Simple, Fast, and Efficient Method for Derivation of Dermal Fibroblasts From Skin Biopsies
Grazia Iannello, Grazia Iannello, Achchhe Patel, Achchhe Patel, Dario Sirabella, Dario Sirabella, Alejandro Garcia Diaz, Alejandro Garcia Diaz, Benjamin N. Hoover, Benjamin N. Hoover, Hemanta Sarmah, Hemanta Sarmah, Barbara Corneo, Barbara Corneo
Abstract
Primary fibroblasts are a precious resource in the field of translational regenerative medicine. Dermal fibroblasts derived from human subject biopsies are being used as donor tissues for the derivation of patient-specific iPSC lines, which in turn are used for disease modeling, drug screening, tissue engineering, and cell transplantation. We developed a fast and simple protocol to grow dermal fibroblasts from skin biopsies. Using this protocol, we simply and firmly fix the biopsy piece on the surface of a tissue culture–treated plate and allow the fibroblasts to grow. This novel method eliminates any need for enzymatic digestion or mechanical dissociation of the biopsy piece. By using this newly developed protocol, we have successfully established around 100 fibroblast lines characterized by the expression of specific markers [Serpin H1 (Hsp-47), F-actin, and Vimentin]. Finally, we have used many of these fibroblast lines as donor tissues to successfully derive iPSC lines. We have developed a method that is simple, fast, convenient, efficient, and gentle on the cells to derive dermal fibroblasts from human skin biopsies. © 2023 The Authors. Current Protocols published by Wiley Periodicals LLC.
Basic Protocol : Skin biopsy collection and fibroblast derivation
Support Protocol 1 : Culturing, freezing, and thawing dermal fibroblasts derived from a skin biopsy
Support Protocol 2 : Characterization of dermal fibroblasts by immunocytochemistry
INTRODUCTION
Patient biopsies represent a unique source of human donor tissues for skin fibroblasts that can be used for several applications. Fibroblasts are functionally and morphologically heterogenous (Haydont et al., 2020; Haydont, Neiveyans, Zucchi, Fortunel, & Asselineau, 2019; Mahmoudi et al., 2019). Alterations in the composition and distribution of the various types of fibroblasts in the skin have been shown to be related to aging (Mine, Fortunel, Pageon, & Asselineau, 2008), with different responses to tissue repair and wound healing (Mahmoudi et al., 2019). Surface markers and high-throughput sequencing have been used to identify fibroblasts with different biological potentials (Huang et al., 2021), and recent work also identifies fibroblasts as mediators of regeneration in major organs (Gomes, Manuel, & Nascimento, 2021), thus highlighting their translational capability in regenerative medicine. Dermal fibroblasts obtained from skin biopsies may be used to study fibroblast-related diseases, such as tissue fibrosis or scarring (Jiang & Rinkevich, 2020), in wound healing (Talbott, Mascharak, Griffin, Wan, & Longaker, 2022), as well as to study mitochondrial impairment in neurological disorders (Olesen, Villavicencio-Tejo, & Quintanilla, 2022). Furthermore, fibroblasts may be used in case of a lack of accessibility to a specific tissue or cell type, as they can be directly converted in tissues such as myoblasts (Davis, Weintraub, & Lassar, 1987), neurons (Vierbuchen et al., 2010), osteoblast-like cells (Yamamoto et al., 2015), multi-lineage blood progenitors (Szabo et al., 2010), dopaminergic neurons (Pfisterer et al., 2011), and other tissues (for review, Xu, Du, & Deng, 2015) through transdifferentiation. Moreover, fibroblasts can be used as donor tissues for derivation of induced pluripotent stem cells (iPSCs) (Takahashi et al., 2007).
Several protocols for growing fibroblasts from biopsy samples have been published in the past years (Hybbinette, Bostrom, & Lindberg, 1999; Mak, Tewari, Tetrud, Langston, & Schule, 2011; Takashima, 2001; Vangipuram, Ting, Kim, Diaz, & Schule, 2013). These protocols either use enzymatic digestion or dissection of the biopsy to grow the fibroblast monolayer (Mak et al., 2011; Vangipuram et al., 2013; Villegas & McPhaul, 2005). However, these procedures have the disadvantage of adding multiple steps to the protocol, while also increasing the potential for contamination. Considering the value of patient biopsies, it is critical to minimize the risk of sample loss. Given the challenges mentioned above, developing new and improved protocols for growing and establishing fibroblast lines is crucial. Here, we describe an easy, fast, convenient, and efficient protocol developed in our laboratory for processing human biopsy samples and establishing fibroblast cultures that can be used for further experimental studies (Fig. 1 and Fig. 2). The present protocol was successfully used by our lab to generate many different fibroblast lines from donors of different ages, sexes, and disease statuses that were then reprogrammed to generate patient-specific iPSC lines (Baulier, Garcia Diaz, Corneo, & Farber, 2018; Patel, Garcia Diaz, Moore, Sirabella, & Corneo, 2020; Riera et al., 2019) plus other iPSC lines (distributed by Primed or the CSCI Stem Cell Core) using a Sendai-based reprogramming strategy (Cytotune 2.0, Life Technologies, Thermo Fisher Scientific) according to the manufacturer's instructions. The papers listed above describe in detail the reprogramming protocol and the characterization of the resulting iPSC lines.
