Tackling the Mouse-on-Mouse Problem in Cochlear Immunofluorescence: A Simple Double-Blocking Protocol for Immunofluorescent Labeling of Murine Cochlear Sections with Primary Mouse Antibodies
Mohamed Bassiouni, Mohamed Bassiouni, Katharina Stölzel, Katharina Stölzel, Alina Smorodchenko, Alina Smorodchenko, Heidi Olze, Heidi Olze, Agnieszka J. Szczepek, Agnieszka J. Szczepek
Abstract
The mouse is the most widely used animal model in hearing research. Immunohistochemistry and immunofluorescent staining of murine cochlear sections have, thus, remained a backbone of inner ear research. Since many primary antibodies are raised in mouse, the problem of “mouse-on-mouse” background arises due to the interaction between the anti-mouse secondary antibody and the native mouse immunoglobulins. Here, we describe the pattern of mouse-on-mouse background fluorescence in sections of the postnatal mouse cochlea. Furthermore, we describe a simple double-blocking immunofluorescence protocol to label mouse cochlear cryosections. The protocol contains a conventional blocking step with serum, and an additional blocking step with a commercially available anti-mouse IgG blocking reagent. This blocking technique virtually eliminates the “mouse-on-mouse” background in murine cochlear sections, while adding only a little time to the staining protocol. We provide detailed instructions and practical tips for tissue harvesting, processing, and immunofluorescence-labeling. Further protocol modifications are described, to shorten the duration of the protocol, based on the primary antibody incubation temperature. Finally, we demonstrate examples of immunofluorescence staining performed using different incubation times and various incubation temperatures with a commercially available mouse monoclonal primary antibody. © 2020 The Authors.
Basic Protocol : Tackling the Mouse-on-Mouse Problem in Cochlear Immunofluorescence: A Simple Double-Blocking Protocol for Immunofluorescent Labeling of Murine Cochlear Sections with Primary Mouse Antibodies
Basic Protocol:
The mouse is the most commonly utilized animal model in hearing research, mainly due to the readily available mouse genome sequence data, the ability to generate transgenic mice, and the relative similarity to the human cochlea (Santi, Rapson, & Voie, 2008). The detection of proteins in mouse cochlear sections has, therefore, remained an essential technique in inner ear research. Unfortunately, a high staining background results when using methods for indirect detection of murine proteins with primary antibodies raised in a mouse. This phenomenon has been termed the “mouse-on-mouse” background. It results from the binding of the anti-mouse secondary antibodies by the (Fc) and antigen-binding (Fab) fragments of the native mouse tissue immunoglobulin (Lu & Partridge, 1998). When using the indirect detection methods, the anti-mouse secondary antibody cannot distinguish the exogenous mouse primary antibodies from the native mouse immunoglobulins. This non-specific binding results in a high background signal that can obscure antigen detection, especially when labeling proteins in the cell membrane or extracellular matrix.
Mouse monoclonal primary antibodies are widely used in immunohistochemical and immunofluorescent staining of murine tissues, and several techniques were developed to eliminate the “mouse-on-mouse” background, such as using directly labeled antibodies (Hierck, Iperen, Gittenberger-De Groot, & Poelmann, 1994; Tse & Goldfarb, 1988), or blocking with Fab fragments (Nielsen, Borup-Christensen, Erb, Jensenius, & Husby, 1987; Yamashita & Korach, 1989). Numerous commercial staining kits have also been made available to detect mouse proteins using primary mouse antibodies. Unfortunately, no blocking method has been universally agreed upon, since the blocking protocol has to be optimized for each application. Indeed, previous studies have shown the “mouse-on-mouse” background to be tissue-specific, suggesting that immunolabeling protocols need to be adjusted for each tissue type (Lu & Partridge, 1998). The latter finding indicates that immunohistochemistry protocols, established on other mouse tissue types, may not always be appropriate to use on murine cochlear sections. Recent histological and ultrastructural studies have shown that a significant portion of the membranous cochlea is acellular and consists of extracellular matrix proteins (Santi & Johnson, 2013; Santi et al., 2016). Thus, the problem of “mouse-on-mouse” background becomes especially challenging when performing cochlear immunohistochemistry and immunofluorescence.
Here, we describe a simple double-blocking immunofluorescence protocol to label mouse cochlear cryosections, which virtually eliminates the “mouse-on-mouse” background. The protocol contains a conventional blocking step with serum, and an additional blocking step with a commercially available anti-mouse-IgG blocking reagent. This technique has been successfully used in recent work for protein detection in the murine cochlear cryosections (Bassiouni, Dos Santos, Avci, Lowenheim, & Muller, 2016). It significantly reduces the “mouse-on-mouse” background in murine cochlear sections while increasing only slightly the duration of the protocol.
