mounting of a whole-body sample for light-sheet imaging using the Blaze microscope: two pieces of black tape are sticked onto the mounting region of the samples holder ( k and l , magenta arrowheads), superglue is applied onto the black tapes ( m , magenta arrowheads), accelerator is injected into the superglue ( n ) and the animal is positioned onto the sample holder at the level of the tapes ( o ). p – r , Strategy to remove air bubbles ( p and r , red arrowheads) from the samples (e.g., a whole body) by inserting a fine needle into the bubble ( q , cyan arrowhead) and sucking the bubble out with the syringe ( r ). Animal experiments followed European directive 2010/63/EU for animal research, reported according to the Animal Research: Reporting of In Vivo Experiments (ARRIVE) criteria, complied with the ‘3Rs’ measure and were approved by the ethical review board of the government of Upper Bavaria (Regierung von Oberbayern, Munich, Germany) and conformed to institutional guidelines of Klinikum der Universität München/Ludwig Maximilian University of Munich). The severity of the procedure was low.
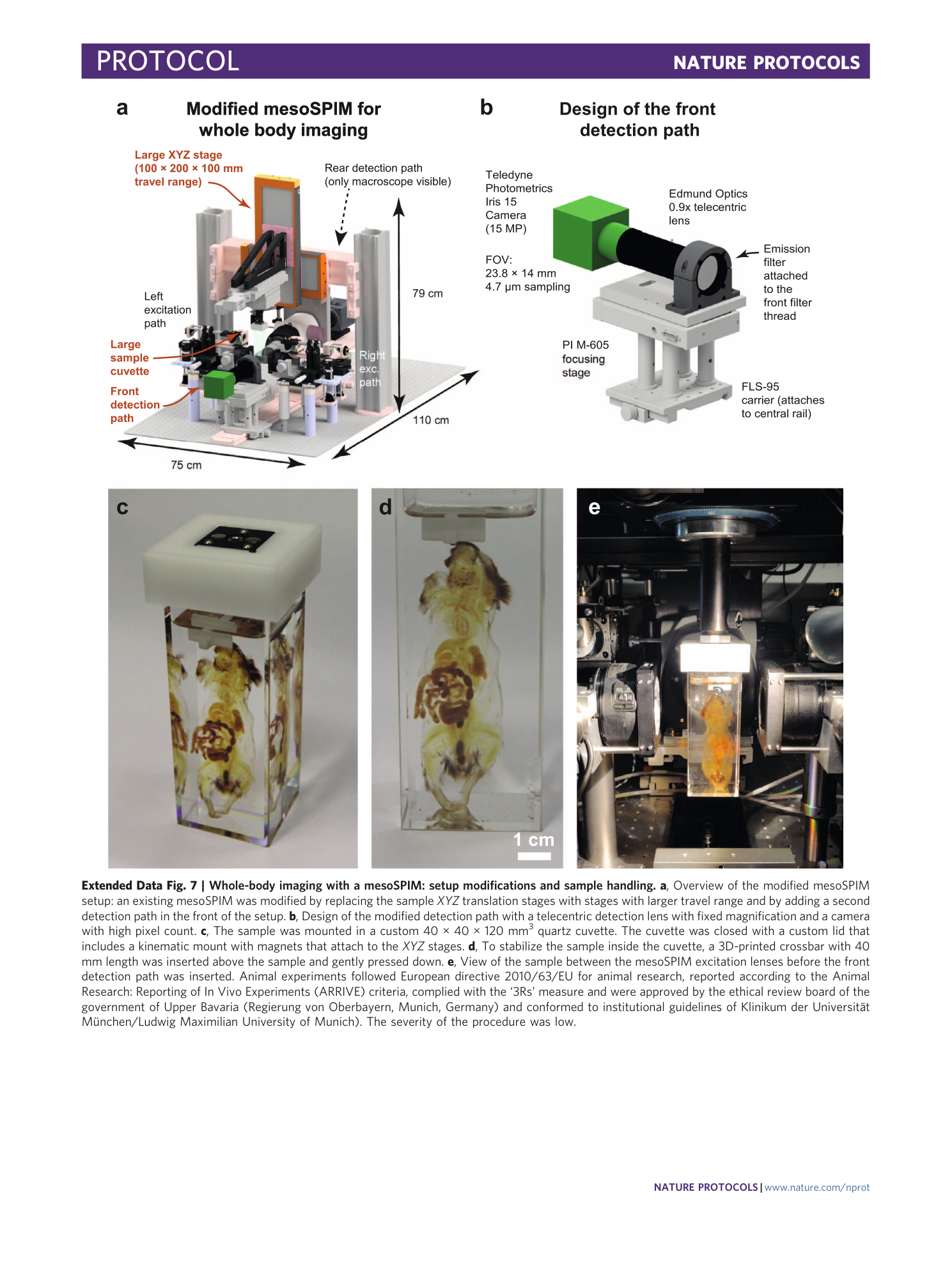
Extended Data Fig. 7 Whole-body imaging with a mesoSPIM: setup modifications and sample handling.