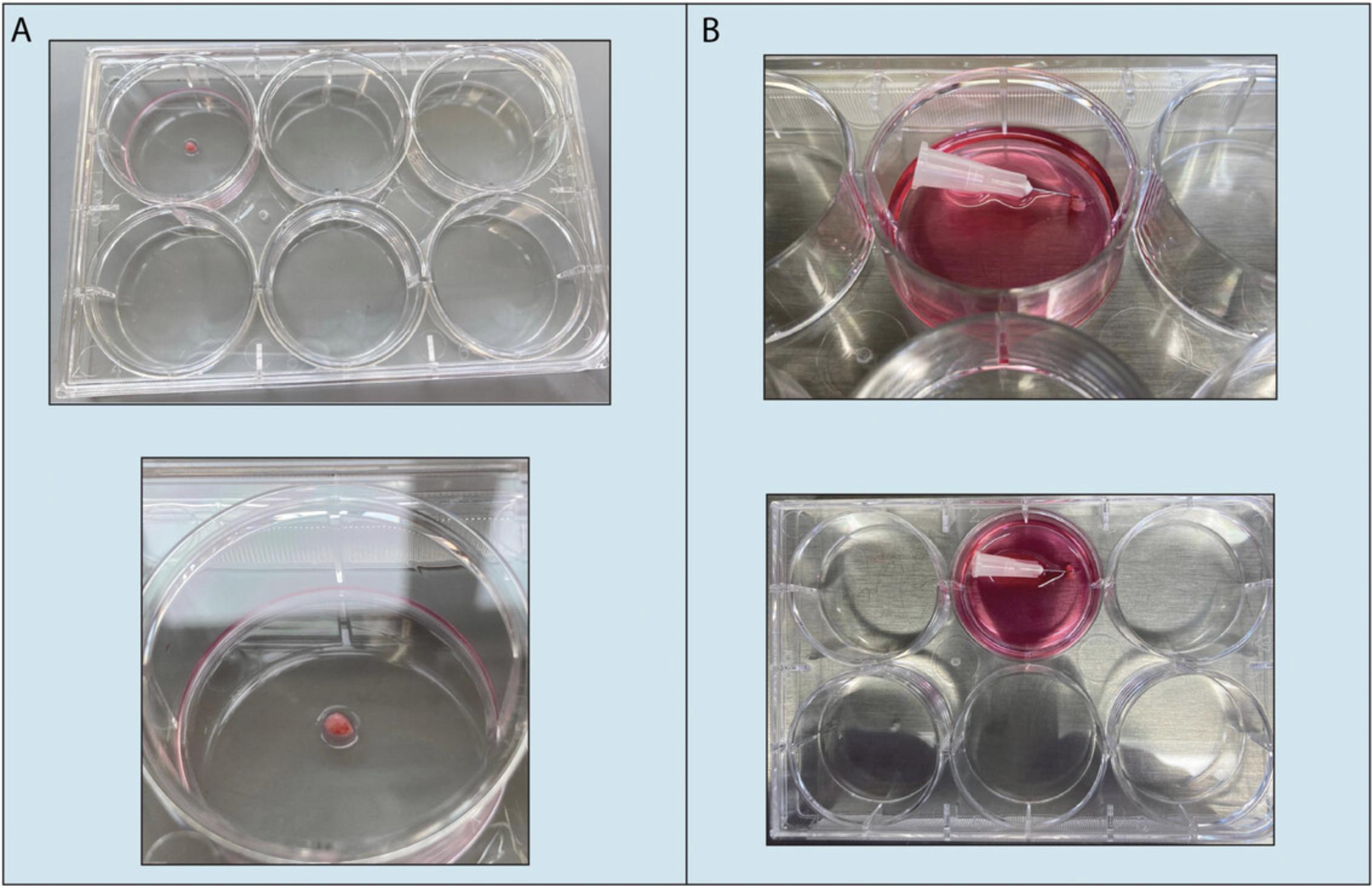
In terms of purity of the dermal fibroblasts isolated when using this protocol, because we do not separate the dermis and epidermis from the biopsy piece, growth of keratinocytes (originating from the epidermis) is often observed within the first few weeks of culture, but these cells disappear pretty fast due to the conventional medium we use, which, by only containing FBS, is formulated to support the growth and expansion of fibroblasts (versus for example using media containing supplements and growth factors supporting keratinocyte growth). Other cell types may not survive due to their low mitotic activity (Schuhmachers, Xu, Bergstresser, & Takashima, 1995). The resulting monolayer of dermal fibroblasts can be expanded by passaging with trypsin (in our hands, fibroblasts still showed the ability to divide up to passage five, but we did not try to passage more than P5). Moreover, the dermal fibroblasts obtained with this protocol express markers such as vimentin, Hsp-47, and F-actin (Table 1 and Fig. 3).