Materials
-
Mice
-
2% paraformaldehyde (PFA; see recipe)
-
Phosphate buffered saline (PBS; see recipe), 1× Ethylenediaminetetraacetic acid (EDTA; see recipe)
-
30% sucrose (see recipe)
-
OCT medium (PolyFreeze cryoembedding medium; SHH0026, Sigma-Aldrich)
-
Citrate retrieval buffer (see recipe)
-
Triton-X (Sigma-Aldrich, cat. no. X100-100ML)
-
Normal goat serum (Jackson Dianova, cat. no. 005-000-121, Hamburg, Germany)
-
Anti-mouse-IgG blocking reagent (see recipe for mouse-on-mouse blocking solution)
-
Mouse anti- Tubulin β3 (TUJ1) (1:500 dilution; R&D Systems, cat. no. MAB1195)
-
Goat anti-mouse 488 (1:400 dilution; Invitrogen, ThermoFisher Scientific, cat. no. A-11001)
-
Antibody dilution solution (see recipe)
-
ProLong™ Gold Antifade Mountant with DAPI (Cell Signaling Technology, cat. no. 8961)
-
Surgical scissors and forceps (Fine Science Tools, CA, USA)
-
Eppendorf pipettes 1-10, 10-100, 20-200, and 100-1000 µl (Eppendorf, Hamburg, Germany)
-
Petri dishes (35 × 10 mm) (CELLSTAR, Greiner Bio-One GmbH, Solingen, Germany)
-
27-G syringe needles (BD Biosciences, Heidelberg, Germany)
-
15- and 50-ml Falcon tubes (Thermo Fisher Scientific, Germany)
-
Cryomolds
-
Cryostat (Leica CM3050, Leica Biosystems, Wetzlar, Germany)
-
Razor blade and paintbrushes for cryosectioning
-
Leica TCS SL confocal microscope (Leica Biosystems)
-
SuperFrost® glass slides (03-0060, Langenbrinck, Emmendingen, Germany)
-
Microscopic cover glasses (24 × 60 mm) (Langenbrinck, Emmendingen, Germany)
-
ImmEdge® Hydrophobic Barrier PAP Pen (H-4000, Vector Laboratories, Burlingame, CA, USA)
-
Microwave oven
-
Humidified chamber
-
Olympus BX51 epifluorescence microscope (Olympus Corp., Tokyo, Japan)
-
Slide immunostaining tray
-
DURAN® screw-cap lab bottles 100, 500, and 1000 ml (DWK Life Sciences GmbH, Wertheim, Germany).
Day 1: Tissue harvest, fixation, and cryoprotection
For early postnatal mice (between postnatal day p0 and p6)
1a. Sacrifice mice by quick decapitation with sharp scissors.
2a. Remove the skin with fine scissors and bisect the head vertically in the median sagittal plane. Proceed to step 6.
For mice p7 and older
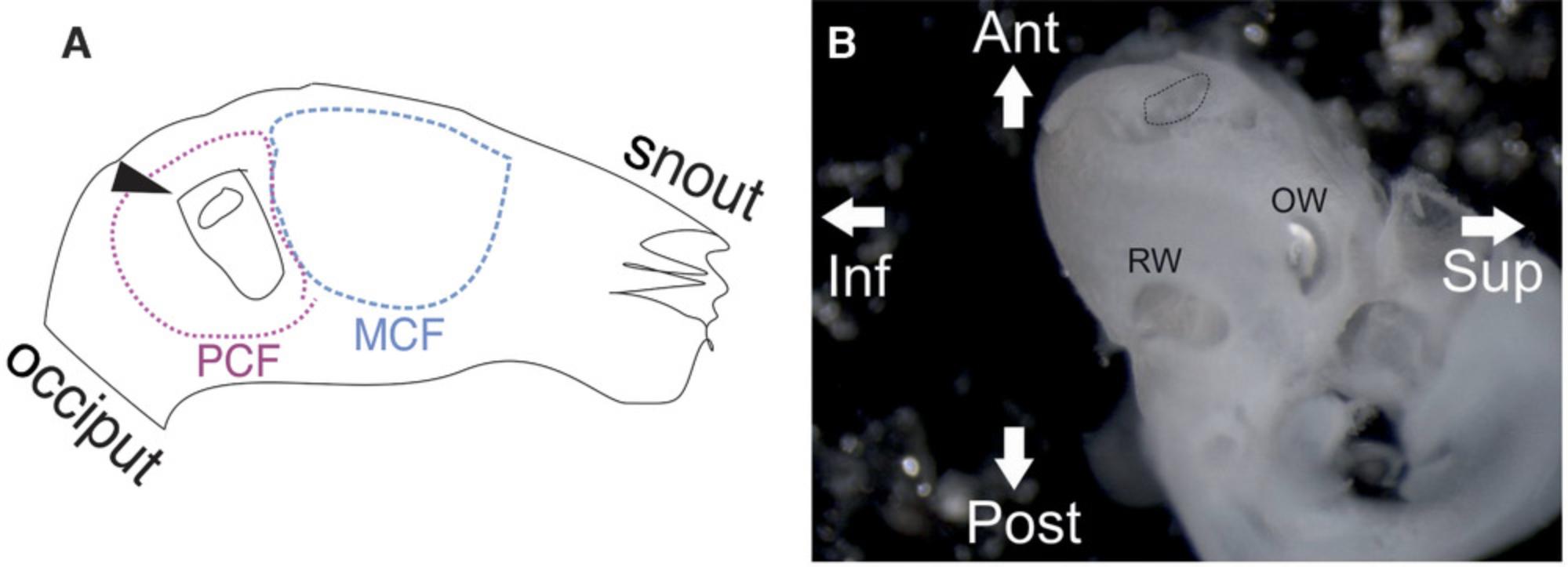
1b. Sacrifice mice by cervical dislocation under anesthesia, or any other method approved by the respective institutional ethics board. Perform micro-dissection of the bony inner ear out of the skull base (Fig. 1).
