The Affective Bias Test and Reward Learning Assay: Neuropsychological Models for Depression Research and Investigating Antidepressant Treatments in Rodents
Justyna K. Hinchcliffe, Justyna K. Hinchcliffe, Emma S. J. Robinson, Emma S. J. Robinson
Abstract
The Affective Bias Test (ABT) quantifies acute changes in affective state based on the affective biases they generate in an associative reward learning task. The Reward Learning Assay (RLA) provides a control assay for the ABT and reward-induced biases generated in this model are sensitive to changes in core affective state. Both tasks involve training animals to associate a specific digging substrate with a food reward. Animals learn to discriminate between two digging substrates placed in ceramic bowls, one rewarded and one unrewarded. In the ABT, the animal learns two independent substrate-reward associations with a fixed reward value following either an affective state or drug manipulation, or under control conditions. Affective biases generated are quantified in a choice test where the animals exhibit a bias (make more choices) for one of the substrates which is specifically related to affective state at the time of learning. The ABT is used to investigate biases generated during learning as well as modulation of biases associated with past experiences. The RLA follows a similar protocol, but the animal remains in the same affective state throughout and a reward-induced bias is generated by pairing one substrate with a higher value reward. The RLA provides a control to determine if drug treatments affect memory retrieval more generally. Studies in depression models and following environmental enrichment suggest that reward-induced biases are sensitive to core changes in affective state. Each task offers different insights into affective processing mechanisms and may help improve the translational validity of animal studies and benefit pre-clinical drug development. © 2024 The Authors. Current Protocols published by Wiley Periodicals LLC.
Basic Protocol 1 : Bowl digging and discrimination training
Basic Protocol 2 : The reward learning assay
Basic Protocol 3 : The affective bias test - new learning
Basic Protocol 4 : The affective bias test - modulation of affective biases associated with past experiences
INTRODUCTION
Modeling clinically relevant symptoms of major depression disorder (MDD) in animals is key to understanding the relationships between the biological and experience-dependent factors that drive the behavioral symptoms of MDD and its treatment. A core feature of MDD is the prevalence of cognitive processing biases associated with negative affective states, termed negative affective biases, which may be a key factor underpinning low mood and negative rumination. In this neuropsychological model of depression, negative affective biases play a causal role in vulnerability, precipitation, and maintenance of MDD (Godlewska & Harmer, 2021). Human studies suggest healthy controls have tendencies to overestimate the likelihood of future positive events and underestimate the negative ones (Bower & Mann, 1992; Korn et al., 2014; Nygren et al., 1996), whereas people in low, negative mood manifest the opposite (Rude et al., 2002; Sharot, 2011; Sharot et al., 2011). Moreover, depressed patients exhibit reward-related learning impairments where they attribute less value to rewarding experiences compared to healthy people (Geugies et al., 2019; Gonda et al., 2015; Lawson et al., 2017).
Conventional animal models and behavioral tests for MDD research have largely focused on chronic stress manipulations associated with readouts of behavioral despair, such as immobility time in the forced swim test (FST) and reward sensitivity changes in the sucrose preference test (SPT). While these methods have established some validity in terms of stress-related behaviors, it is not clear how well they translate to human mood disorders. The FST was also originally developed as a pharmacological screening tool rather than a test sensitive to changes in phenotypic models, despite it now being quite widely used for this objective. Although stress is a known risk for MDD, models associated with other risk factors, such as early life adversity and chronic inflammation, do not consistently result in impairments in these readouts. Furthermore, significant sex differences have been observed in most of the stress models of depression: learned helplessness, chronic mild stress, and chronic psychosocial stress, or lipopolysaccharide-induced depression model and in behavioral tests that quantify depressive-like behaviors, such as the FST or SPT (Dalla et al., 2005, 2010; Kokras & Dalla, 2014; Kokras et al., 2015; Palanza, 2001). Where sex differences have been presented, the manifestation of symptoms or depressive-like behaviors in females is in the opposite direction to what is observed in human clinical diagnoses (Lopez & Bagot, 2021; Ma et al., 2019).
Work over the last 20 years has sought to develop more translational rodent models designed to recapitulate aspects of human neuropsychological deficits that can be objectively measured in different species (Mendl et al., 2009). The first study using the ABT was published in 2013 (Stuart et al., 2013). The underlying concept of ABT was translated from clinical observations in depressed patients who exhibit negative affective biases in relation to reward-related learning and memory. These observations suggest that the affective state during a rewarding experience may bias how the memory of the experience is encoded and subsequently retrieved, which the ABT aims to quantify in non-human animals (Robinson, 2018, Fig. 1). The ABT recapitulates in animals a clinically relevant symptom of MDD and has been shown to achieve constructive, predictive, mechanistic, homological, and translational validity (Hinchcliffe et al., 2017, 2020; Hinchcliffe, Jackson et al., 2022; Stuart et al., 2013, 2015, 2017; Fig. 2). The bowl-digging task is based on associative learning and memory between a specific digging substrate and a food reward. During the learning phase, animals undergo two substrate-reward pairing sessions for each condition: one memory is experienced during an affective state manipulation or test treatment, and the other memory is experienced under control conditions. The value of each experience is kept equal, and the only factor that drives the bias is the affective state change induced at the time of learning. The affective biases generated are quantified using a choice test which takes place at least 24 hr after the last pairing session. During the choice test, the animal is presented with the two previously reinforced digging substrates at the same time, and biases are observed as an increase or decrease in the relative value of the treatment-paired experience (Fig. 1).
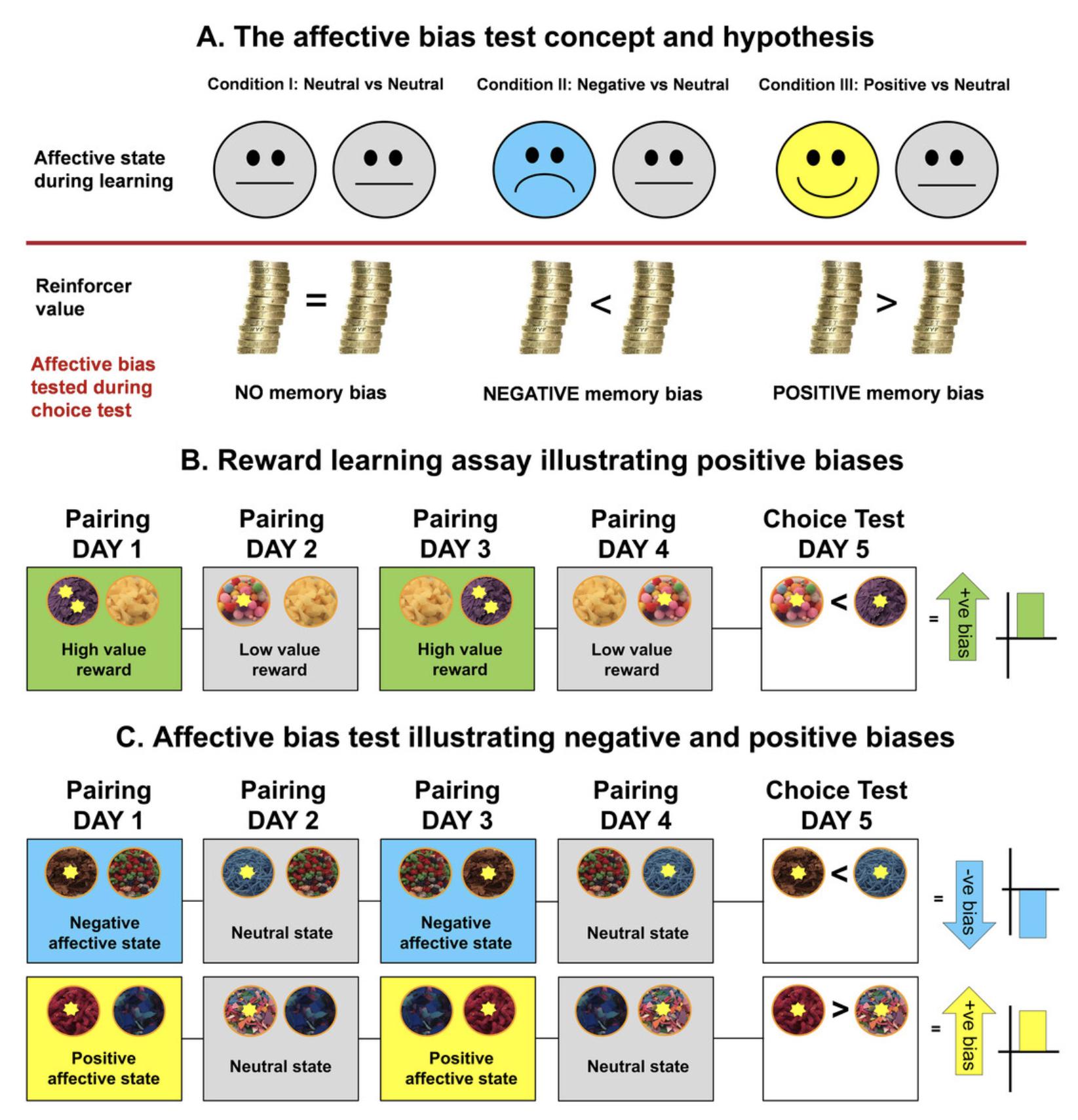
As part of the validation of the original ABT, we developed a modified version of the task in which animals remain in the same affective state throughout the protocol, and a reward-induced bias is generated by pairing one experience with a higher value reward. The RLA is used as part of the training for all cohorts to ensure they have successfully learned the rules of the task before proceeding to the test. The method is also useful as a control for studies that are testing the acute effects of treatment of the retrieval of biased memories in the ABT. The RLA can be used as a control measure to establish the specificity of treatments to affective state-induced biases or if the treatment causes more general effects on memory. Observations during preparation for ABT studies in a depression model suggested RLA was sensitive to the core affective state resulting in a reward learning deficit. Further exploration in different rodent depression models, including early life adversity, chronic corticosterone, chronic interferon-alpha, chronic pro-depressant drug, and chronic neuropathic pain, have found similar reward learning impairments (Phelps et al., 2021; Robinson, 2018; Stuart et al., 2019). Alongside our MDD-related research, we have also used the ABT and RLA to explore animal welfare-related questions using these readouts as an objective measure of affective state to assess refined housing and handling methods (Hinchcliffe et al., 2020; Hinchcliffe, Jackson et al., 2022).
In this article, we describe the methods used to train animals in preparation for the ABT and RLA. Basic Protocol 1 describes the training and basic discrimination learning protocol. Basic Protocol 2 describes the RLA protocol. Basic Protocol 3 describes the ABT protocol and Basic Protocol 4 describes variations that can be used to investigate modulation of biased memories.
STRATEGIC PLANNING
Animals and equipment require preparation before a study can begin.
Animals
- 1.Studies involving living animals require ethical approval from the relevant National/Institutional Animal Ethics Committee and must follow local regulations for the care and use of laboratory animals.
- 2.After animals have been delivered to the facility, provide 1 week for acclimatization to the new environment and recovery from transportation before starting the behavioral experiments. Transportation stress can lead to significant alterations in the immune, endocrine, cardiovascular, nervous, and reproductive systems, as well as behavior (Obernier & Baldwin, 2006; Tuli et al., 1995). During this acclimatization period, the animals can begin habituation to handling and rewards. If the study plan involves changing the light:dark cycle, time is needed for the animals' circadian rhythms to adapt. The adaptation period should be no less than 7 days for a 12 hr shift in cycle.
- 3.House rats in suitable cages with adequate ventilation, e.g., metal mesh lid and at least 18 cm height to allow rearing and at least the minimum floor space area for age and weight (<600 g = 800 cm2 and ∼1500 cm2 for animals over 600 g). Rats also benefit from access to playpens, which can help maintain a more positive affective state and provide more normal control subjects (Hinchcliffe, Jackson et al., 2022).
- 4.The number of rats per cage should avoid overcrowding stress (no more than 4 rats per cage), which can lead to an increase of corticosteroids and anxiety-related behaviors (Gavrilov et al., 2022), but avoid individual housing whenever possible, as rats benefit from social contact (Harper et al., 2002).
- 5.Animal holding rooms should be maintained under controlled temperature (21.5 ± 1°C) and humidity (55 ± 10%) conditions. The light:dark cycle should be programmed for 12 hr:12 hr with lights off in the morning (8:00 am), to enable testing of the animals during their active phase.
- 6.Clean the home cages once a week to ensure a clean environment, but transfer enrichment to maintain the olfactory cues within the cage. Cage changing should be conducted after the animals have finished behavioral procedures for the day.
- 7.Provide food and water ad libitum (unless the specific experimental design requires animals to be kept on a food-restricted regimen).
- 8.Reserve an appropriate amount of time (at least 1 week) to handle the animals prior to training and testing to reduce their handling stress and aversion to the experimenter. Animals should be calm and easy to handle, without overt signs of distress (e.g., audible vocalizations, signs of struggle when picked up, fecal pellets). For our refined handling protocols, see the 3Hs Initiative: Housing, Handling, and Habituation (www.3hs-initiative.co.uk). Rats are unlikely to perform the ABT correctly if they remain stressed by human contact even if food restriction motivates them to forage for rewards.
- 9.Plan your study design carefully in advance, including counterbalancing of all the factors (e.g., substrate, bowl position, treatments). Tables 2, 3, and 4, presented in Basic Protocols 2, 3 and 4, detail a counterbalanced design for the ABT and RLA. Whenever possible, the experimenter should be blind to treatment to avoid unintentional biases influencing the animal's behavior.