NOTE : All solutions and equipment coming into contact with cells must be sterile, and proper sterilizing techniques should be used accordingly. All work with cells should be performed in a biosafety cabinet.
Basic Protocol: SKIN BIOPSY COLLECTION AND FIBROBLAST DERIVATION
This protocol describes how to perform a skin biopsy on a patient and the different steps for obtaining a primary fibroblast cell line from the biopsy (Fig. 1 and Fig. 2). The method is straightforward and does not require the use of enzymatic digestion; moreover, fibroblast cultures can be easily established in less than a month. The operator needs to be trained in basic tissue culture techniques.
Time needed
Overall, the protocol requires 2 to 3 months: 30 to 60 min for performing the skin biopsy on a patient; 10 to 30 min to anchor the skin biopsy to the corner of the plate; 1 to 2 months to generate fibroblasts; 1 to 1.5 months to expand and freeze the established dermal fibroblasts.
Support Protocol 1: CULTURING, FREEZING, AND THAWING DERMAL FIBROBLASTS DERIVED FROM A SKIN BIOPSY
In this section, we describe how to expand the newly derived fibroblast line (passage 0) using enzymatic digestion. Once the cells reach 100% confluency, they can be passaged at 1:6 (1 confluent well of a 6-well plate into 6 wells of a 6-well plate) for further expansion (passage 1). When they reach confluency, cells can be trypsinized and collected to make frozen stocks for long-term storage in liquid nitrogen. Live or frozen cells can be used for further culturing (we have expanded these cells up to passage 5 only; as they are primary cultures, we try to minimize population doubling) and reprogramming.
Time needed
We need 1 hr to split the fibroblasts, 1 to 3 weeks to expand and make stocks, and 1 hr to thaw and plate frozen fibroblasts.
Support Protocol 2: CHARACTERIZATION OF DERMAL FIBROBLASTS BY IMMUNOCYTOCHEMISTRY
The dermal fibroblasts generated from the skin biopsy can be characterized by confirming the expression of specific markers such as Serpin H1 (Hsp-47), F-actin, and Vimentin using immunocytochemistry. Moreover, given the invaluable role of fibroblasts for downstream experiments (for example reprogramming), it is important to regularly test the newly generated cells for the absence of mycoplasma contamination.
Time needed
We need 2 days. On the first day, cells are fixed and incubated overnight with primary antibodies; on the second day, cells are incubated with the secondary antibody (when needed) and DAPI, and staining is visualized by microscopy. In parallel, cells are tested by PCR for absence of mycoplasma contamination (∼ 6 hr).
REAGENTS AND SOLUTIONS
Fibroblast medium (500 ml)
- 435 ml DMEM, high glucose (Life Technologies, cat. no. 11960077)
- 50 ml (10%) fetal bovine serum (FBS), heat inactivated (Life Technologies, cat. no. 10082147)
- 5 ml (1×) Penicillin-Streptomycin (100×, Life Technologies, cat. no. 15140122)
- 5 ml (1×) L-glutamine (100×, Life Technologies, cat. no. 25030081)
- 5 ml (1×) sodium pyruvate (100×, Life Technologies, cat. no. 11360070)
Filter the medium using a 0.2-µm 500-ml sterile disposable filter unit.
Freezing medium (10 ml)
- 5 ml (50%) DMEM, high glucose (Life Technologies, cat. no. 11960077)
- 4 ml (40%) fetal bovine serum (FBS), heat inactivated (Life Technologies, cat. no. 10082147)
- 1 ml (10%) dimethyl sulfoxide (DMSO, Hybri-Max, Sigma, cat. no. D2650)
Make sure the freezing medium is ice cold (can be stored in the fridge).