| Step | Problem | Troubleshooting |
|---|---|---|
| 1-2 | Insufficient quality of harvested tissue (e.g., broken cochlear bone or torn cochlear duct tissue) | Beginners should practice the micro-dissection and tissue harvesting rigorously. Refer to previously published protocols and video tutorials for the technique of harvesting and processing inner ear tissue (Akil & Lustig, 2013; Bako et al., 2015; Fang, Wu, Chai, & Sha, 2019; Haque, Pandey, Kelley, & Puligilla, 2015; Landegger, Dilwali, & Stankovic, 2017; Montgomery & Cox, 2016; Mulvaney & Dabdoub, 2014; Ogier, Burt, Drury, Lim, & Nayagam, 2019; Parker, Brugeaud, & Edge, 2010; Tung, Di Marco, Lim, Brichta, & Camp, 2013). |
| 5-6 | Poor antigenicity with good morphological preservation | Consider reducing tissue fixation or perform antigen retrieval . |
| 5-6 | Poor morphological preservation (e.g., degeneration of the organ of Corti or stria vascularis) | Consider stronger tissue fixation or better perfusion of the perilymphatic space with the fixative (in adult cochleae). |
| 9 | Fine morphology suboptimal (e.g., tears in the Reissner's membrane) | Instead of a single dehydration step with 30% sucrose, the specimens can be submerged in serial dilutions of sucrose for 1 hr of each step (5%, 10%, 20%, and then 30% sucrose) at RT, after which, the specimens are incubated overnight at 4°C. This serial dehydration is not necessary for embryonic and postnatal samples and may provide better morphological preservation only in the ossified fluid-filled adult cochlea. |
| 15-20 | Blurry morphology (tissue sections too thick) | Reduce slice thickness during cryosectioning. Excellent morphology can be obtained with 10-12-µm thick sections. |
| 15-20 | Sectioning artifacts (cuts and tears) | Change the cutting blade and the glass cover. If sectioning artifacts still persist, consider increasing the slice thickness. |
| 26 | Sections washing off during antigen retrieval | Adult cochlear sections tend to be washed out from the slides during the heat-induced antigen retrieval. One solution to this problem is to apply mechanical compression to the coverslipped slide during the antigen retrieval procedure, as described recently (Eckhard, O'Malley, Nadol, & Adams, 2019). |
| 26 | Hydrophobic pen barrier washing out after antigen retrieval | Using a surfactant such as Triton X-100 and the antigen retrieval may wash off the hydrophobic barrier around the sections. In such case, apply the hydrophobic pen again, before proceeding to the blocking step. |
| 28-37 | Specific antibody signal too weak (with otherwise good antigenicity) | Decrease the dilution of the primary and/or secondary antibody (i.e., higher concentration). Consider antigen retrieval. |
| 28-37 | High fluorescence background | Perform control experiments to determine the source of background. Mouse-on-mouse background shows a consistent pattern in the cochlea (Fig. 5), which should not be mistaken for non-specific binding. According to the underlying cause of the background, either increase the dilution of the secondary antibody or optimize blocking. Old secondary antibodies tend to aggregate and form precipitates, which may lead to high non-specific fluorescence. In this case, researchers may try centrifuging the antibody and recovering the supernatant. Otherwise, use a new lot of the antibody (or a new, unused frozen aliquot). |
| 28-37 | High mouse-on-mouse background | Usually, the double-blocking protocol strongly reduces the mouse-on-mouse background, which practically eliminates the problem for most antibody staining. If there is still a high mouse-on-mouse background after double-blocking, this usually means that the primary antibody signal is weak, since it shows a similar brightness to the mouse-on-mouse background. In this case, it may help to increase the concentration of the primary antibody or to perform antigen retrieval. |
| 28-37 | Sections dry out during the protocol | The slides should be incubated with a wet tissue paper in a humidified chamber. If sections frequently dry out, consider adding more antibody solution to each slide, to sufficiently immerse the slides. The only disadvantage to adding more solution is the cost of the antibodies. Ideally, a slide should be covered with at least 100 µl, and preferably 200 µl of the antibody solution. |
2b. Transfer the isolated inner ear samples into a culture dish with a pre-chilled 2% paraformaldehyde (PFA solution).