Daily Preparation of Experimental Set Up
- 10.Perform behavioral procedures and testing during the animals' active phase between 08:00 hr and 20:00 hr.
- 11.Print your pairing sheets and have them ready to record each animal's choices and latencies to dig (Tables S1 and S2).
- 12.Use an arena with opaque sides to reduce external distraction (or cover a transparent arena with an external liner). Do not use an arena with high sides that cause an approaching handler to appear to the rat from above, as this can induce a stress response.
- 13.Put a clean paper liner inside the arena and keep it for 5 days of training or testing, only removing fecal pellets. Remove the liner only if it gets very dirty. It is best to avoid sawdust or other substrates that can distract the animal from the bowls.
- 14.Use two identical ceramic bowls. Place both bowls filled with appropriate substrates inside the arena and against the back wall, about 5 cm (1 rat width) apart (Fig. 3A).
- 15.Always choose a trio of digging substrates (reward-paired substrates - ‘A’ or ‘B’ versus unrewarded substrate - ‘C’) matched for digging effort and with rewarded substrates counterbalanced across experimental subjects (see Table 1).

| Substrate ‘A’ | Substrate ‘B’ | Substrate ‘C’ | |
|---|---|---|---|
| Test 1 | Felt | Shredded dishcloth blue | Exfoliating gloves |
| Test 2 | Absorbent fiber | String | Foam shapes |
| Test 3 | Dusters | Tissue paper balls | Yellow bath sponge |
| Test 4 | Black satin | Cardboard | Rope |
| Test 5 | Fur | Polyester | Pompoms |
| Test 6 | Cellulose sponge | Corrugated paper | Perlite |
| Test 7 | Purple ribbon | Green raffia ribbon | Sparkly pompoms |
| Test 8 | Brown pet bedding | Cork | Hessian sack |
| Test 9 | Cotton wool balls | Stringy cloth | Hairbands |
| Test 10 | Organza | Silk | Shredded paper |
| Test 11 | Bin liner | Plastic scourer | Straws |
| Test 12 | Cotton mix | Leather | Balloons |
| Test 13 | Chubby wool | Shoelaces | Velcro |
| Test 14 | Brown partition paper | Dishcloth squares | Polyester lining |
| Test 15 | Aspen | Cypress | Colored matchsticks |
| Test 16 | Christmas ribbon | Umbrella | Tights |
| Test 17 | Towel | Canvas | Pipe cleaners |
| Test 18 | Newspaper | Paper pet bedding | Confetti |
| Test 19 | Suede | Chenille strands | Yellow fleece |
| Test 20 | Poster squares | Polystyrene | Sequins |
| Test 21 | Crepe paper squares | Scarf yarn | Sparkling fiber |
| Test 22 | Denim | Rucksack strap | Foam pad |
| Test 23 | Maple tree leaves | Moss | Black lining |
| Test 24 | Silver birch leaves | Silver birch bark | Hemp bedding |
| Test 25 | Swimming suit | Straw hat | Suede fabric |
The digging substrate provides a multidimensional cue, i.e., texture and olfactory cues, which the animals learn to associate with a food reward. Before starting an experiment, prepare the digging substrates (see Table 1 ), such as by cutting them to a size small enough to provide good coverage of the food reward when placed in the ceramic bowls.
- 16.Use a lid to cover at least half of the arena (whole arena for 1st habituation) to prevent the animals from jumping onto the rim.
- 17.For each trial, place the rat near the bowls in the middle of the arena to help reduce any side bias (Fig. 3A).
- 18.Do not remove the non-selected bowl until the animal has shown a committed choice (when the animal has obviously and actively started to dig or search within one of the substrates), then remove the bowl in a single action. It is better to be too slow than too fast.
- 19.Randomly place pellets in locations equivalent to clock positions 10, 12, and 2.Remember to bait the bowl, position it in the arena, and then press down the substrates in both bowls from left to right to ensure no differences in handler-related olfactory cues.
- 20.Between trials, either place the rat in a holding box (Fig. 3C) while you reset the bowls or gently hold the animal on your chest (Fig. 3B).
Pharmacological Studies
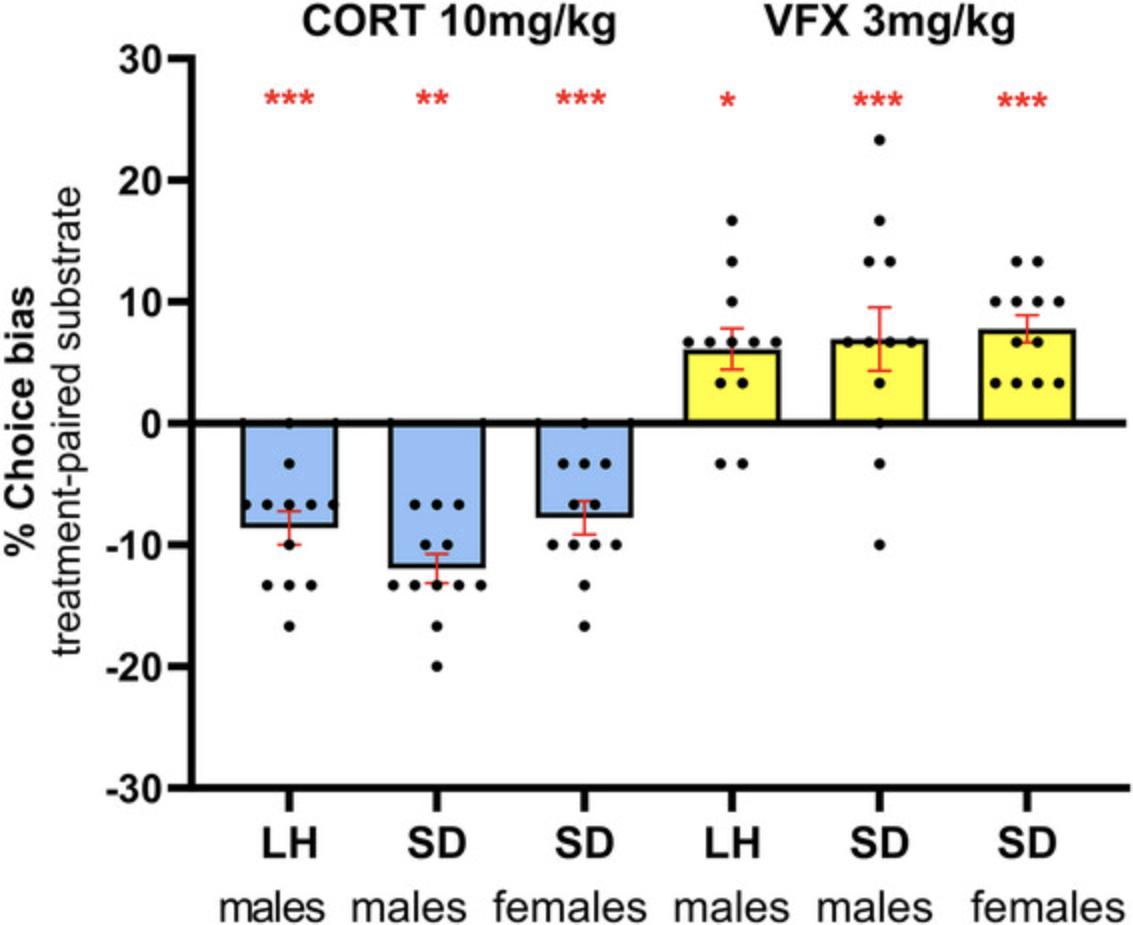
Carefully consider the dose range when using pharmacological treatments in the ABT or RLA, as higher doses can lead to non-specific effects. Ideally, pharmacokinetic data should be used to inform dose selection based on predicted receptor occupancy, but where this is not available, estimations based on clinical data and scaling for animal weight can be useful (Nair & Jacob, 2016). It should be noted that previous studies with antidepressant drugs suggest effective doses are lower than those used in conventional models of depression and align more closely with clinical doses (Stuart et al., 2013). Higher doses of antidepressants tend to induce non-specific effects in the task (Hinchcliffe et al., 2024). Compounds that bind irreversibly with the target receptor are not suitable for the induction of affective biases in the ABT due to the within-subject design, although they can be tested in the retrieval protocol of the ABT or RLA using a between-subject design.
To induce a negative affective bias in Basic Protocol 4, the most frequently used manipulations are corticosterone (stress hormone, 10 mg/kg subcutaneous) or FG7142 (anxiogenic benzodiazepine partial inverse agonist, 3 mg/kg subcutaneous). For both treatments, a pre-treatment time of 30 min before the pairing session induces a reliable negative affective bias in the choice test (Hinchcliffe et al., 2024). Both compounds can be purchased from commercial suppliers (we purchase them from Merck, previously Sigma-Aldrich, UK). We dissolve corticosterone first in 5% DMSO (VWR Chemicals, UK) and then add 95% sesame oil (Merck, UK). FG7142 is suspended in sterile saline with one drop per 1 ml of Tween 80 (Merck, UK). For both drugs, use a 1 ml/kg dose volume. Make sure the drugs are well mixed and vortex before dosing, especially the FG7142 suspension. Always prepare the drugs fresh on the day of use and, if required, store them at 4°C.
NOTE : All protocols involving animals must be reviewed and approved by the appropriate Animal Care and Use Committee and must follow regulations for the care and use of laboratory animals.
Basic Protocol 1: BOWL DIGGING AND DISCRIMINATION TRAINING
This protocol describes how to first train rats to retrieve rewards contained in ceramic bowls and presented in a testing arena. Once animals have learned to reliably retrieve buried rewards from a bowl containing substrate versus an empty bowl, a single discrimination learning session is used to train the animals to associate one digging substrate with a reward and the other with no reward, and to select the correct substrate to the required learning criteria. A final training stage for all cohorts involves the RLA protocol, which is used to check the cohort is performing the assay correctly and develops a reward-induced bias (see Basic Protocol 2).
Materials
-
Rats (In our laboratory, we routinely use male Lister Hooded rats; RR ID:RGD_2312466, Envigo, UK) that weigh approximately 275-300 g at the beginning of the study; however, albino strains Sprague Dawley (RR ID:RGD_734476, Charles River, UK) (Hinchcliffe et al., 2017, 2020) and Wistar (RR ID:RGD_2312504, Charles River, UK) (Phelps, unpublished) and females have also been used (Hinchcliffe et al., 2017, 2020)
-
Standard laboratory rat chow (e.g., TestDiet, UK, or equivalent)
-
Rodent reward pellets (e.g., 45 mg purified rodent tablets, containing sucrose, casein, maltodextrin, corn starch, corn oil, minerals, silicon dioxide, vitamins, magnesium stearate, DL-methionine, Test Diet, Sandown Scientific, UK, or equivalent)
-
Nontoxic detergent for cleaning (e.g., non-fragranced Anistel, UK, or equivalent)
-
Water bottles for drinking water (500 ml, from e.g., Techniplast, UK, or equivalent)
-
Type opaque NK cages (55 × 35 × 21 cm, from e.g., Techniplast, UK, or equivalent), with metal mesh lids and food hoppers, lined with bedding (such as aspen woodchip) and a handful of nesting material such as paper wool
-
Environmental enrichment cages (In our laboratory, we house rats with paper bedding, cotton rope, wood chew, aspen balls, cardboard tube, and a red Perspex® house (30 × 17 × 10 cm))
-
Scale for weighing the rats (e.g., Weighwell, UK, or equivalent)
-
Nontoxic, permanent marker pen for tail marking (e.g., Pentel N850)
-
Perspex® testing arena with removable lid (40 × 40 × 25 cm)
-
Three ceramic identical bowls (10 cm, e.g., pet drinking bowls)
-
Absorbent paper-based liner cut to the size of the arena (40 × 40 cm)
-
Small plastic container for reward pellets
-
Appropriate personal protective equipment (face mask, hair net, gloves, scrubs/animal facility gown, clogs/overshoes, etc.)
-
Laboratory notebook (for manual recording of the experimental details) and folder for storage of printed pairing sessions and choice test sheets
-
Personal computer to use Microsoft Excel package (to prepare experimental design plans, dosing sheets, sheets for data acquisition, and processing) and GraphPad Prism (data processing, visualization).
-
Data handling software (e.g., Microsoft Excel, RR ID:SCR_016137, GraphPad Prism; RR ID:SCR_002798)
-
Statistical analysis software (e.g., GraphPad Prism; RR ID:SCR_002798 or SPSS; RR ID:SCR_002865)
Housing
1.After the animals arrive at the animal facility, allow them to habituate to their new environment, diet, and light-dark cycle for at least a week before beginning any procedures or experimental manipulations.
2.During the acclimatization period, provide water and standard laboratory chow ad libitum for all animals.
3.House animals in pairs in standard enriched laboratory cages under a 12:12 hr reverse light-dark cycle (e.g., lights off at 08:00 hr).
Husbandry, handling, and feeding
4.Prior to any behavioral experiments with laboratory animals, experimenters need to undertake the appropriate training, including theoretical background, handling, daily care, and obtain any necessary approvals to work with animals for scientific purposes.
5.Following a week of acclimatization, weigh and tail mark each animal. Gently transfer each rat from its home cage to a scale, weigh it individually, and then record its body weight in a weight record book. Before returning it to the home cage, mark the rat's tail with a unique and easy-to-identify number, code, or letter using a nontoxic, permanent marker. Continue to weigh the rats at least weekly to monitor their weight gain and mark their tails on the same day. As animals reach maturity, it can be useful to use body condition scores to help maintain a healthy weight.
