Pseudorabies Virus (PRV) injection into interscapular brown adipose tissue
Heike Muenzberg
Abstract
Pseudorabies Virus (PRV) injection into interscapular brown adipose tissue.
Before start
Institutional requirements before you start: - Approval of Institutional Biosafety Committee (IBC) to work with PRV.- Approval of Institutional Animal Care and Use Committee (IACUC) to perform PRV injections in animals.
Steps
Record the mouse’s body weight to prepare syringes for saline and analgesia treatment.Warm saline (1-1.5 ml for 25 g body weight); bupivacaine/lidocaine (2.5-12.5 mg/kg body weight); carprofen (5-10 mg/kg body weight).
Induce anesthesia with 5% isoflurane in the induction chamber.
Place the mouse on the surgery platform and maintain anesthesia via a nose cone at 1.5-2% Isoflurane/oxygen (adjust for each mouse accordingly to breathing and anesthetic depth (e.g. loss of withdrawal reflex)).
Apply eye lubricant to the mouse’s eyes.
Disinfect the lower back of the mouse with 70% ethanol prior to subcutaneous carprofen injection.
Check for full withdrawal reflex to ensure anesthetic depth.
Use a clipper to shave the interscapular region of the mouse and remove all hair.
Disinfect the skin by three alternating scrubs of Novalsan followed by 70% Isopropyl rubbing alcohol.
Make a midline incision with sharp scissors from the scapula to the neck.
Remove connective tissue underneath the skin incision using a sterile cotton swab and teethed forceps until the interscapular brown adipose tissue (iBAT) depot is fully accessible.
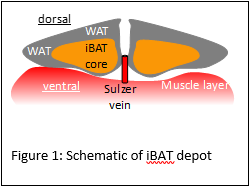
Cover the iBAT with a saline soaked sterile gauze to keep the tissue from drying, then prepare the Hamilton syringe for injection.
Fill the Hamilton syringe with 500nl PRV-152 virus, return to the surgery platform with the Hamilton syringe to begin the injections.
Remove the saline-soaked gauze from the brown adipose tissue and locate the brown adipose tissue pad.
Place injections (5x100nl) into the right BAT and hold the syringe in place for ~30 seconds after each injection to minimalize backflow, slowly pull the syringe out of the BAT while holding a sterile cotton swab on the site of injection to dry of any possible virus leakage.
Place sterile saline-soaked gauze on top of the brown adipose tissue and clean the syringe under the biological safety cabinet with 70% ethanol. Flush the syringe about 10x with 70% ethanol. After that flush the syringe about 10x with sterile H2O.
Remove the saline-soaked gauze from the brown adipose tissue and with a sterile cotton swab and teethed forceps, reposition the overlaying white adipose tissue back to its original location prior to your incision.
With the teethed and tissue forceps align both sides of the incision properly, and apply wound clips (~25 mm apart, to ensure proper blood circulation).
Inject bupivacaine/lidocaine subcutaneously around the incision site.
Inject warm saline intraperitoneal for rehydration.
Place the unconscious mouse in a fresh cage with approximately one half of the cage on a heating mat and monitor until startling reflexes are restored.
Use 70% ethanol to clean surgery tools and place in glass bead sterilizer for 15-20 seconds before additional surgeries.