3b. Puncture the bony otic capsule at the cochlear apex in the region of the helicotrema, with fine forceps, to allow the drainage of the perilymph from the scala vestibuli and the scala tympani.
4b. Open the oval window by removing the footplate of the stapes, and then open the round window by perforating the round window membrane with a pair of sharp forceps. (Fig. 1)
5b. Perfuse the perilymphatic fluid spaces slowly with cold 2% PFA through the round and oval windows using a thin (27-G) needle syringe. Proceed to step 6.
6.Transfer the separated half-heads (animals p0-p7) or isolated inner ears (animals older than p7) into a 50-ml Falcon tube filled with paraformaldehyde (PFA) 4% solution for 2 hr at 4°C for tissue fixation.
7.Rinse the specimens at room temperature (RT) three times, each time in a 50-ml Falcon tube filled with 50 ml of 1× PBS for 2 min.
8.Decalcify the bony inner ear specimens of juvenile and adult mice (starting from p7) in 10% (w/v) EDTA in 1× PBS for 24 hr at 4°C.
9.Dehydrate the specimens in 30% sucrose in PBS overnight at 4°C, or until the specimens sink.
Day 2: Cryoembedding
10.Fill the cryomolds with the OCT medium (e.g., PolyFreeze cryoembedding medium), and transfer the specimens into the filled cryomolds.
- Orient the early postnatal half-head specimens, with the head (or half-head) lying horizontally flat on the bottom of the mold (resembling an axial cut). Usually, the postnatal heads (or half-heads) can be oriented by eye without the need for a microscope.
- Orient the adult inner ear specimens lying horizontally flat on the bottom of the mold, with the cochlear nerve facing down, such that the modiolus is mostly parallel to the surface of the mold. This orientation facilitates obtaining “mid-modiolar” sections during cryosectioning. Since the adult inner ear specimens are rather small, they should be placed in the correct orientation under the microscope.
11.Remove air bubbles manually with a pair of forceps. Alternatively, use vacuuming devices to remove the air bubbles. In our experience, the vacuuming step is not essential, especially not with the early postnatal (p0-p7) specimens.
12.Allow the specimen to settle in the OCT medium for 3-5 min at RT before freezing them. This step is crucial in the fluid-filled cochlea of adult animals.
13.Place the cryomolds at −80°C, where they can be stored for several months to years.
Day 3: Cryosectioning and immunostaining
14.Place the cryomolds in the cryostat for 30 min to adapt to the chamber temperature.
15.Adjust the chamber temperature to the desired temperature for each experiment
16.Remove the tissue blocks from the cryomolds by holding the sides of the molds while applying finger pressure from below the molds until the tissue blocks slip out. Apply OCT medium on the metal chucks, which fit into the fixed cooling units of the cryostat (only one tissue block can be placed on one chuck and processed at one time). This semisolid OCT medium acts as a “glue” to fix the tissue blocks onto the metal chucks. Quickly place the tissue block on the chuck before it freezes. After this extra “OCT glue” gets completely frozen, the tissue block should be firmly fixed to the metal chucks.
17.Proceed to section the block in a usual fashion, which may differ according to the cryostat being used. Typically, razor blades are used to trim excess OCT medium from the tissue blocks, and paintbrushes are used to scrape away excess OCT residues.
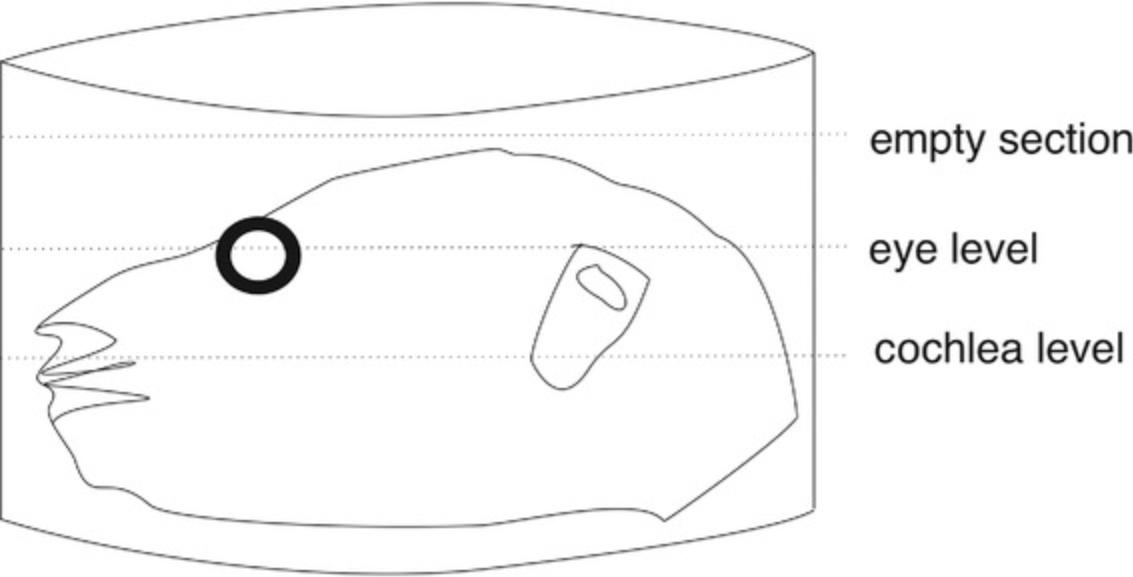
18.Start sectioning from the upper part of the half-head specimen by cutting “empty sections” containing only OCT medium (without any tissue) first.