6.Three days before the start of training, restrict food for all rats to initiate a regime to maintain body weight at approximately 90% of their free-feeding weights matched to the normal growth curve (∼18 g food per rat/day laboratory chow) and provide water ad libitum. Once they reach maturity, use body condition scores and maintain the animals in good condition (4/5) but not obese. Greater levels of food restriction can overly motivate the animals for food and impact the reliability of the behavior.
7.At the end of the acclimatization period, habituate rats to reward pellets in a ceramic bowl. For 3 days, place a small number of pellets and one bowl in their home cage and observe to make sure all rats have eaten the pellets. Ensure all rats are habituated to handling with positive reinforcement and familiarized with the travel box/holding cage (Fig. 3C) before they start training. Handling-induced stress will confound results and limit the reliability of both the ABT and RLA.
Training
The first 2 days comprise habituation to the testing arena. The animals are then introduced to digging training for at least the next 5 days with increasing levels of difficulty. On each day, bring rats (with cage mate(s)) into in the behavioral room for 5-10 min, either in a transport box or cage or in their home cage. Return animals to the holding room once all the subjects for that cage have completed the session for that day.
8.Habituation 1 (usually on Thursday of week 1)
-
Place two rats from the same cage in the arena together with both bowls (no substrate) containing pellets and allow them to explore.
-
Observe animals and record if they both eat pellets and score the number of fecal pellets.
-
Leave both animals in the arena for 10 min.
9.Habituation 2 (usually on Friday of week 1)
-
Place one rat in the arena with both bowls (no substrate) containing a few pellets and allow to explore.
-
Observe the animal: record if it eats pellets and score the number of fecal pellets.
-
Rebait bowls each time pellets are eaten for up to 10 min.
10.Shuttle training (usually on Monday of week 2)
-
Use two bowls with no digging substrate.
-
Place one pellet in one of the three reward locations in one bowl (10, 12, and 2 on a clock).
-
Place one rat in the arena with both bowls (no substrate) and allow to explore.
-
Once the rat has consumed the pellet in the bowl, place a pellet into the left-hand corner of the arena and wait for the animal to find it, then pick up the animal and remove it from the arena to a holding box (or gently hold) between trials.
-
Re-bait the bowl with one pellet in one of the three locations and repeat the trial. Bait either the left or the right bowl in a pseudo-random spatial order to avoid animals developing a side bias and to train animals that either bowl could contain a reward.
-
Repeat for 12 trials. Omissions do not count toward the total number of trials.
-
If animals fail to collect pellets (explore bowls) after 30 s, remove them from the arena and place them in a holding box for 5 s before restarting.
-
Record trial outcomes (‘correct’ or ‘omission’) and latencies.
-
At the end of the day, feed ∼2/3 of the normal food ration.
-
Replace tray liner and wash bowls with water. Let it dry to be ready for the next day.
Criteria for progression: The rat performs at least 10 trials consuming the pellets from the bowl and the left corner of the arena. Any animal that fails to achieve the criteria should be re-run later in the day or the next day. Do not progress animals to the next stage until criteria have been met.
11.Digging training 1 (usually on Tuesday of week 2)
- a.Bring the assigned rats (with cage mates) from their holding room. While you are setting up your bowls with substrates and the pairing paper sheets, the rats can habituate to the testing room.
- b.Continue to use the pellet in the left-hand corner to train recall (animal return) and remove the animal from the arena between trials.
- c.Record trial outcomes (‘correct’, ‘incorrect’, or ‘omission’) and latencies.
Trial 1
- d.Use two bowls with no digging substrate.
- e.Place one pellet in one of the three locations in one bowl.
- f.Place an individual rat in the arena and wait until the pellet is found and consumed. Do not remove the other bowl from the arena.
- g.Once the rat has consumed the pellet in the bowl, place a pellet in the left-hand corner of the arena and wait for the animal to find it, then pick up the animal and remove it from the arena to a holding box (or gently hold) between trials.
Trial 2-3
- h.Place the pellet in the same location as trial 1, but cover it with 1 cm sawdust (Fig. 3D).
- i.Leave the other bowl empty with no sawdust.
- j.Place an individual rat in the arena and wait until the pellet is found and consumed.
- k.If the rat does not start digging in the bowl after ∼30 s, remove the animal and place it in the holding box (or gently hold it) for 5 s and mark the trial as an ‘omission’. The cut-off time may need to be increased if rats are still exhibiting any fear-related behaviors. For animals that fail to dig, repeat trials 2-3 until they dig within 30 s. Placing the pellet on top of the sawdust for one trial before burying may re-engage animals not performing the task.
- l.Once the rat has consumed the pellet in the bowl, place a pellet into the left-hand corner of the arena and wait for the animal to find it, then pick up the animal and remove it from the arena to a holding box (or gently hold) between trials.
Trial 4-xx
- m.Continue with 1 cm sawdust in one bowl and place one pellet in one of the three locations. Randomize the position of the baited bowl with sawdust between the left and right positions.
- n.Continue to leave the other bowl empty.
- o.Place an individual rat in the arena until the pellet is found and consumed.
- p.Once the rat has consumed the pellet in the bowl, place a pellet in the left-hand corner of the arena and wait for the animal to find it, then pick up the animal and remove it from the arena to a holding box (or gently hold) between trials.
- q.If the rat does not start digging in the bowl for 15 s, remove the animal to a holding box (or gently hold) for 5 s and mark trial as an ‘omission’.
- r.If you get two consecutive omissions, consider increasing the latency cut-off for a couple of trials e.g., 30 s.
- s.Repeat until 12 trials are completed (‘omissions’ do not count as completed trials).
- t.
Record trial outcomes (‘correct’, ‘incorrect’, or ‘omission’) and latency to dig (time from placing in the arena to time to dig).
If the rat has 5 consecutive ‘omissions’, repeat trial 1 and trial 2.
Criteria for progression: The rat performs at least 12 trials. Any animal that fails to achieve the criteria should be re-run later in the day or the next day. Do not progress animals to the next stage until criteria have been met.
12.Digging training 2 (usually on Wednesday of week 2)
- a.Bring the assigned rats (with cage mates) from their holding room. While you are setting up your bowls with substrates, the rats can habituate to the testing room.
- b.Continue to use the pellet in the left-hand corner to train recall and remove the animals from the arena between trials.
- c.Record trial outcomes (‘correct’, ‘incorrect’, or ‘omission’) and latencies to dig.
Trial 1
- d.Bait one bowl with one pellet and no sawdust. Leave the other bowl empty.
- e.Let the rat explore both bowls until the pellet is collected.
- f.Once the rat has consumed the pellet in the bowl, place a pellet in the left-hand corner of the arena and wait for the animal to find it, then pick up the animal and remove it from the arena to a holding box (or gently hold) between trials.
Trial 2
- g.Place the pellet in the same location and place 1 cm sawdust in that bowl. Leave the other bowl empty.
- h.Remove the empty bowl once the rat has committed to digging in the sawdust bowl. It is very important that bowl removal occurs after the animal has started digging. For the first couple of trials, bowl removal may distract the rat, so leave them for long enough to return to the baited bowl and find the reward.
- i.Once the rat has consumed the pellet in the bowl, place a pellet in the left-hand corner of the arena and wait for the animal to find it, then pick up the animal and remove it from the arena to a holding box (or gently hold) between trials.
- j.If the rat does not start digging within ∼30 s, remove the animal to a holding box (or gently hold) for 5 s and mark trial as an ‘omission’.
Trial 3
- k.Place pellet in the same location and place 2 cm sawdust in that bowl. Leave the other bowl empty.
- l.Remove the empty bowl once the rat has committed to digging in the sawdust bowl and eaten the pellet.
- m.Once the rat has consumed the pellet in the bowl, place a pellet in the left-hand corner of the arena and wait for the animal to find it, then pick up the animal and remove it from the arena to a holding box (or gently hold) between trials.
- n.If the rat does not start digging within ∼10 s, remove the animal to a holding box (or gently hold) for 5 s and mark trial as an ‘omission’.
Trial 4-xx
- o.Continue with 2 cm sawdust in one bowl and place one pellet in one of the three locations.
- p.Randomize the position of the baited bowl with sawdust between the left and right positions.
- q.Continue to leave the other bowl empty.
- r.Wait for the rat to find the reward. If the rat does not start digging ∼10 s, remove the animal to the holding box (or gently hold) for 5 s, then repeat the trial and mark the first attempt as an ‘omission’.
- s.Once the rat has consumed pellet in the bowl, place a pellet in the left-hand corner of the arena and wait for the animal to find it, then pick up the animal and remove it from the arena to a holding box (or gently hold) between trials.
- t.
Repeat until 12 trials are completed; ‘omissions’ do not count as completed trials.
If the rat has 3 consecutive omissions, the cut-off time may need to be adjusted but should be adapted for each rat.
Criteria for progression: The rat performs at least 12 trials. Any animal that fails to achieve the criteria should be re-run later in the day or the next day. Do not progress animals to the next stage until criteria have been met.
13.Digging training 3 (usually on Thursday of week 2)
-
Repeat Digging training 2 , but stop using the pellet in the left-hand corner (rats should be trained to return to hand by now).
-
Continue to remove the animal from the arena between trials.
-
If the rat does not start digging within ∼5 s, remove the animal to the holding box or gently hold for 5 s, then replace it in the arena (mark trial as an ‘omission’).
-
If the rat has 3 consecutive omissions, the cut-off time may need to be adjusted but should be adapted for each rat.
-
Record trial outcomes (‘correct’, ‘incorrect’, or ‘omission’) and latencies.
The cut-off time is used to encourage the animals to approach the bowls quickly and dig. It is gradually reduced through the training sessions but may need to be adjusted for each animal based on their confidence in the task and performance. If animals have a slow average latency (on the border of the cut-off time) from the previous session, it may be necessary to use a longer cut-off time than described in this protocol. If >5 consecutive omissions occur, the animal can lose interest and stop performing the trials.
Before progressing further with training, it is important that the rat has learned the trial sequence (approach bowl – choose substrate – dig for reward – return to the handler) and is reliably approaching the bowls, digging for reward, eating the reward, and returning to the handler. They may have the odd trial when they are distracted, but any animal that is not reliably performing the trial sequence will need further digging training before they progress to the discrimination stage. If animals are not returning to the handler, use additional training sessions with a reward placed in the left corner after the bowl is removed. Although it can be frustrating waiting for animals to leave the bowls and return to the handler, patience at this stage will benefit running the task in the longer term.
In our lab, more than 95% of rats will learn the task with one day of training at each stage and meet criteria after a single session. If rats are not meeting criteria the most likely cause is the handler-animal interaction and relates more to the pre-training habituation than training.
14.Discrimination (usually on Friday of week 2)
The discrimination session is used to train animals to associate the reward location with one of two different digging substrates not previously encountered. This stage also enables the experimenter to establish whether the rats have met the required learning criteria to progress.
-
Bring the assigned rats (with cage mates) from their holding room. While you are setting up your bowls with substrates and the pairing paper sheets, the rats can habituate to the testing room.
-
Fill one bowl with 2 cm of substrate ‘A’, e.g., chopped tissue bedding, and the other bowl with 2 cm of substrate ‘B’, e.g., shredded dishcloth (Fig.3E).
-
Mix finely crushed reward pellets into both substrates to reduce animals' ability to use olfactory cues to locate the reward.
-
Bait substrate ‘A’ bedding (odd-numbered animals) or substrate B (even-numbered animals) with one pellet as ‘rewarded’ substrate.
-
Use the same bowls and substrates for all rats that day.
-
Trials are run using the same method established during digging training, and animals should approach and dig in the bowl within ∼ 5 s and return to the handler after consuming the reward or failing to find a reward. Animals that fail to show robust trial behavior may still be experiencing a degree of stress and may benefit from additional handling habituation and digging training. Remove animals from the arena between trials.
-
Record trial outcomes (‘correct’, ‘incorrect’, or ‘omission’) and latencies to dig. Choice of the reward-paired substrate is marked as a ‘correct’ trial, digging in the unrewarded substrate is classified as an ‘incorrect’ trial, and if an animal fails to approach and explore the bowls within 10 s, the trial is recorded as an ‘omission’.
Trial 1
- h.Allow the animal to explore both bowls, find the pellet, and leave the bowl. Do not restrict to 30 s if the animal is still exploring. If the animal is not engaging with the task for more than 30 s, remove for 5 s, then repeat.
Trial 2-xx
- i.
Place rat in the arena (Fig. 3A), wait for the animal to ‘choose’ a substrate, then remove the other bowl (Movie 1).
When has the animal made a choice? When the animal has obviously started to dig or search within one of the substrates. The animal should be allowed to investigate both bowls and should not be rushed. If the animal does not make an obvious choice after ∼10 s, remove the rat from the arena to a holding box or hold for 5 s, then re-run the trial.
When you place a rat to approach a bowl and make a choice, do not give away any cues (e.g., lifting your arm to remove the other bowl) to the animal until it has made a choice.
- j.Use ∼5 s cut-off time for removing the animal and marking it as ‘omission’ but be prepared to adapt to each subject.
- k.If a rat has 3 consecutive omissions, use a longer cut-off for the next trial before going back to the 5 s cut-off time.
- l.Omissions do not end a series of correct trials (if a rat has 4 correct trials followed by an omission, they still only need 2 more correct trials to meet the criterion)
- m.
Run until the animal achieves 6 consecutive correct trials (maximum 20 trials).
Criteria for progression: If an animal achieves 6 consecutive correct trials in less than 20 trials in total, it is considered trained and correctly performing the task. The probability of achieving this criterion by chance is 0.0156.Once animals successfully reach the criteria in the discrimination session, they are considered trained and can progress to testing in the RLA.