a , Overview of the modified mesoSPIM setup: an existing mesoSPIM was modified by replacing the sample XYZ translation stages with stages with larger travel range and by adding a second detection path in the front of the setup. b , Design of the modified detection path with a telecentric detection lens with fixed magnification and a camera with high pixel count. c , The sample was mounted in a custom 40 × 40 × 120 mm 3 quartz cuvette. The cuvette was closed with a custom lid that includes a kinematic mount with magnets that attach to the XYZ stages. d , To stabilize the sample inside the cuvette, a 3D-printed crossbar with 40 mm length was inserted above the sample and gently pressed down. e , View of the sample between the mesoSPIM excitation lenses before the front detection path was inserted. Animal experiments followed European directive 2010/63/EU for animal research, reported according to the Animal Research: Reporting of In Vivo Experiments (ARRIVE) criteria, complied with the ‘3Rs’ measure and were approved by the ethical review board of the government of Upper Bavaria (Regierung von Oberbayern, Munich, Germany) and conformed to institutional guidelines of Klinikum der Universität München/Ludwig Maximilian University of Munich). The severity of the procedure was low.
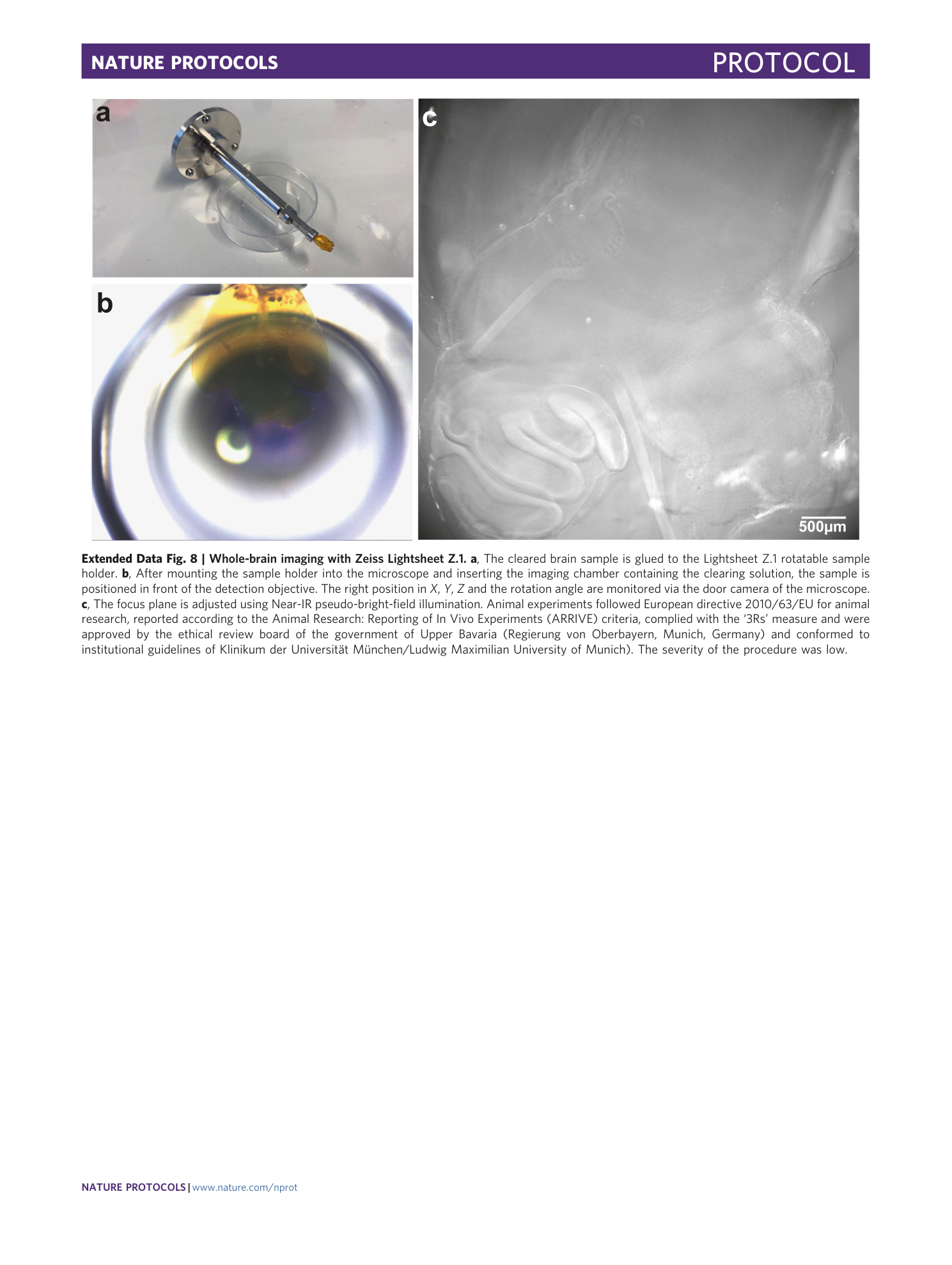
Extended Data Fig. 8 Whole-brain imaging with Zeiss Lightsheet Z.1.