19.Keep adjusting the sectioning conditions until homogeneous slices without cracks or tears are obtained, and then continue cutting (and discarding) thick sections in a “trim” mode, until reaching the eye, which can be easily macroscopically identified in the tissue block. The eye is positioned slightly higher than the cochlea; thus, it can serve as an orientation point for the position of the cochlea (Fig. 2). After reaching the eye, it is advisable to cut thin sections (12 µm) and examine them under a light microscope, until you have “usable” cochlear sections.
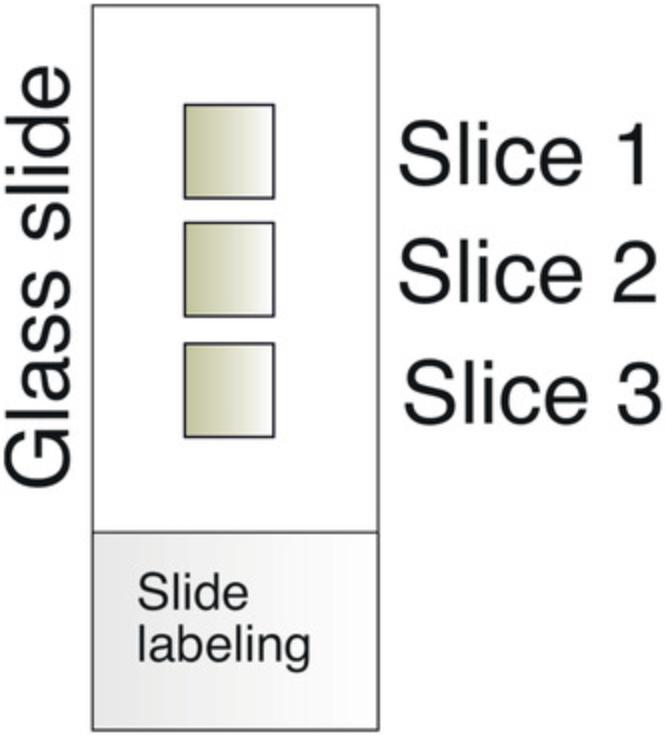
20.To collect a section onto the microscope slide, gently touch the room-temperature SuperFrost Plus™ slides to the frozen sections, allowing the tissue to stick to the slides. Each slide may hold three to five tissue slices (Fig. 3). In this manner, one mouse cochlea is usually collected in 10-12 slides. It is advisable to place one section on the first slide, then one on the second slide, and so on, until all the slides contain one tissue section, before returning to place the next section on the first slide. This procedure aims to make the slides as similar as possible to each other, which provides a better comparison between slides after staining and fluorescence microscopy.
21.Leave the slides to dry for 1 hr at RT.
22.Store the slides at –80°C in a standard slide container until immunostaining, if the staining will not start on the same day. The slide containers do not need to be airtight.
23.Air-dry the slides for 20 min at RT in a standard immunostaining tray (if they were previously stored at –80°C).
24.Circle the sections with a hydrophobic barrier pen (e.g., PAP Pen or ImmEdge® pen).
25.Rehydrate the sections with 1× PBS for 5 min at RT.
26.Perform heat-induced epitope retrieval (HIER), if needed.
27.After retrieval, wash each slide with 500 µl of 1× PBS for 5 min at RT. Aspirate the 1× PBS.
28.Add 200 µl of 0.1% Triton X-100 in 1× PBS onto each slide for 20 min at RT.
29.Perform the first blocking step with 200 µl 5% serum in 1× PBS (or 5% serum in 0.1% Triton X-100).
30.Wash three times, each time with 500 µl 1× PBS for 5 min at RT.
31.Perform the second blocking step using a commercially available anti-mouse-IgG blocking reagent for 1 hr at RT, according to the manufacturer's instructions.
32.Wash again three times, each time with 500 µl of 1× PBS for 5 min at RT.
33.Dilute the primary antibodies in 0.5% serum in PBS.
34.Incubate with the primary antibody overnight at 4°C in a humidified chamber. Place 100-200 µl of an antibody solution on each slide (depending on the number of sections on the slide).
Day 4: Secondary antibody incubation, mounting, and coverslipping
35.Wash three times, each time with 500 µl 1× PBS for 5 min at RT. These are the most critical washing steps.
36.Dilute the fluorescence-labeled secondary antibody (1:400) in antibody dilutions solution (0.5% serum in PBS). Incubate the slides with the secondary antibody solution for 1 hr at RT in the dark.