Basic Protocol 2: THE REWARD LEARNING ASSAY
The RLA protocol is carried out over 5 days (usually week 3 for an experienced experimenter) and is used to check that the cohort is correctly performing the task and, at the population level, a reward-induced positive bias should be observed before progressing the cohort to testing. Using a within-subject design, animals learn to associate one of the two reward-paired digging substrates with either a high (2 pellet) or low (1 pellet) value reward. Three objectives can be achieved using the RLA: a) to determine whether the animals correctly perform the task post-discrimination training, b) to investigate the animals' core affective state, and c) to test for any acute, non-specific effects on memory following an acute drug treatment prior to the choice test (Fig. 4). The results for the cohort are analyzed statistically to verify that a significant positive bias is observed. At the end of this protocol, the animals are trained and ready to undergo testing following acute pharmacological or non-pharmacological manipulations in the ABT (Basic Protocols 3 and 4).

See Basic Protocol 1 for details on materials, housing, husbandry, handling, feeding, and training.
Pairing sessions
1.Using Table 3, run substrate-reward pairing sessions using a fully counterbalanced design that follows that of the basic discrimination training stage but does not include an exploratory first trial.
2.Fill bowl 1 with substrate ‘A’, bowl 2 with substrate ‘B’, and bowl 3 with substrate ‘C’.
3.Use the same substrates and bowls for the whole week but remove contaminants.
4.Mix finely crushed reward pellet into substrate ‘C’ to prevent animals from sniffing out the reward pellet.
5.Record trial outcomes (‘correct’, ‘incorrect’, or ‘omission’) and latencies to dig. Choice of the reward-paired substrate is marked as a ‘correct’ trial, digging in the unrewarded substrate is classified as an ‘incorrect’ trial, and if an animal fails to approach and explore the bowls within 5 s, the trial is recorded as an ‘omission’.
6.Bring the assigned rats (with cage mates) from their holding room. While you set up your bowls with substrates and the pairing session paper sheets, the rats can habituate to the testing room.
7.Place an individual rat in the arena and run the pairing session.
8.Make sure the two pellets used for the high reward value substrate are placed close together and check animals are finding and consuming both pellets. Some rats may leave briefly and then return to find the second pellet before returning to the handler.
9.Remove the animal from the arena between trials.
10.If a rat has 3 consecutive omissions (trial >5 s), use a longer cut-off for the next trial before going back to the 5 s cut-off time.
11.Omissions do not end a series of correct trials (if a rat has 4 correct trials followed by an omission, it still only needs 2 more correct trials to meet the criterion).
12.Run until the animal achieves 6 consecutive correct trials (maximum 20 trials).
13.Return all animals to their holding room immediately after the pairing session.
Choice test
Reward-induced biases generated in this protocol are quantified during a choice test performed at least 24 hr after the last pairing session. During the choice test, the two previously rewarded substrates (‘A’ and ‘B’) are presented at the same time over 30 trials (Fig. 4). In order to keep rats motivated to dig, but without providing new associative information, a single 45 mg food pellet is placed in either substrate using a random schedule with a probability of one in three, so that rats randomly receive a reward (i.e., substrate ‘A’ contained a pellet on 10 of the 30 trials, and likewise for substrate ‘B’; and no trials were both bowls baited).
14.Run individual animals using the same procedure as the pairing sessions but bait bowls using random reinforcement as per schedule. Template suitable for recording data
15.Mix finely crushed reward pellets into both substrates ‘A’ and ‘B’ to reduce the likelihood of the animal using odor to find the reward.
16.Use only 1 pellet on each rewarded trial.
17.Make sure animals are given enough time to make a choice (let them briefly explore both bowls but avoid letting them sniff out the pellet), but aim to keep to <5 s as much as possible, taking each rat's behavior into consideration.
18.Place a rat in the arena and wait for the animal to choose a substrate, then remove the other bowl.
19.When you place a rat to approach a bowl and make a choice, do not give any cues (e.g., lifting your arm to remove the other bowl) to the animal until it makes a clear choice.
20.Record the animals' choices and latency to dig. There are no ‘correct’ or ‘incorrect’ trials, only choices for either substrate ‘A’ or ‘B’. If an animal fails to make a choice within 10 s, record trial as an ‘omission’. If ‘omissions’ occur, mark them down, but do not count into 30 trials that animals need to complete.
21.Return all animals to the holding room.
22.For testing animals' core affective state, a between-subject study design is used with animals from each treatment group being trained and tested in the RLA in a randomized study design. For studies involving the induction of a depression-like phenotype using a chronic manipulation such as repeated stress or a pro-depressant drug, it is better to pre-train the animals and undertake an RLA before allocating them to treatment groups. At the end of the planned treatment period, a repeat of the RLA can be run using new substrates. Normally, treatment continues throughout the second RLA, but this will depend on the specific experimental objectives (Fig. 4B).
23.For testing the acute, non-specific effects of a drug, dose animals with the drug or vehicle and required pre-treatment time prior to the choice test (Fig. 4C).
Basic Protocol 3: THE AFFECTIVE BIAS TEST - NEW LEARNING
The ABT protocol is used to investigate the neuropsychology of affective state-induced biases associated with specific cue-reward memories. This protocol can be used in different ways to investigate different aspects of affective bias modification. To investigate affective biases induced at the time of learning, the affective state manipulation or drug treatment is combined with the learning experience and the arising choice bias generated is quantified at least 24 hr after the last treatment.
For details on materials, housing, husbandry, handling, feeding, and training, see Basic Protocol 1.
Pairing sessions
Each week is comprised of four pairing sessions (one per day, usually Monday to Thursday) to generate two independent cue-specific memories (Fig. 5A) and a choice test performed not less than 24 hr after the last pairing session. Using a within-subject design, each animal acquires two substrate-reward association memories under different treatment or control conditions. The value of the reward is kept the same for both experiences, and the experiment design counterbalances all other factors, so any biases observed during the subsequent choice test can be attributed to the treatment at the time of learning.
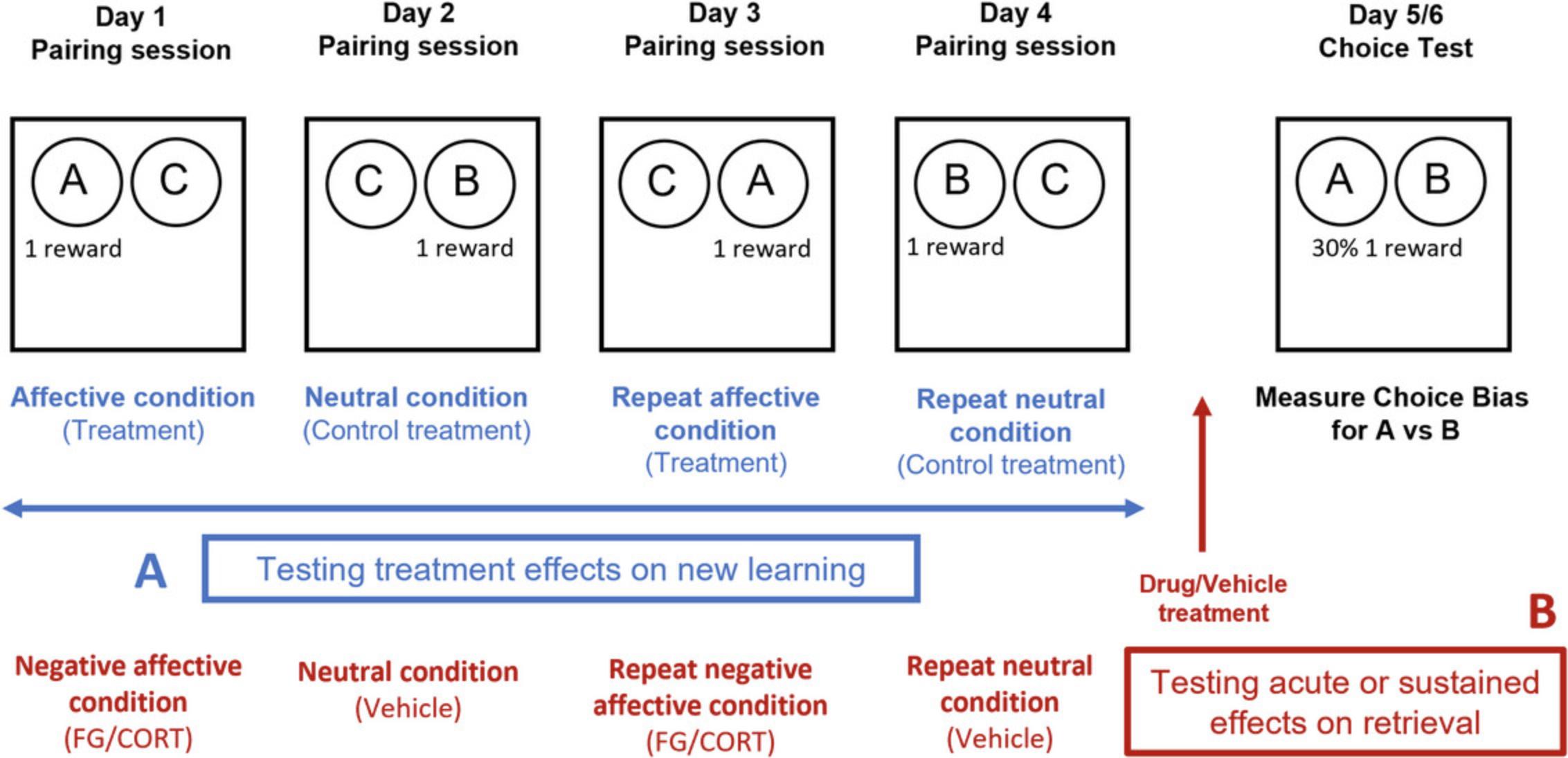
During the pairing sessions, each trial involves presenting the rat with a choice between two bowls containing two different digging substrates (make sure they are matched for a digging effort), one of which is reward-paired (substrate ‘A’ or ‘B’, counterbalanced across subjects and manipulations) and containing a single 45-mg reward pellet, and the other of which is unrewarded (substrate ‘C’). Substrate ‘C’ is kept the same for all four pairing sessions. Either substrate ‘A’ or ‘B’ is presented during pairing sessions on days 1 and 3, and the other is presented on days 2 and 4, with the order counterbalanced across subjects (see Table 2).
| Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | |
|---|---|---|---|---|---|
| Pairing 1 | Pairing 2 | Pairing 3 | Pairing 4 | Choice test | |
| Group 1 |
A vs. C Drug |
B vs. C Vehicle |
A vs. C Drug |
B vs. C Vehicle |
A vs. B, 30 trials |
| Group 2 |
B vs. C Drug |
A vs. C Vehicle |
B vs. C Drug |
A vs. C Vehicle |
A vs. B, 30 trials |
| Group 3 |
A vs. C Vehicle |
B vs. C Drug |
A vs. C Vehicle |
B vs. C Drug |
A vs. B, 30 trials |
| Group 4 |
B vs. C Vehicle |
A vs. C Drug |
B vs. C Vehicle |
A vs. C Drug |
A vs. B, 30 trials |
- a
Each animal receives manipulation/drug treatment or control/vehicle counterbalanced over the four substrate-reward pairing sessions. Substrate (reward-paired substrates—“A” or “B” versus unrewarded substrate—“C”) and day are also counterbalanced, resulting in four different groups.
| Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | |
|---|---|---|---|---|---|
| Pairing 1 | Pairing 2 | Pairing 3 | Pairing 4 | Choice Test | |
| Group 1 |
A vs. C 2 pellets |
B vs. C 1 pellet |
A vs. C 2 pellets |
B vs. C 1 pellet |
A vs. B, 30 trials |
| Group 2 |
B vs. C 2 pellets |
A vs. C 1 pellet |
B vs. C 2 pellets |
A vs. C 1 pellet |
A vs. B, 30 trials |
| Group 3 |
A vs. C 1 pellet |
B vs. C 2 pellets |
A vs. C 1 pellet |
B vs. C 2 pellets |
A vs. B, 30 trials |
| Group 4 |
B vs. C 1 pellet |
A vs. C 2 pellets |
B vs. C 1 pellet |
A vs. C 2 pellets |
A vs. B, 30 trials |
- a
Each animal receives 2 pellets or 1 pellet counterbalanced over the four substrate-reward pairing sessions. Substrate (reward-paired substrates—“A” or “B” versus unrewarded substrate—“C”) and day are also counterbalanced, resulting in four different groups.
New trios of substrates are used for each week of the study, and animals can complete multiple studies as long as new substrates are used. Where a study involves comparing multiple treatments or doses of a drug, the same trio of substrates is used for all animals for that week, but the treatments are counterbalanced over the course of the study. Templates suitable for recording data from pairing sessions are provided in Table S1 and Table S2.
1.Following the trial details in Table 2, perform substrate-reward pairing sessions on days 1-4.
2.Dose animals with the drug or vehicle at the required pre-treatment time prior to pairing sessions or subject animals to non-pharmacological manipulation prior to and/or after pairing sessions.
3.Fill bowl 1 with substrate ‘A’, bowl 2 with substrate ‘B’, and bowl 3 with substrate ‘C’.
4.Use the same substrates and bowls for the whole week but remove contaminants.
5.Mix finely crushed reward pellet into substrate ‘C’ to prevent animals from sniffing out the reward pellet.
6.Record trial outcomes (‘correct’, ‘incorrect’, or ‘omission’) and latencies to dig. Choice of the reward-paired substrate is marked as a ‘correct’ trial, digging in the unrewarded substrate is classified as an ‘incorrect’ trial, and if an animal fails to approach and explore the bowls within 5 s, the trial is recorded as an ‘omission’.