Troubleshooting
Table 2 is list common problems encountered during the various stages of operation of this protocol.
Acknowledgments
The authors would like to thank the fibroblast donors; Dr Neil Shneider, Dr Matthew Harms, and their team at the Eleanor and Lou Gehrig ALS Center at Columbia UniversityIrving Medical Center for performing the skin biopsies, including Jessica Singleton, previously in charge of consent forms and IRB approval, and Simone Norris for organizing the sample collection; all past members of the Stem Cell Core for their help and dedication; and all the users of the core. The authors acknowledge the support of the Columbia Stem Cell Initiative. Biorender.com was used for some of the figures.
Author Contributions
Grazia Iannello : conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, project administration, software, supervision, validation, visualization, writing original draft, writing review and editing; Achchhe Patel : conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, project administration, resources, supervision, validation, visualization, writing original draft, writing review and editing; Dario Sirabella : conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, project administration, resources, supervision, validation, visualization, writing review and editing; Alejandro Garcia Diaz : conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, project administration, resources, supervision, validation, visualization, writing review and editing; Benjamin N Hoover : data curation, resources, validation; Hemanta Sarmah : writing review and editing; Barbara Corneo : conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, project administration, resources, supervision, validation, visualization, writing original draft, writing review and editing
Conflict of Interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
Data sharing is not applicable to this article, as no new data were created or analyzed in this study.
Literature Cited
- Baulier, E., Garcia Diaz, A., Corneo, B., & Farber, D. B. (2018). Generation of a human Ocular Albinism type 1 iPSC line, SEIi001-A, with a mutation in GPR143. Stem Cell Research , 33, 274–277. doi: 10.1016/j.scr.2018.11.016
- Davis, R. L., Weintraub, H., & Lassar, A. B. (1987). Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell , 51, 987–1000. doi: 10.1016/0092-8674(87)90585-x
- Gomes, R. N., Manuel, F., & Nascimento, D. S. (2021). The bright side of fibroblasts: Molecular signature and regenerative cues in major organs. NPJ Regenerative Medicine , 6, 43. doi: 10.1038/s41536-021-00153-z
- Haydont, V., Neiveyans, V., Perez, P., Busson, E., Lataillade, J., Asselineau, D., & Fortunel, N. O. (2020). Fibroblasts from the human skin dermo-hypodermal junction are distinct from dermal papillary and reticular fibroblasts and from mesenchymal stem cells and exhibit a specific molecular profile related to extracellular matrix organization and modeling. Cells , 9, 368. doi: 10.3390/cells9020368
- Haydont, V., Neiveyans, V., Zucchi, H., Fortunel, N. O., & Asselineau, D. (2019). Genome-wide profiling of adult human papillary and reticular fibroblasts identifies ACAN, Col XI alpha1, and PSG1 as general biomarkers of dermis ageing, and KANK4 as an exemplary effector of papillary fibroblast ageing, related to contractility. Mechanisms of Ageing and Development , 177, 157–181. doi: 10.1016/j.mad.2018.06.003
- Huang, X., Khoong, Y., Han, C., Su, D., Ma, H., Gu, S., … Zan, T. (2021). Targeting dermal fibroblast subtypes in antifibrotic therapy: Surface marker as a cellular identity or a functional entity? Frontiers in Physiology , 12, 694605. doi: 10.3389/fphys.2021.694605
- Hybbinette, S., Bostrom, M., & Lindberg, K. (1999). Enzymatic dissociation of keratinocytes from human skin biopsies for in vitro cell propagation. Experimental Dermatology , 8, 30–38. doi: 10.1111/j.1600-0625.1999.tb00345.x
- Jiang, D., & Rinkevich, Y. (2020). Scars or regeneration?—Dermal fibroblasts as drivers of diverse skin wound responses. International Journal of Molecular Sciences , 21, 617. doi: 10.3390/ijms21020617