37.Wash again three times, each time with 200 µl 1× PBS for 5 min at RT.
38.Mount the slides in one to two drops of ProLong™ Gold Antifade Mountant (with DAPI) and cover them with glass coverslips.
39.Perform epifluorescence or confocal microscopy on the slides, after leaving the mounting medium to polymerize and dry for at least 2 hr at RT or, preferably, overnight at 4°C.
40.Store the slides up to a few weeks at 4°C.
REAGENTS AND SOLUTIONS
Antibody dilution solution
Prepare an antibody dilution solution (0.5% serum in 1× PBS). For five slides, add 5 µl serum to 995 µl of 1× PBS in a 1.5-ml or 2-ml Eppendorf tube then mix gently for 5 sec in a vortex mixer. Prepare fresh.
Citrate retrieval buffer
Add 15 ml of the antigen unmasking solution (citrate-based, Vector Laboratories, cat. no. H-3300) to 1600 ml ddH2O. Store the stock reagent up to 1 week at 4°C. The working solution should be prepared freshly for each immunostaining experiment.
EDTA solution, 10%
Add 50 g EDTA tetrasodium salt dihydrate to 500 ml of 1× PBS in a 500-ml or 1000-ml glass bottle, warm to 37°C and stir until the EDTA powder completely dissolves. Store EDTA powder at room temperature (RT). After preparation of 10% EDTA solution, store up to 12 months at 4°C.
Mouse-on-mouse blocking solution
Use the pipetting vessel delivered by the manufacturer (Vector Laboratories). Add two drops of the Anti-mouse-IgG blocking reagent (Vector Laboratories, cat. no. MKB-2213) to 2.5 ml of 1× PBS. This working solution should be prepared freshly for each immunostaining experiment. Store the stock reagent up to 1 week at 4°C.
Paraformaldehyde (PFA), 4%
ROTI®Histofix 4% solution (Carl Roth, cat. no. P087) is ready to use without modification. If a 2% PFA solution is desired, dilute 1:1 in 1× PBS (add 20 ml of 1× PBS to 20 ml ROTI®Histofix 4% solution in a 50-ml Falcon tube). Store at RT till use.
Phosphate-buffered saline (PBS), 1×
Add one tablet of PBS (Gibco®, cat. no. 11510546, Thermo Fisher Scientific, Darmstadt, Germany) to 500 ml ddH2O in a 500-ml or 1000-ml glass bottle. Store up to 3 months at 4°C.
Primary antibody solution
Dilute the primary antibody(ies) to the desired concentration in the antibody dilution solution. This solution should be freshly prepared for each immunostaining experiment. In the experiment illustrated in Figure 6, the TUJ1 antibody was diluted 1:500 in the antibody dilution solution.
Secondary antibody solution
Dilute the secondary antibody(ies) 1:400 in the antibody dilution solution. For example, add 2.5 µl of the secondary antibody to 997.5 µl of the antibody dilution solution to stain five slides.
Serum blocking solution
To stain five slides, prepare a 1000 µl solution (200 µl per slide). Add 50 µl serum to 950 µl of 0.1% Triton-X in PBS. In the examples shown in Figure 5 and Figure 6, normal goat serum (5%) was used.
Sucrose solution, 30%
Add 30 g sucrose (Merck-Millipore, Thermo Fisher Scientific) to 100 ml of 1× PBS in a 100-ml glass bottle, adjust the temperature to 37°C and stir until the sucrose powder completely dissolves. Store sucrose powder at room temperature (RT). After preparation of 30% sucrose solution, store at 4°C for up to 6 months.
Triton-X, 0.1%
Add 1 ml Triton-X onto a 1000 ml glass bottle filled with 999 ml 1× PBS. Store up to 6 months at 4°C.
COMMENTARY
Background Information
Previous studies have proposed various solutions to the mouse-on-mouse problem in immunohistochemistry. One solution is to use primary conjugated antibodies (Goodpaster & Randolph-Habecker, 2014; Hierck et al., 1994; Tse & Goldfarb, 1988). The main disadvantage of direct immunoglobulin with conjugated antibodies is the lower sensitivity, due to the large size of the resulting immune complexes (Goodpaster & Randolph-Habecker, 2014; Hierck et al., 1994; Tse & Goldfarb, 1988). Nevertheless, a recent modification of this approach produced excellent results, even allowing simultaneous labeling with multiple mouse primary antibodies (Goodpaster & Randolph-Habecker, 2014). An alternative approach is to block the binding of Fab fragments of the anti-mouse secondary antibodies to the tissue immunoglobulins (Nielsen et al., 1987; Yamashita & Korach, 1989). This approach, however, resulted only in a partial reduction of the “mouse-on-mouse” background, suggesting the possible contribution of Fc fragments (Lu & Partridge, 1998). Indeed, blocking both Fab and Fc fragments of the secondary antibody further reduced the background signal, and adding additional anti-Fc antibodies has dramatically reduced the background staining (Lu & Partridge, 1998).