7.Bring the assigned rats (with cage mates) from their holding room. While you are setting up your bowls with substrates and the pairing sessions paper sheets, the rats can habituate to the testing room.
8.Place the rat in the arena (Fig. 3A), wait for the animal to choose a substrate, and then remove the other bowl.
9.When you place a rat to approach a bowl and make a choice, do not move or give any cues (e.g., lifting your arm to remove the other bowl) to the animal until it makes a clear choice.
10.Remove the animal from the arena when it returns to the handler, as in training and between trials.
11.If a rat has 3 consecutive omissions, increase the cut-off time for the next trial before returning to the 5 s cut-off time.
12.Omissions do not end a series of correct trials (if a rat has 4 correct trials followed by an omission, they still only need 2 more correct trials to meet the criterion)
13.Run independent trials with the baited bowl placed in either the left or right location to maintain a pseudo-random spatial order until the animal achieves 6 consecutive correct trials (maximum 20 trials if the animal does not achieve the criterion within a total of 20 trials).
14.Return all animals to their holding room immediately after the pairing session.
15.Between animals, clear the arena of any fecal pellets or bits of remaining substrate.
16.Animals should complete 2 pairing sessions for each substrate over days 1-4 with one substrate used on days 1 and 3 and the other on days 2 and 4 and counterbalanced across the cohort. Failing to meet learning criteria for at least one session for both rewarded substrates is rare and used as an exclusion criterion.
Choice test
Affective biases generated by this protocol are quantified during the choice test on day 5 when the two previously rewarded substrates (‘A’ and ‘B’) are presented at the same time for 30 trials. In order to sustain the rat's engagement during the choice test, place a single 45 mg food pellet is placed in either bowl using a random schedule with a probability of one in three, so that rats randomly receive a reward (i.e., substrate ‘A‘ contained a pellet on 10 of the 30 trials, and likewise for substrate ‘B’; and no trials where both bowls are baited). An example of a choice test data sheet is given in Table S3.
17.Run individual animals using the same procedure as per pairing sessions but bait bowls using random reinforcement as per schedule.
18.Mix a finely crushed reward pellet into both substrates ‘A’ and ‘B’.
19.Use just 1 pellet on each rewarded trial.
20.Make sure animals are given enough time to make a choice but remain engaged with the task.
21.Place a rat in the arena and wait for the animal to choose a substrate, then remove the other bowl.
22.When you place a rat to approach a bowl and make a choice, do not give any cues (e.g., lifting your arm to remove the other bowl) to the animal until it makes a clear choice.
23.Record the animals' choices and latency to dig. There are no ‘correct’ or ‘incorrect’ trials, only choices for either substrate ‘A’ or ‘B’. If an animal fails to make a choice within 10 s, record trial as an ‘omission’. If ‘omissions’ occur, mark them down but do not count into 30 trials that animals need to complete.
24.Return all animals to their holding room.
Basic Protocol 4: THE AFFECTIVE BIAS TEST - MODULATION OF AFFECTIVE BIASES ASSOCIATED WITH PAST EXPERIENCES
To investigate modulation of biased memories, an affective-state-induced bias is first generated and then the treatment is given either shortly before (acute) or 24 hr (sustained) before the choice test. The same protocol as New Learning (Basic Protocol 3) is used to generate a biased memory using a treatment known to induce the desired affective bias e.g., the benzodiazepine inverse agonist FG7142 at 3mg/kg subcutaneously induces a reliable negative affective bias. The protocol is run over four consecutive pairing sessions (one per day, usually Monday to Thursday) with a choice test on day 5 (acute modulation) or day 6 (sustained modulation). Using a within-subject design, each animal is tested with or without drug pre-treatment counterbalanced over the weeks of the study. For studies involving the 24 hr (or later) timepoint, the treatment is given in the home cage. In a recent study, we also combined the drug treatment with a cue reactivation protocol to further investigate the interactions between the treatment and memory reactivation (Hinchcliffe et al., 2024).
For details on materials, housing, husbandry, handling and feeding, training, and Reward Learning Protocol see Basic Protocol 1.
Pairing sessions
1.Pairing sessions are run using the same protocol as in New Learning protocol detailed above (see Basic Protocol 3, section Pairing sessions) but with an additional intervention step post-learning but pre-choice test (Table 4, Fig. 5B).
| Day 1 | Day 2 | Day 3 | Day 4 | Day 5 or 6 | Day 5 or 6 | |
|---|---|---|---|---|---|---|
| Group | Pairing 1 | Pairing 2 | Pairing 3 | Pairing 4 | Treatment group | Choice test |
| 1 |
A vs. C FG7142 |
B vs. C Vehicle |
A vs. C FG7142 |
B vs. C Vehicle |
Drug |
A vs. B, 30 trials |
| 2 |
B vs. C FG7142 |
A vs. C Vehicle |
B vs. C FG7142 |
A vs. C Vehicle |
Vehicle |
A vs. B, 30 trials |
| 3 |
A vs. C Vehicle |
B vs. C FG7142 |
A vs. C Vehicle |
B vs. C FG7142 |
Drug |
A vs. B, 30 trials |
| 4 |
B vs. C Vehicle |
A vs. C FG7142 |
B vs. C Vehicle |
A vs. C FG71422 |
Vehicle |
A vs. B, 30 trials |
- a
Each animal receives drug (FG7142/corticosterone) or vehicle counterbalanced over the four substrate-reward pairing sessions. Substrate (reward-paired substrates - “A” or “B” versus unrewarded substrate - “C”) and day are also counterbalanced resulting in four different groups. On day 5, each animal receives treatment prior to the choice test (on day 5) to investigate the acute modulation of the negative biases, while to test sustained modulation, each animal receives treatment on day 5 and is tested on day 6 in the choice test.
Choice test
2.To test acute modulation, on day 5, animals were dosed with the drug or vehicle, and pre-treatment time prior to the choice test was also required on day 5 (Fig. 5B).
3.To test sustained modulation, on day 5, dose animals with the drug or vehicle ∼24 hr prior to the choice test, which should be conducted on day 6 (Fig. 5B).
4.The choice test is performed as described for the new learning protocol (see Basic Protocol 3, section Choice test).
COMMENTARY
Background Information
It is widely acknowledged that conventional methods for quantifying depression-related behavior and predicting antidepressant efficacy are limited (Gururajan et al., 2019; Planchez et al., 2019). The approach we and others have taken involved looking to neuropsychological impairments observed in patients that are quantified using objective measures which could be translated to non-human species. Development of the rodent tests involves a shift from using emotional stimuli, such as faces, words, or sentences, to cues that are suitable for non-human animals. Building from the observation that cognitive processes such as learning and memory, decision-making, and attention are negatively biased in MDD (Mendl et al., 2009; Paul et al., 2005), two key areas have emerged: decision-making biases using a judgement bias task (Harding et al., 2004) and reward learning and memory biases using an affective bias test (Stuart et al., 2013).
The first cognitive bias task for rodents was reported by Mendl's group in 2004 when they developed an operant version of a go/no-go judgment bias task for rats (Harding et al., 2004). Rats were trained to respond to a specific auditory cue to obtain a reward and refrain from responding to avoid punishment (white noise). Rats subjected to a chronic mild stress regime were less likely to anticipate a positive/rewarding outcome in response to intermediate ambiguous cues suggesting a negative bias (Harding et al., 2004). Studies in phenotypic models suggest a good correlation between predicted affective state changes and optimistic or pessimistic choices in the judgment bias task (Enkel et al., 2010; Harding et al., 2004; Papciak et al., 2013). However, the pharmacological findings published so far are less consistent with the human emotional interpretation task, particularly in terms of the time course of antidepressant effects (Joormann & Gotlib, 2006; Harmer, Goodwin et al., 2009; Harmer, O'Sullivan et al., 2009; Lagisz et al., 2020; Neville et al., 2020). The disparity might be linked to the difference in the nature of the tasks; human studies rely on emotional stimuli that provoke innate thoughts and responses, while animal task uses long training procedures to teach animals the reference cue-affective associations. Although there are inconsistent pharmacological findings from judgement bias tasks, they have shown some promise as a tool to investigate the decision-making process under ambiguity associated with changes in core affective state (Lagisz et al., 2020), particularly for animal welfare research.
The ABT and RLA were developed to quantify affective-state or reward-induced biases associated with learning and memory. The ABT is suitable for quantifying short-term changes in affective state associated with a specific reward memory (substrate cue-reward association), while the RLA has been shown to be sensitive to changes in the core affective state. The ABT is normally run in healthy subjects but has been combined with a phenotypic model to investigate vulnerability to the acute effects of short-term affective state manipulations. This type of study involves a more complex design with phenotype as a between-subject factor and acute affective state manipulation as a within-subject factor. Pharmacological and psychosocial manipulations of affective states have been used to demonstrate the validity of the new learning protocol for the ABT. These included testing acute treatment with a variety of conventional delayed onset antidepressants, social and environmental enrichment, psychosocial stress, pro-depressant drugs, and immune challenges, with the direction of the affective bias included consistent with the predicted effects on animals' affective state (Hinchcliffe et al., 2017, 2020, 2024; Hinchcliffe, Jackson et al., 2022; Stuart et al., 2013, 2015, 2017, 2019). Drugs of abuse (cocaine, amphetamine, nicotine), an antidepressant that failed in clinical trials (aprepitant, a neurokinin 1 antagonist), and diazepam (benzodiazepine anxiolytic) did not produce any effects in the ABT (Stuart et al., 2013). Whether the treatment was given prior to or immediately after substrate-reward learning did not change the observed bias for the antidepressant venlafaxine or psychosocial stress (Stuart et al., 2013) suggesting a complex integration of experiences and affective state. The magnitude of an affective bias amplifies with each successive experience (Stuart et al., 2013), and negative affective biases induced by FG7142 can be attenuated by post-treatment induction of a positive affective state (Hinchcliffe, Jackson et al., 2022).
The reward learning assay (RLA), described in Basic Protocol 2 is similar to the ABT and was used in the initial validation experiments conducted while developing the ABT protocol. Animals remain in the same affective state throughout the pairing sessions and choice test, but one experience is paired with a higher value reward, and in normal animals, this results in more choices for high reward-paired substrate and a reward-induced positive bias (Hinchcliffe et al., 2017, 2024; Stuart et al., 2013, 2015). Running the RLA is helpful during training to check animals are correctly performing the task, but the reward-induced positive bias has also been found to be impaired in animals in a depression-like state associated with risk factors including stress, pro-depressant drug treatment, and early life adversity (Stuart et al., 2017, 2019). In these studies, we also quantified reward sensitivity using the SPT and found that only chronic corticosterone treatment also affects performance in the SPT, suggesting the reward learning impairments in this assay may be a more reliable translation method to study reward-related impairments in MDD. Negative affective states are associated with other psychiatric disorders and a study using sub-chronic phencyclidine (PCP) treatment to model Schizophrenia found similar reward learning impairments in the RLA (Sahin et al., 2016). In our most recent work investigating RAADs, we have focused on the retrieval of biased memories and used the RLA as a control assay to establish if the observed effects are specific to an affective state-induced bias or involve more general disruptions in memory processes (Hinchcliffe et al., 2024). Studies utilizing female rats and different strains revealed a consistent picture in terms of both positive and negative affective bias modification across both pharmacological and psychosocial manipulations of the affective state in both ABT and RLA (Hinchcliffe et al., 2017).
Having established the translational validity of both the ABT and RLA, we have been able to use these methods to explore novel hypotheses relating to antidepressant efficacy as well as the fundamental neurobiology of affective bias modification. These studies have revealed important differences between conventional delayed-onset antidepressants and RAADs in terms of the way they interact with affective biases. Conventional delayed onset antidepressants positively bias new learning consistent with the human neuropsychological model of antidepressant efficacy (Godlewska & Harmer, 2021; Harmer, Goodwin et al., 2009; Harmer, O'Sullivan et al., 2009). RAADs such as ketamine and psilocybin attenuated negative biases associated with past experiences (Hinchcliffe et al., 2024; Stuart et al., 2015). When the choice test is carried out 24 hr after treatment with ketamine and psilocybin, the negative bias becomes positive consistent with a re-learning effect (Hinchcliffe et al., 2024). The conventional antidepressant venlafaxine has no effect on biases associated with past experiences, and ketamine lacks the ability to positively bias new experiences (Stuart et al., 2015). The psychedelic psilocybin positively biased new experiences, suggesting its neuropsychological effects combined features of both conventional and RAADs (Hinchcliffe et al., 2024). These differences may be important factors contributing to the time course of their effects on mood. This contrasts with the FST, where both conventional and RAADs similarly reduce immobility time. Further exploring the re-learning effects at 24 hr, we found that the effects of ketamine were dependent on protein synthesis localized to the rat medial prefrontal cortex and could be modulated by cue-reactivation, consistent with experience-dependent neural plasticity (Hinchcliffe et al., 2024). These findings potentially link their effects on neural plasticity with affective bias modification and experience-dependent learning and memory.
We have also begun to investigate the underlying neural mechanisms that modulate affective biases and how these are affected by different classes of antidepressants. Studies using targeted brain lesions or temporary pharmacological lesions suggest the involvement of both the amygdala and medial prefrontal cortex in the modulation of affective biases (Stuart et al., 2015). Amygdala lesions inhibited the formation of a venlafaxine-induced positive bias, and FG7142- or stress-induced negative bias was also reduced. Temporary inactivation of the mPFC with muscimol micro-infusion, ketamine, or the 5-HT2A agonist DOI resulted in attenuation of FG7142-induced negative bias (Hinchcliffe et al., 2024; Stuart et al., 2015). These findings align favorably with human imaging studies, which suggest both prefrontal and amygdala regions are involved in affective bias modification and exhibit changes in activity in patients with MDD (Mayberg, 2003; Ressler & Mayberg, 2007).
