Stereotaxic Injections
Lindsay Meyerdirk, Michael Henderson
Abstract
This protocol details stereotaxic injections for any purpose, but in this case is primarily used for the injection of misfolded proteins.
Attachments
Steps
Preparing Mouse
Turn on the heating box so the pad starts warming.
Make sure surgery area is sterilized then set up entire station, leaving all sterilized tools covered until ready to start procedure.
Anaesthetize mouse per IACUC protocol, mouse weight and genetic background.
Once mouse has reached an anesthetic plane, shave the head up to between the eyes. When using isoflurane place the mouse back into the chamber until it no longer responds to a toe pinch.
Place the mouse head in the stereotaxic mount by placing the teeth in the bite bar and sliding the cone over the nose. Once the mouse is not responsive to toe pinch, place the ear bars into the ear canal of the mouse very carefully so as to not crack the bone or puncture the tympanic membrane.
Cover the eyes generously with eye ointment.
Place a sterile drape over the entire mouse, and cut a small hole where the incision will be.
Clean skull with sterile cottons swabs.
Place needle in holder with the bevel facing toward the front. Needle holder should have an alignment of 0.
Head Alignment
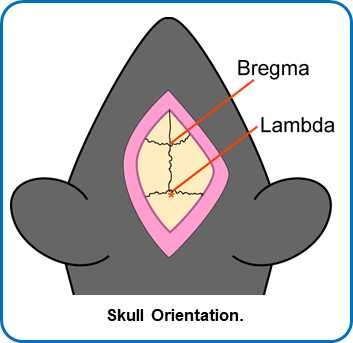
With needle directly over Bregma, write down anterior-posterior (A-P), left-right (L-R), dorsal-ventral (D-V) position.
Go to Lambda. Check the height here and level with Bregma to within 0.1 mm tolerance by raising or lowering the ear bars slowly and evenly.
Go 2 mm to the right of Bregma and record the D-V measure. Go 2 mm to the left of Bregma and record the D-V measure. Level the skull within a tolerance of 0.1 mm.
Injection
Move the needle far from the injection site.
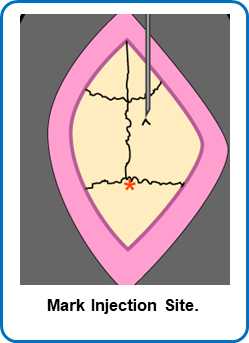
Drill a hole with a 28 g burr (slightly bigger than the injection needle).
Move the injection needle back into place. Carefully move the needle to the top of the dura, without puncturing the tissue.
Calculate how far down to go.
Lower needle to the site of injection.
Inject material at 0.4 μL/min.
Wait 3 minutes after the injection has finished
Raise needle out of the brain.
Use an iodine swab to moisten the edge of the incision site and tissue, careful not to get any iodine in the drilled hole. Suture the skin closed with 3-4 stitches. Each stitch has 3 knots. Front-to-back, back-to-front, front-to-back. Follow sutures with a small amount of VetBond to secure the wound together.
Post-operative Care
Place the mouse back into a clean cage on a warming pad and watch for the mouse to wake up.
Once awake place a Tylenol water bottle (1 mg/mL) on the cage for the next 3 days.
Monitor the mice once daily for open wounds or adverse side-effects, recording on the surgical card for 72h 0m 0s.
After day 3 remove the water bottle.