It is important to note that those abovementioned mouse-on-mouse staining efforts, albeit generally successful, cannot be universally applied to all tissue types since the blocking protocol needs to be optimized for each application, protein, and tissue type (Lu & Partridge, 1998). To prevent incorrect interpretation of immunofluorescence signals in future studies, we describe the “mouse-on-mouse” non-specific fluorescence pattern in the murine cochlear sections. This undesired background fluorescence signal can be strongly reduced with a double-blocking protocol described here. Using this protocol allows reliable visualization of a target antigen in the mouse cochlea with primary mouse antibodies and indirect detection methods. It should be noted that the double-blocking protocol does not entirely abolish “mouse-on-mouse” background, and may, thus, be insufficient alone for the detection of some weakly-expressed antigens. In such a case, our protocol should be combined with other known blocking measures, or even with antigen retrieval. However, the main advantage of our protocol is its simplicity and flexibility, since it only adds one blocking step and a few washing steps to already-established staining protocols. Thus, this approach can be applied to different detection methods, including multicolor immunofluorescence. In our experience, the double-blocking protocol practically eliminates the problem of the “mouse-on-mouse” background in cochlear sections for the majority of antigens tested. We, therefore, propose this cost-effective and straightforward protocol for future studies using primary mouse antibodies for the immunostaining of the murine cochlea. The immunofluorescence protocol is summarized in Table 2.
| Solution | Incubation time | Repetitions | Temperature |
|---|---|---|---|
| 1× PBS | 20 min | RT | |
| Optional: heating in citrate buffer | 5 min | 90°C-95°C | |
| 1× PBS | 20 min | RT | |
| 0.1% Triton-X in 1× PBS | 20 min | RT | |
| 1× PBS | 5 min | ×3 | RT |
| 5% Serum | 30 min | RT | |
| 1× PBS | 5 min | ×3 | RT |
| Anti-mouse-IgG blocking reagent | 60 min | RT | |
| 1× PBS | 5 min | ×3 | RT |
| Primary antibody(ies) | Overnight | 4°C | |
| 1× PBS | 5 min | ×3 | RT |
| Secondary antibody(ies) | 60 min | RT | |
| 1× PBS | 5 min | ×3 | RT |
| Incubate in ProLong™ Gold with DAPI | – | RT |
Critical Parameters
- Researchers should follow their local and institutional ethical guidelines concerning animal sacrifice for scientific purposes.
Tissue harvest
Immediately after early postnatal mouse sacrifice, there are several possible ways for tissue processing. Researchers may harvest whole heads of mice up to p7, or half-heads. We recommend processing half-heads, instead of the entire heads. Harvesting half-heads doubles the number of stored samples but reduces the specimen size, which is advantageous during cryosectioning. Some researchers remove the brain tissue from the half-head specimens before fixation, leaving empty half-skulls with the temporal bones, including the inner ear. We recommend not to remove the brain tissue from the half-head specimens during early tissue processing. After cryosectioning and immunostaining, the brain tissue may serve as an internal positive control for some antibodies on the same slide. The same principle applies to the eye tissue, since many proteins characteristic of sensory systems, are also expressed in the retina. The researchers should choose the samples for tissue processing, according to the experimental setup and the research questions.
Tissue fixation
There is a compromise between morphological preservation and antigenicity, concerning the tissue fixation. The most commonly used fixatives in mouse cochlear research belong to the group of aldehyde fixatives, such as formaldehyde/paraformaldehyde. During fixation, protein cross-linking may cause masking of some antigens. Should that happen, an antigen retrieval step is necessary. Too strong tissue fixation may compromise the specimen antigenicity, whereas too weak fixation may compromise the tissue morphology. In our experience, tissue fixation with 4% PFA for 2 hr (at 4°C) is suitable for the vast majority of antigens. However, for a small group of antigens that are especially susceptible to cross-linking, 2% PFA for 2 hr (at 4°C) results in better preservation of antigenicity (especially if no antigen retrieval is performed), with excellent preservation of gross morphology. Thus, unless the investigated antigen is particularly sensitive to fixation and cross-linking, we recommend routine fixation of the postnatal and adult mouse cochleae in 4% PFA for 2 hr at 4°C. We found that, for mice cochlear tissues, it is not necessary to use transcardiac, or whole-body perfusion with formaldehyde.
Thickness of cryosection
Slice thickness depends on the application, tissue type, and fixation. As a general rule, thicker sections are obtained after cryoembedding compared to other methods, such as paraffin embedding. Too thin sections often have mechanical sectioning artifacts and poor morphology. Sections that are too thick display blurry morphology due to the three-dimensional effect of having multiple cell layers on the same tissue slice. An ideal section would contain only one layer of cells, corresponding to about 5 µm thickness. In our experience, it is not possible to obtain mouse cochlear cryosections that thin, while preserving proper morphology. For most qualitative applications, especially if using a confocal microscope, a slice thickness of 10-12 µm has shown to be the most suitable.