The ABT method does not require expensive equipment and the training period is much shorter than in tasks based on traditional instrumental-based learning protocols. The ABT and RLA are more refined methods than the FST/TST, particularly when combined with refined methods for substance administration. The ABT is useful for differentiating the effects of delayed versus rapid-acting antidepressants, including novel compounds. The RLA has shown construct validity as a phenotypic model and to study changes in the core affective state, including for assessing the animal welfare of laboratory rodents (Hinchcliffe et al., 2020; Hinchcliffe, Jackson, 2022).
Critical Parameters
The paramount factors to achieve reliable data from the ABT are refined handling techniques, habituation facilitated with positive reinforcement, and providing standardized enriched housing. Include enough time in the study design to handle animals prior to training and testing to minimize distress and aversion to the experimenter and minimize the use of physical restraint. Animals should be calm and easy to handle, without overt signs of distress e.g., audible vocalizations, signs of struggling when picked up, fecal pellets. The handler should be the experimenter and should be the same person for the cohort throughout the experiment. Changing handlers within a study is not recommended, and if a change is needed, animals should be habituated to a new handler before starting further experiments. For more details on our refined handling, habituation, and housing protocols, see our website 3Hs Initiative: Housing, Handling, and Habituation (www.3hs-initiative.co.uk). Acute stress is a potential confound in both the ABT and RLA as the experience of stress can generate a negative affective state and negative affective bias. This is particularly an issue with substance administration procedures. Oral dosing in palatable solutions is the route of administration least likely to generate a stress response but is not suitable for all compounds, and where intraperitoneal and/or subcutaneous injection procedures are required, these should be performed using a low-restraint method. In our group, injections are performed using a low-stress, minimal restraint method such as the one developed in our research group (Stuart & Robinson, 2015, 3Hs website protocols). All animals are habituated to the holding position required for injection for five days prior to the experiments. Subcutaneous injections are performed with minimal physical restraint and applied to the left or right flank (changing daily) (3Hs website protocols). In all experiments, the experimenter should be blind to treatment with treatments administered for within-subject studies using a fully counterbalanced experimental design, where all animals receive a vehicle (0 mg/kg), and all drug doses.
The handler, i.e., the human factor, can have a very large effect on the animal's behavior and requires experience with animal behavior to be able to successfully train animals and achieve reliable performance. It is important that the handler is calm and patient and does not rush the animals during training and testing. It is crucial to assign enough time to run the pairing sessions and choice testing and adjust the size of the cohort to achieve this. Care should be taken not to provide inadvertent cues to the animal about the location of the reward or try to rush the animal. By rushing to run rats in the pairing sessions, there is a higher likelihood of making an error or causing a stress response in the animal.
Following the principles of the 3Rs, calculate your sample size with sufficient statistical power for the predicted effect size. The sample size (N = 12-16) for our experiments is based on our previous affective bias test studies and power calculation with α = 0.05 and β = 0.8 (Stuart et al., 2013, 2015). A meta-analysis suggested a medium to large effect size for the drug-induced negative bias and reward-induced bias in Lister Hooded and Sprague Dawley rats (Hinchcliffe et al., 2017). Another factor to consider is the likelihood of exclusion from the study. This is low for experienced handlers but may be higher for inexperienced handlers. Exclusion criteria used for training and testing are: when an animal fails to meet the training criterion, fails to learn the substrate-reward association during at least one pairing session, fails to complete 30 trials in the choice test (usually only seen with acute manipulations before the choice test), has a % choice bias more than 2 SDs from the mean, or the positive control did not generate the predicted affective bias suggesting a cohort level issue.
Troubleshooting
See Table 5 for common problems and troubleshooting solutions.
| Problem | Possible cause | Solution |
|---|---|---|
| Animals do not dig for rewards | Animals not habituated enough and are showing a stress response. | Do additional handling sessions and increase food restriction for the next couple of training sessions. |
| Animals not returning to the handler |
Animals not habituated enough and are showing a stress response. Animals are rushed and not given time to learn the trial sequence. |
Do additional handling sessions. Give animals time to find the reward pellet in the left corner and then pick up. Continue to reward the left corner until the trial sequence is well established. You can try to “wiggle” your fingers at the closer edge of the arena to encourage returning to the starting point and/or picking up the animal after the return cut-off time is ∼1 min. For trained animals, run reminder discrimination session with new pair of substrates and reward with pellet in the corner to encourage returning to the starting point. |
| Animals developing substrate bias or side bias in the choice test | This can confound the result and studies with a substrate or side bias should be excluded. These biases are usually seen when animals are trying to solve the reward location rule within the choice test rather than making choices based on their memories. | Re-run with new substrates and preferably ones that have worked for previous studies (see Table 1 for examples). If a cohort has a side or substrate bias in the first RLA at the end of training, it suggests they have not learned the task correctly. |
| Animals do not complete choice test | Either they are not motivated enough to dig and forage for pellets, or they experience sedative/non-specific effects of the drug manipulations. | Lower the dose of the drug used. Make sure animals are mildly food-restricted to maintain their motivation in the task. |
| Problems with animals' low performance during training sessions or pairing sessions | Animals are not motivated enough to dig or too anxious and not habituated to the experimenter or ABT arena. In the acute and sustained modulation studies make sure you use the doses previously validated and reliable to induce the negative bias i.e., corticosterone 10 mg/kg or FG7142 3 mg/kg. Increasing the dose will induce non-specific effects in animals and e.g., FG7142 at higher doses 5-6 mg/kg carrying risk of triggering seizures in rats. | Mildly increase food restriction and/or spend more time to handle and habituate rats. |
| An animal consistently achieves more than 10 rewards during a choice test or cohort mean shows progressive increase above chance. | Animals may be using olfactory cues to locate rewards. | Monitor performance of individual animals and check choice latencies. If animal has a mean latency higher than the population mean, use a training session with reduced cut-off time to speed up decision-making and encourage the animal to use the substrate cue. This may need to be repeated over several sessions. |
Statistical Analysis
Analyze the data using appropriate statistical software such as SPSS 28 or GraphPad Prism 10.0 (GraphPad Software, USA). Figures should summarize mean and variance and illustrate individual data points.
To calculate a choice bias for each animal, sum the number of choices made for the drug-paired (affective bias test) or 2 pellets-paired (reward learning assay) substrate, divide by the total number of trials, and multiply by 100 to give a percentage value. Then subtract 50 to give a score where a choice bias toward the drug-paired substrate gives a positive value and a bias toward the control-paired substrate gives a negative value.
Calculate the side bias, substrate bias, and the number of pellets collected during the choice test to control for non-specific effects and test whether animals are performing the task correctly.
To calculate the % side bias, manually score the number of times the animal chose the bowl located on the left (or right, reciprocally) side and use the equation:
To calculate the % substrate bias, manually score the number of times the animal chose the bowl with substrate A (or B reciprocally) and use the equation:
To calculate the number of pellets collected during the choice test, manually score the number of times the animal chose the rewarded substrate.
Depending on the number of groups and factors included in the study design, use a suitable statistical test such as a two-tailed t-test (for two treatment groups) or Repeated Measures ANOVA (for three or more treatment groups) with treatment as the within-subject factor. A 2-factor ANOVA is used for studies involving a between-subject and within-subject factor e.g., phenotype and drug treatment. As a post-hoc analysis, perform pairwise comparisons by using two-tailed paired t-tests (for two treatment groups) or Dunnett's test (for three or more treatment groups). To analyze choice bias data against a theoretical mean of 0% choice bias for each manipulation, use a one-sample t -test against a null hypothesized mean of 0% choice bias for each manipulation.
Understanding Results
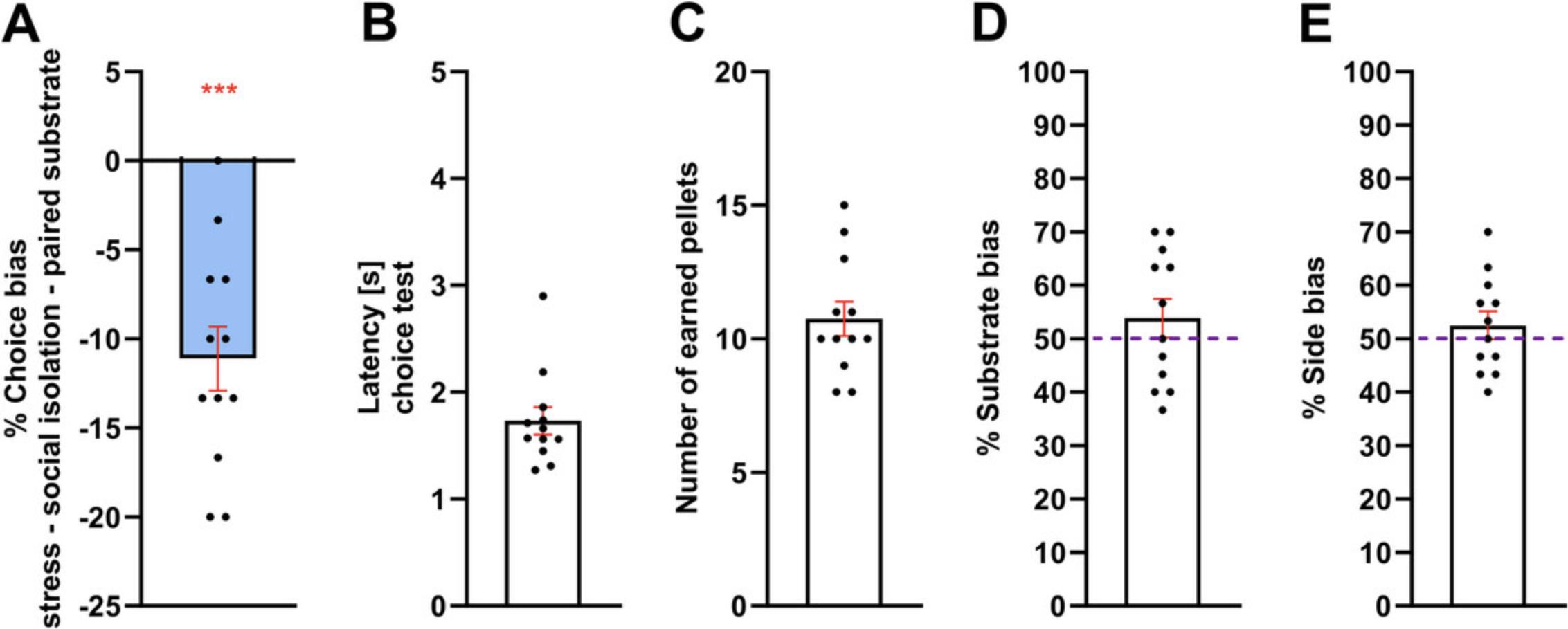
The example data in Figure 6 and Basic Protocol 3 illustrate a significant preference for the control-paired substrate representing a negative affective bias in rats following psychosocial stress, restraint stress, and socialisolation. If animals prefer the manipulation-paired substrate, this is a positive affective bias, e.g., following an antidepressant or social play (Hinchcliffe et al., 2017; Stuart et al., 2013). Response latencies for well-trained rats should be in the range of 1-5 seconds (Fig. 6B). Differences in response latencies during pairing sessions and choice tests, as well as increased omissions and number of trials to criteria during pairing sessions, can be observed in drug studies where pharmacological manipulations can cause non-specific effects, like sedation, changes in motivation or disrupted learning rate (Fig. 6B and Tables 6 and 7). Figure 6C shows the number of pellets consumed during the 30 trials of the ABT choice test, used to verify that the performance of the cohort remains at chance (10/30 trials). Cohorts earning, on average, more than 14 pellets per session may be using other cues, such as olfaction, to find reward. No significant substrate and side bias (Figure 6D and E) indicate rats performing the task correctly and the affective bias arises only from changes in their emotional experience. In case of animals demonstrating the substrate or side bias, see Troubleshooting.
| ID rat | Substrate A | Substrate B | Substrate paired with RSSI | Pellets earned | Mean latency [s] | % Substrate bias (A) | % Side bias (Left) | % Choice bias |
|---|---|---|---|---|---|---|---|---|
| 1 | 13 | 17 | A | 11 | 1.86 | 43.33333 | 43.33333 | −6.67 |
| 2 | 21 | 9 | B | 10 | 1.71 | 70 | 56.66667 | −20.00 |
| 3 | 11 | 19 | A | 13 | 1.27 | 36.66667 | 50 | −13.33 |
| 4 | 19 | 11 | B | 14 | 1.56 | 63.33333 | 46.66667 | −13.33 |
| 5 | 12 | 18 | A | 10 | 1.45 | 40 | 43.33333333 | −10.00 |
| 6 | 20 | 10 | B | 10 | 2.19 | 66.66667 | 63.33333333 | −16.67 |
| 7 | 21 | 9 | B | 8 | 2.90 | 70 | 56.66667 | −20.00 |
| 8 | 12 | 18 | A | 10 | 1.66 | 40 | 60 | −0.00 |
| 9 | 17 | 13 | B | 11 | 1.74 | 56.66667 | 40 | −6.67 |
| 10 | 14 | 16 | A | 8 | 1.31 | 46.66667 | 46.66667 | −3.33 |
| 11 | 19 | 11 | B | 9 | 1.56 | 63.33333 | 70 | −13.33 |
| 12 | 15 | 15 | A | 15 | 1.57 | 50 | 53.33333 | 0.00 |
- a Choice bias data: number of substrate A choices, number of substrate B choices, substrate paired with manipulation, i.e., RSSI, number of pellets earned, mean latency to make a choice (s), % substrate bias, % side bias, and % choice bias. Data are shown as individual animals.
| Restraint stress and psychosocial isolation | Response latency (s) | Number of trials to criterion | ||
|---|---|---|---|---|
| Rat ID | Vehicle | Manipulation | Vehicle | Manipulation |
| 1 | 2.0289 | 1.4431 | 8 | 7 |
| 2 | 2.5300 | 2.2453 | 8 | 7.5 |
| 3 | 1.7020 | 2.2560 | 9 | 10 |
| 4 | 1.6367 | 1.5424 | 6 | 11 |
| 5 | 1.8615 | 1.9123 | 6.5 | 6.5 |
| 6 | 2.9129 | 3.4896 | 9 | 12.5 |
| 7 | 2.0125 | 4.1850 | 12.5 | 10 |
| 8 | 1.9710 | 1.4920 | 8.5 | 8.5 |
| 9 | 1.8876 | 1.8233 | 7 | 7.5 |
| 10 | 2.3454 | 1.3640 | 9.5 | 6.5 |
| 11 | 2.9663 | 2.6226 | 6.5 | 10.5 |
| 12 | 3.1778 | 1.6625 | 8 | 7 |
- a Pairing session data: response latency to make a choice (s) and number of trials to criterion. Data is shown as mean from the two pairing sessions for each substrate-reward association, vehicle, and manipulation for individual animals.