- Mahmoudi, S., Mancini, E., Xu, L., Moore, A., Jahanbani, F., Hebestreit, K., … Brunet, A. (2019). Heterogeneity in old fibroblasts is linked to variability in reprogramming and wound healing. Nature , 574, 553–558. doi: 10.1038/s41586-019-1658-5
- Mak, S. K., Tewari, D., Tetrud, J. W., Langston, J. W., & Schule, B. (2011). Mitochondrial dysfunction in skin fibroblasts from a Parkinson's disease patient with an alpha-synuclein triplication. Journal of Parkinson's Disease , 1, 175–183. doi: 10.3233/JPD-2011-11025
- Mine, S., Fortunel, N. O., Pageon, H., & Asselineau, D. (2008). Aging alters functionally human dermal papillary fibroblasts but not reticular fibroblasts: A new view of skin morphogenesis and aging. PLoS ONE , 3, 4066. doi: 10.1371/journal.pone.0004066
- Olesen, M. A., Villavicencio-Tejo, F., & Quintanilla, R. A. (2022). The use of fibroblasts as a valuable strategy for studying mitochondrial impairment in neurological disorders. Translational Neurodegeneration , 11, 36. doi: 10.1186/s40035-022-00308-y
- Patel, A., Garcia Diaz, A., Moore, J. C., Sirabella, D., & Corneo, B. (2020). Establishment and characterization of two iPSC lines derived from healthy controls. Stem Cell Research , 47, 101926. doi: 10.1016/j.scr.2020.101926
- Pfisterer, U., Kirkeby, A., Torper, O., Wood, J., Nelander, J., Dufour, A., … Parmar, M. (2011). Direct conversion of human fibroblasts to dopaminergic neurons. Proceedings of the National Academy of Sciences of the United States of America , 108, 10343–10348. doi: 10.1073/pnas.1105135108
- Riera, M., Patel, A., Corcostegui, B., Chang, S., Sparrow, J. R., Pomares, E., & Corneo, B. (2019). Establishment and characterization of an iPSC line (FRIMOi001-A) derived from a retinitis pigmentosa patient carrying PDE6A mutations. Stem Cell Research , 35, 101385. doi: 10.1016/j.scr.2019.101385
- Schuhmachers, G., Xu, S., Bergstresser, P. R., & Takashima, A. (1995). Identity and functional properties of novel skin-derived fibroblast lines (NS series) that support the growth of epidermal-derived dendritic cell lines. Journal of Investigative Dermatology , 105, 225–230. doi: 10.1111/1523-1747.ep12317512
- Szabo, E., Rampalli, S., Risueno, R. M., Schnerch, A., Mitchell, R., Fiebig-Comyn, A., … Bhatia, M. (2010). Direct conversion of human fibroblasts to multilineage blood progenitors. Nature , 468, 521–526. doi: 10.1038/nature09591
- Takahashi, K., Tanabe, K., Ohnuki, M., Narita, M., Ichisaka, T., Tomoda, K., & Yamanaka, S. (2007). Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell , 131, 861–872. doi: 10.1016/j.cell.2007.11.019
- Takashima, A. (2001). Establishment of fibroblast cultures. Current Protocols in Cell Biology , 00, 2.1.1–2.1.12. doi: 10.1002/0471143030.cb0201s00
- Talbott, H. E., Mascharak, S., Griffin, M., Wan, D. C., & Longaker, M. T. (2022). Wound healing, fibroblast heterogeneity, and fibrosis. Cell Stem Cell , 29, 1161–1180. doi: 10.1016/j.stem.2022.07.006
- Vangipuram, M., Ting, D., Kim, S., Diaz, R., & Schule, B. (2013). Skin punch biopsy explant culture for derivation of primary human fibroblasts. Journal of Visualized Experiments , 77, e3779. doi: 10.3791/3779
- Vierbuchen, T., Ostermeier, A., Pang, Z. P., Kokubu, Y., Sudhof, T. C., & Wernig, M. (2010). Direct conversion of fibroblasts to functional neurons by defined factors. Nature , 463, 1035–1041. doi: 10.1038/nature08797
- Villegas, J., & McPhaul, M. (2005). Establishment and culture of human skin fibroblasts. Current Protocols in Molecular Biology , 71, 28.3.1–28.3.9. doi: 10.1002/0471142727.mb2803s71
- Xu, J., Du, Y., & Deng, H. (2015). Direct lineage reprogramming: Strategies, mechanisms, and applications. Cell Stem Cell , 16, 119–134. doi: 10.1016/j.stem.2015.01.013
- Yamamoto, K., Kishida, T., Sato, Y., Nishioka, K., Ejima, A., Fujiwara, H., … Mazda, O. (2015). Direct conversion of human fibroblasts into functional osteoblasts by defined factors. Proceedings of the National Academy of Sciences of the United States of America , 112, 6152–6157. doi: 10.1073/pnas.1420713112
Citing Literature
Number of times cited according to CrossRef: 1
- Cecilia Evangelisti, Sherin Ramadan, Antonio Orlacchio, Emanuele Panza, Experimental Cell Models for Investigating Neurodegenerative Diseases, International Journal of Molecular Sciences, 10.3390/ijms25179747, 25 , 17, (9747), (2024).