a , The cleared brain sample is glued to the Lightsheet Z.1 rotatable sample holder. b , After mounting the sample holder into the microscope and inserting the imaging chamber containing the clearing solution, the sample is positioned in front of the detection objective. The right position in X , Y , Z and the rotation angle are monitored via the door camera of the microscope. c , The focus plane is adjusted using Near-IR pseudo-bright-field illumination. Animal experiments followed European directive 2010/63/EU for animal research, reported according to the Animal Research: Reporting of In Vivo Experiments (ARRIVE) criteria, complied with the ‘3Rs’ measure and were approved by the ethical review board of the government of Upper Bavaria (Regierung von Oberbayern, Munich, Germany) and conformed to institutional guidelines of Klinikum der Universität München/Ludwig Maximilian University of Munich). The severity of the procedure was low.
Supplementary information
Supplementary Information
Supplementary Figs. 1–3.
Reporting Summary
Supplementary Video 1
Stomach incision and cleaning as a sample preparation step in vDISCO whole-body clearing protocol
Supplementary Video 2
Gut incision and cleaning as a sample preparation step in vDISCO whole-body clearing protocol
Supplementary Video 3
vDISCO clearing protocol pump set-up and demonstration of how to refresh the pumping reference tube slot
Supplementary Video 4
The vDISCO processed Prox1-eGFP mouse with details of lymphatic vessels in the whole body, neurons in the hippocampus and satellite cells in the muscle tissue
Supplementary Video 5
The vDISCO processed brain from an adult Emx1-Cre x ROGT injected with a EnvA-pseudotyped G-deleted rabies virus expressing GFP (SADB1969) that is visible in the neocortex and in the striatum