Healthy animals develop a significant reward-induced positive bias at the population level in the RLA (Fig. 7A). If rats fail to develop a reward-induced positive bias, they are not performing tasks correctly and require further digging training. When the RLA is used in an animal model of depression, two weeks of chronic treatment with interferon alpha-induced reward learning impairments and animals failed to develop a positive bias toward the high reward cue during the choice test (Fig. 7B). The results for substrate bias, side bias, pellets consumed, response latencies, number of pairing sessions to criteria during the RLA choice test are analyzed the same way and with the same interpretation as the ABT choice test (Table 8).
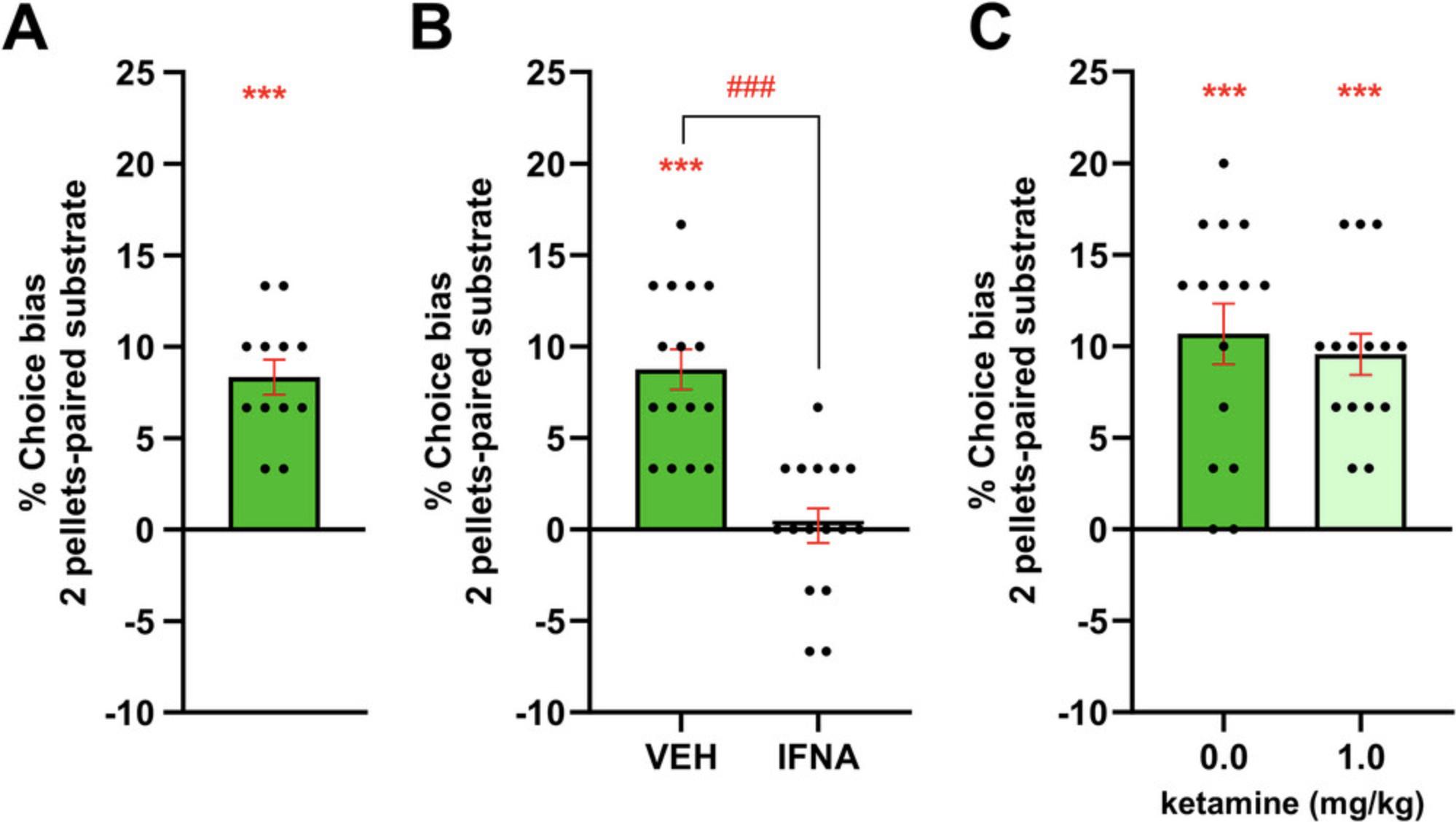
| ID rat | Substrate A | Substrate B | Substrate paired with 2 pellets | Pellets earned | Mean latency [s] | % Substrate bias (A) | % Side bias (left) | % Choice bias |
|---|---|---|---|---|---|---|---|---|
| 1 | 12 | 18 | B | 11 | 2.32 | 40 | 40 | 10 |
| 2 | 19 | 11 | A | 13 | 1.88 | 63.3333 | 50 | 13.3333 |
| 3 | 17 | 13 | A | 11 | 2.43 | 53.3333 | 50 | 6.66667 |
| 4 | 13 | 17 | B | 11 | 1.91 | 46.6667 | 46.6667 | 6.66667 |
| 5 | 14 | 16 | B | 12 | 2.17 | 46.6667 | 53.3333 | 3.33333 |
| 6 | 18 | 12 | A | 12 | 2.00 | 53.3333 | 46.6667 | 10 |
| 7 | 17 | 13 | A | 8 | 2.36 | 53.3333 | 60 | 6.66667 |
| 8 | 13 | 17 | B | 14 | 1.94 | 50 | 40 | 6.66667 |
| 9 | 14 | 16 | B | 9 | 1.78 | 40 | 43.3333 | 3.33333 |
| 10 | 18 | 12 | A | 7 | 1.68 | 66.6667 | 50 | 10 |
| 11 | 18 | 12 | A | 12 | 1.67 | 63.3333 | 50 | 10 |
| 12 | 11 | 19 | B | 12 | 1.63 | 50 | 53.3333 | 13.3333 |
- a Choice bias data: number of substrate A choices, number of substrate B choices, substrate paired with manipulation, i.e., 2 pellets, number of pellets earned, mean latency to make a choice (s), % substrate bias, % side bias, and % choice bias. Data are shown as individual animals.
The results in Figure 8 illustrate the effects of the RAAD ketamine administered at 1 mg/kg, 1 hr (panel A) or 24 hr (panel B) before the choice test. All animals receive FG7142 during the pairing sessions and would be expected to exhibit a negative affective bias during the choice test (Fig. 8A and B). This is what is observed in the vehicle-treated groups at both time points, with a significant negative affective bias observed with a one-sample t -test. Ketamine significantly attenuates this negative bias at 1 hr with no bias observed in the one-sample t -test and pairwise comparisons revealing a significant attenuation (Fig. 8A, Table 9). At 24 hr, a positive bias is observed with the one sample t -test and pairwise comparisons (or ANOVA if more than one test group) show a significant difference from the vehicle control (Fig. 8B). Treatments that fail to modulate biased memories exhibit similar negative biases to the vehicle group and no difference from vehicle treatment is observed. To investigate the specificity of any affective bias modulation observed similar analyses are used for the same treatments in animals performing the RLA. Where treatments have no effects in the RLA they are considered to selectively modulate affective state-induced biases (Fig. 7C). If treatments impair performance in the RLA, they are considered to have general effects on memory retrieval and act in a non-specific manner.
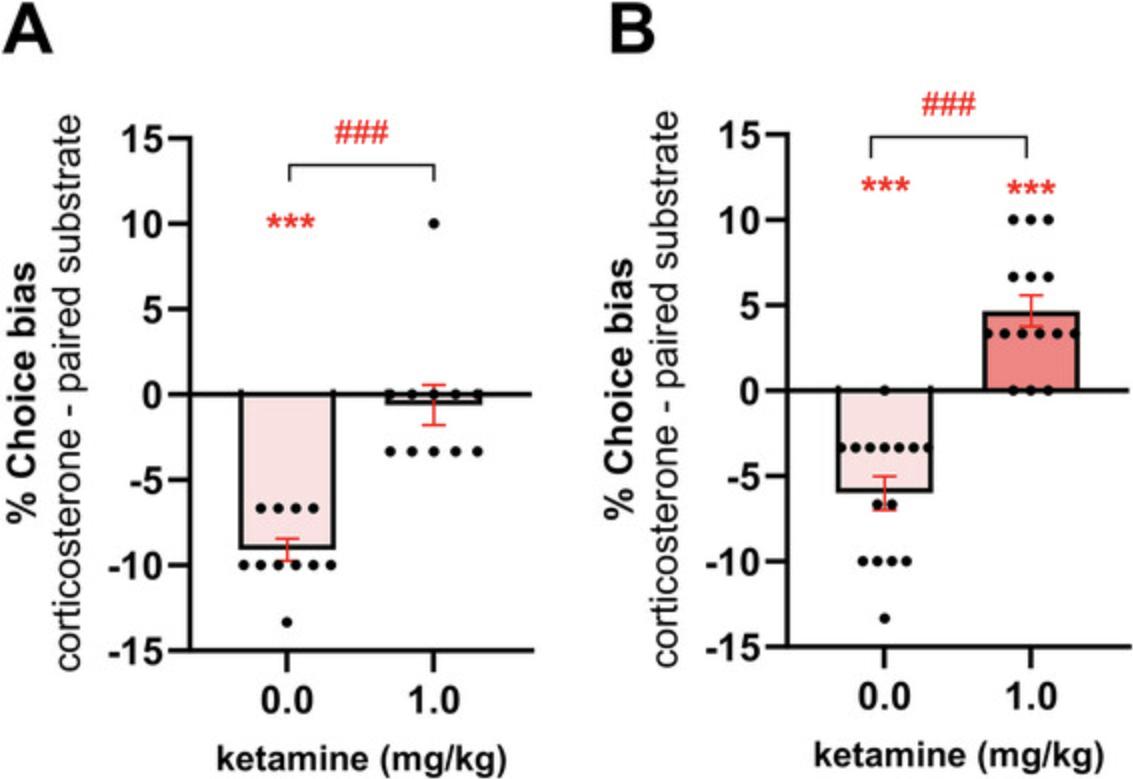
Open Research
Data Availability Statement
The data that support the protocol are openly available in Open Science Framework at https://osf.io/w5ufe/, DOI 10.17605/OSF.IO/W5UFE.
Supporting Information
| Filename | Description |
|---|---|
| cpz11057-sup-0001-SuppMat.xlsx13.3 KB | Table S1. Pairing sheet for recording data for ABT protocol pairing sessions (Left). |
| cpz11057-sup-0002-SuppMat.xlsx21.5 KB | Table S2. Pairing sheet for recording data for ABT protocol pairing sessions (Right). |
| cpz11057-sup-0003-SuppMat.xlsx16.6 KB | Table S3. Data entry template for ABT choice test sessions. |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
Literature Cited
- Bower, G. H., & Mann, T. (1992). Improving recall by recoding interfering material at the time of retrieval. Journal of Experimental Psychology. Learning, Memory, and Cognition , 18(6), 1310–1320.
- Dalla, C., Antoniou, K., Drossopoulou, G., Xagoraris, M., Kokras, N., Sfikakis, A., & Papadopoulou-Daifoti, Z. (2005). Chronic mild stress impact: Are females more vulnerable? Neuroscience , 135(3), 703–714. https://doi.org/10.1016/j.neuroscience.2005.06.068
- Dalla, C., Pitychoutis, P. M., Kokras, N., & Papadopoulou-Daifoti, Z. (2010). Sex differences in animal models of depression and antidepressant response. Basic & Clinical Pharmacology & Toxicology, 106(3), 226–233. https://doi.org/10.1111/j.1742-7843.2009.00516.x
- Enkel, T., Gholizadeh, D., von Bohlen Und Halbach, O., Sanchis-Segura, C., Hurlemann, R., Spanagel, R., Gass, P., & Vollmayr, B. (2010). Ambiguous-cue interpretation is biased under stress- and depression- like states in rats. Neuropsychopharmacology , 35, 1008–1015.
- Gavrilov, V. V., Onufriev, M. V., Moiseeva, Y. V., Alexandrov, Y. I., & Gulyaeva, N. V. (2022). Chronic social isolation stress and crowding in rats have different effects on learning an operant behavior and the state of the hypothalamo-hypophyseal-adrenocortical system. Neuroscience and Behavioral Physiology , 52(5), 698–704. https://doi.org/10.1007/s11055-022-01295-3
- Geugies, H., Mocking, R. J. T., Figueroa, C. A., Groot, P. F. C., Marsman, J.-B. C., Servaas, M. N., Steele, J. D., Schene, A. H., & Ruhé, H. G. (2019). Impaired reward-related learning signals in remitted unmedicated patients with recurrent depression. Brain , 142(8), 2510–2522. https://doi.org/10.1093/brain/awz167
- Godlewska, B. R., & Harmer, C. J. (2021). Cognitive neuropsychological theory of antidepressant action: A modern-day approach to depression and its treatment. Psychopharmacology , 238(5), 1265–1278. https://doi.org/10.1007/s00213-019-05448-0
- Gonda, X., Pompili, M., Serafini, G., Carvalho, A. F., Rihmer, Z., & Dome, P. (2015). The role of cognitive dysfunction in the symptoms and remission from depression. Ann Gen Psychiatry , 14(1), 27. https://doi.org/10.1186/s12991-015-0068-9
- Gururajan, A., Reif, A., Cryan, J. F., & Slattery, D. A. (2019). The future of rodent models in depression research. Nature Reviews Neuroscience , 20(11), 686–701. https://doi.org/10.1038/s41583-019-0221-6
- Harding, E. J., Paul, E. S., & Mendl, M. (2004). Animal behaviour: Cognitive bias and affective state. Nature , 427(6972), 312. https://doi.org/10.1038/427312a
- Harmer, C. J., Goodwin, G. M., & Cowen, P. J. (2009). Why do antidepressants take so long to work? A cognitive neuropsychological model of antidepressant drug action. The British Journal of Psychiatry , 195, 102–108. https://doi.org/10.1192/bjp.bp.108.051193
- Harmer, C. J., O'Sullivan, U., Favaron, E., Massey-Chase, R., Ayres, R., Reinecke, A., Goodwin, G. M., & Cowen, P. J. (2009). Effect of acute antidepressant administration on negative affective bias in depressed patients. The American Journal of Psychiatry , 166(10), 1178–1184. https://doi.org/10.1176/appi.ajp.2009.09020149
- Harper, D., Hunt, M., & Patterson-Kane, E. G. (2002). Rats demand social contact. Animal Welfare , 11(3), 327–332. https://doi.org/10.1017/S0962728600024908
- Hinchcliffe, J. K., Jackson, M. G., & Robinson, E. S. J. (2022). The use of ball pits and playpens in laboratory Lister Hooded male rats induces ultrasonic vocalisations indicating a more positive affective state and can reduce the welfare impacts of aversive procedures. Laboratory Animals , 56(4), 370–379. https://doi.org/10.1177/00236772211065920
- Hinchcliffe, J. K., Mendl, M., & Robinson, E. S. J. (2020). Rat 50 kHz calls reflect graded tickling-induced positive emotion. Current Biology , 30(18), R1034–R1035. https://doi.org/10.1016/j.cub.2020.08.038
- Hinchcliffe, J. K., Thomas, C. W., Golden, C., Selimbeyoglu, A., & Robinson, E. S. J. (2022). COMP360 psilocybin restores reward learning impairments in rats caused by chronic interferon-alpha treatment. FENS FORUM conference proceeding , BOARD NUMBER: S07–136.
- Hinchcliffe, J. K., Stuart, S. A., Mendl, M., & Robinson, E. S. J. (2017). Further validation of the affective bias test for predicting antidepressant and pro-depressant risk: Effects of pharmacological and social manipulations in male and female rats. Psychopharmacology , 234(20), 3105–3116. https://doi.org/10.1007/s00213-017-4687-5
- Hinchcliffe, J. K., Stuart, S. A., Wood, C. M., Bartlett, J. M., Kamenish, K., Arban, R., Thomas, C. W., Selimbeyoglu, A., Hurley, S., Hengerer, B., Gilmour, G., & Robinson, E. S. J. (2024). Rapid-acting antidepressant drugs modulate affective bias in rats. Science Translational Medicine , 16(729), eadi2403. https://doi.org/10.1126/scitranslmed.adi2403
- Joormann, J., & Gotlib, I. H. (2006). Is this happiness I see? Biases in the identification of emotional facial expressions in depression and social phobia. Journal of Abnormal Psychology , 115(4), 705–714. https://doi.org/10.1037/0021-843X.115.4.705
- Kokras, N., Antoniou, K., Mikail, H. G., Kafetzopoulos, V., Papadopoulou-Daifoti, Z., & Dalla, C. (2015). Forced swim test: What about females? Neuropharmacology , 99, 408–421. https://doi.org/10.1016/j.neuropharm.2015.03.016
- Kokras, N., & Dalla, C. (2014). Sex differences in animal models of psychiatric disorders. British Journal of Pharmacology , 171(20), 4595–4619. https://doi.org/10.1111/bph.12710
- Korn, C. W., Sharot, T., Walter, H., Heekeren, H. R., & Dolan, R. J. (2014). Depression is related to an absence of optimistically biased belief updating about future life events. Psychological Medicine , 44(3), 579–592. https://doi.org/10.1017/s0033291713001074
- Lagisz, M., Zidar, J., Nakagawa, S., Neville, V., Sorato, E., Paul, E. S., Bateson, M., Mendl, M., & Løvlie, H. (2020). Optimism, pessimism and judgement bias in animals: A systematic review and meta-analysis. Neuroscience and Biobehavioral Reviews , 118, 3–17. https://doi.org/10.1016/j.neubiorev.2020.07.012
- Lawson, R. P., Nord, C. L., Seymour, B., Thomas, D. L., Dayan, P., Pilling, S., & Roiser, J. P. (2017). Disrupted habenula function in major depression. Molecular Psychiatry , 22(2), 202–208. https://doi.org/10.1038/mp.2016.81
- Lopez, J., & Bagot, R. C. (2021). Defining valid chronic stress models for depression with female rodents. Biological Psychiatry , 90(4), 226–235. https://doi.org/10.1016/j.biopsych.2021.03.010
- Ma, L., Xu, Y., Wang, G., & Li, R. (2019). What do we know about sex differences in depression: A review of animal models and potential mechanisms. Progress in Neuro-Psychopharmacology and Biological Psychiatry , 89, 48–56. https://doi.org/10.1016/j.pnpbp.2018.08.026
- Mayberg, H. S. (2003). Modulating dysfunctional limbic-cortical circuits in depression: Towards development of brain-based algorithms for diagnosis and optimised treatment. British Medical Bulletin , 65, 193–207. https://doi.org/10.1093/bmb/65.1.193
- Mendl, M., Burman, O. H. P., Parker, R. M. A., & Paul, E. S. (2009). Cognitive bias as an indicator of animal emotion and welfare: Emerging evidence and underlying mechanisms. Applied Animal Behaviour Science , 118, 161–181.
- Nair, A., & Jacob, S. (2016). A simple practice guide for dose conversion between animals and human. Journal of Basic and Clinical Pharmacy , 7(2), 27. https://doi.org/10.4103/0976-0105.177703
- Neville, V., Nakagawa, S., Zidar, J., Paul, E. S., Lagisz, M., Bateson, M., Løvlie, H., & Mendl, M. (2020). Pharmacological manipulations of judgement bias: A systematic review and meta-analysis. Neuroscience and Biobehavioral Reviews , 108, 269–286. https://doi.org/10.1016/j.neubiorev.2019.11.008
- Nygren, T. E., Isen, A. M., Taylor, P. J., & Dulin, J. (1996). The influence of positive affect on the decision rule in risk situations: Focus on outcome (and Especially Avoidance of Loss) rather than probability. Organizational Behavior and Human Decision Processes , 66(1), 59–72. https://doi.org/10.1006/obhd.1996.0038
- Obernier, J. A., & Baldwin, R. L. (2006). Establishing an appropriate period of acclimatization following transportation of laboratory animals. ILAR Journal , 47(4), 364–369. https://doi.org/10.1093/ilar.47.4.364
- Palanza, P. (2001). Animal models of anxiety and depression: How are females different? Neuroscience and Biobehavioral Reviews , 25(3), 219–233.
- Papciak, J., Popik, P., Fuchs, E., & Rygula, R. (2013). Chronic psychosocial stress makes rats more 'pessimistic' in the ambiguous-cue interpretation paradigm. Behavioural Brain Research , 256, 305–310.
- Paul, E. S., Harding, E. J., & Mendl, M. (2005). Measuring emotional processes in animals: The utility of a cognitive approach. Neuroscience and Biobehavioral Reviews , 29(3), 469–491. https://doi.org/10.1016/j.neubiorev.2005.01.002
- Phelps, C. E., Lumb, B. M., Donaldson, L. F., & Robinson, E. S. (2021). The partial saphenous nerve injury model of pain impairs reward-related learning but not reward sensitivity or motivation. Pain , 162(3), 956–966. https://doi.org/10.1097/j.pain.0000000000002177
- Planchez, B., Surget, A., & Belzung, C. (2019). Animal models of major depression: Drawbacks and challenges. Journal of Neural Transmission (Vienna) , 126(11), 1383–1408. https://doi.org/10.1007/s00702-019-02084-y
- Ressler, K. J., & Mayberg, H. S. (2007). Targeting abnormal neural circuits in mood and anxiety disorders: From the laboratory to the clinic. Nature Neuroscience , 10(9), 1116–1124. https://doi.org/10.1038/nn1944
- Robinson, E. S. J. (2018). Translational new approaches for investigating mood disorders in rodents and what they may reveal about the underlying neurobiology of major depressive disorder. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences , 373(1742), 20170036. https://doi.org/10.1098/rstb.2017.0036
- Rude, S. S., Wenzlaff, R. M., Gibbs, B., Vane, J., & Whitney, T. (2002). Negative processing biases predict subsequent depressive symptoms. Cognition and Emotion , 16(3), 423–440. https://doi.org/10.1080/02699930143000554
- Sahin, C., Doostdar, N., & Neill, J. C. (2016). Towards the development of improved tests for negative symptoms of schizophrenia in a validated animal model. Behavioural Brain Research , 312, 93–101. https://doi.org/10.1016/j.bbr.2016.06.021
- Sharot, T. (2011). The optimism bias. Current Biology , 21(23), R941–945. https://doi.org/10.1016/j.cub.2011.10.030
- Sharot, T., Korn, C. W., & Dolan, R. J. (2011). How unrealistic optimism is maintained in the face of reality. Nature Neuroscience , 14(11), 1475–1479. https://doi.org/10.1038/nn.2949
- Stuart, S. A., Butler, P., Munafo, M. R., Nutt, D. J., & Robinson, E. S. (2013). A translational rodent assay of affective biases in depression and antidepressant therapy. Neuropsychopharmacology , 38(9), 1625–1635. https://doi.org/10.1038/npp.2013.69
- Stuart, S. A., Butler, P., Munafo, M. R., Nutt, D. J., & Robinson, E. S. J. (2015). Distinct neuropsychological mechanisms may explain delayed- versus rapid-onset antidepressant efficacy. Neuropsychopharmacology , 40(9), 2165–2174. https://doi.org/10.1038/npp.2015.59
- Stuart, S. A., Hinchcliffe, J. K., & Robinson, E. S. J. (2019). Evidence that neuropsychological deficits following early life adversity may underlie vulnerability to depression. Neuropsychopharmacology , 44(9), 1623–1630. https://doi.org/10.1038/s41386-019-0388-6
- Stuart, S. A., & Robinson, E. S. (2015). Reducing the stress of drug administration: Implications for the 3Rs. Scientific Reports , 5, 14288. https://doi.org/10.1038/srep14288
- Stuart, S. A., Wood, C. M., & Robinson, E. S. J. (2017). Using the affective bias test to predict drug-induced negative affect: Implications for drug safety. British Journal of Pharmacology , 174(19), 3200–3210. https://doi.org/10.1111/bph.13972
- Tuli, J. S., Smith, J. A., & Morton, D. B. (1995). Stress measurements in mice after transportation. Laboratory Animals , 29(2), 132–138. https://doi.org/10.1258/002367795780740249
Key References
- Stuart et al. (2013). See above.
Validation of the affective bias test and reward learning assay, new learning studies
- Stuart et al. (2015). See above.
Validation of the affective bias test and reward learning assay, new learning and acute modulation of negative affective biases studies
- Hinchcliffe et al. (2017). See above.
Validation of the affective bias test and reward learning assay
- Stuart et al. (2017). See above.
Validation of the affective bias test and reward learning assay, new learning studies
- Robinson (2018). See above.
Validation of the affective bias test and reward learning assay
- Stuart et al. (2019). See above.
Validation of the affective bias test and reward learning assay, new learning studies
- Hinchcliffe et al. (2020). See above.
Affective biases in animal welfare studies
- Hinchcliffe, J. et al. (2022). See above.
Affective biases in animal welfare studies
- Hinchcliffe (2024). Rapid-acting antidepressant drugs modulate affective bias in rats. Science Translational Medicine , 16(729), eadi2403. https://doi.org/10.1126/scitranslmed.adi2403
Acute and sustained modulation of the negative affective biases, new learning studies and reward learning assay