Troubleshooting
Tips for troubleshooting the protocol are given in Table 1.
Understanding the Results
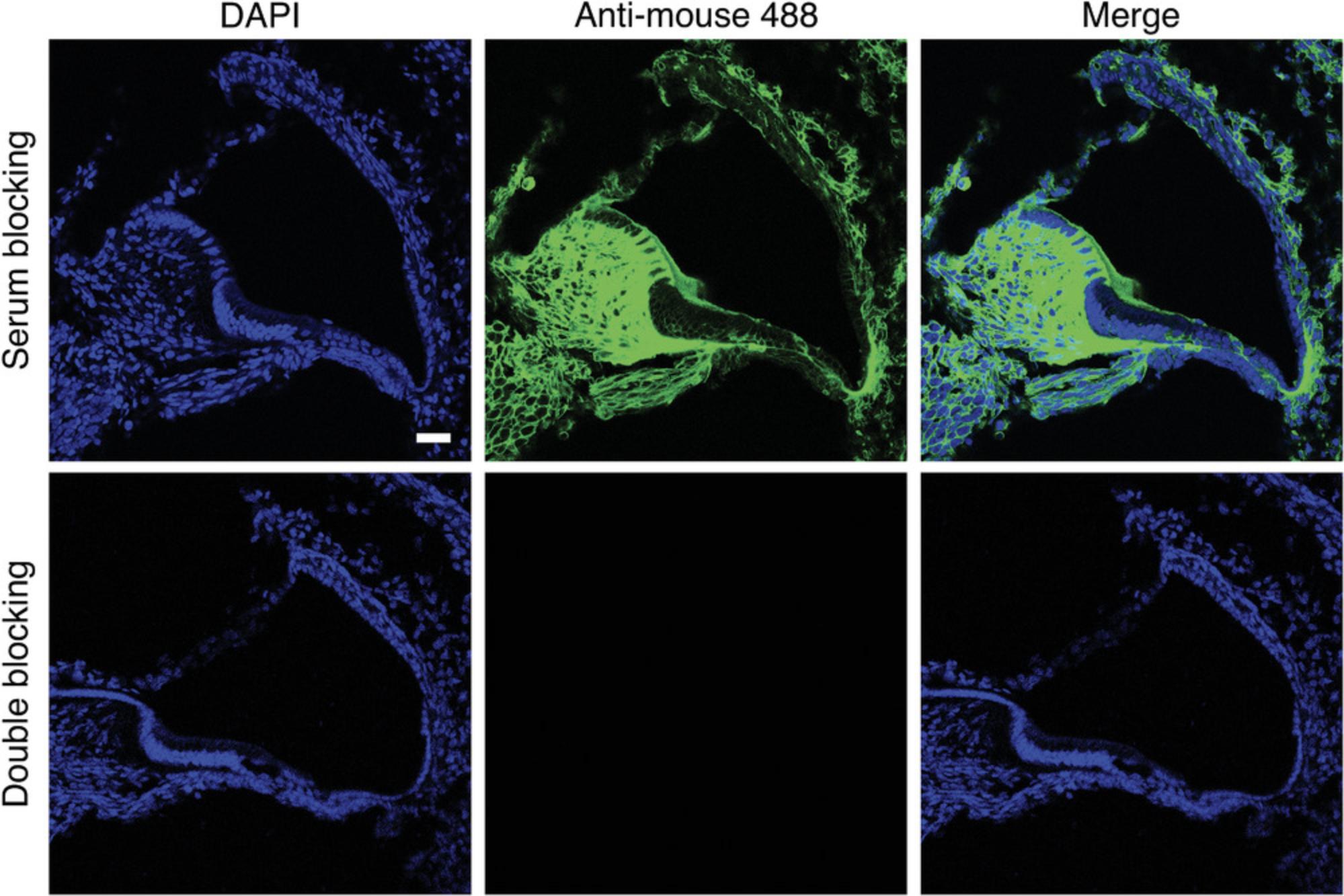
The labeling of murine cochlear sections with the anti-mouse secondary antibody alone allows determination of the extent and pattern of the “mouse-on-mouse” background. Blocking is performed either with serum alone or using the double-blocking protocol described above. To determine the pattern of a non-specific “mouse-on-mouse” background staining, we incubated both groups of sections with a secondary antibody directed against the primary antibody and conjugated with Alexa-488, but without a primary antibody. Sections that were blocked with serum alone showed a strong fluorescence signal in the spiral limbus, basilar membrane and spiral ligament, and a moderate signal in the spiral lamina and intercellular borders (Fig. 5). This background signal may mask or completely obscure the detection of antigens located in the cytoplasm or cell membrane with a specific primary antibody and is especially problematic for antibodies directed against extracellular matrix proteins and collagens. Sections stained according to the double-blocking protocol have a substantial reduction or even abolishment of the background fluorescence in all regions (Fig. 5). This finding suggests that the double-blocking protocol practically eliminates the problem of the non-specific signal produced by the interaction of the secondary antibody and the native mouse immunoglobulins.
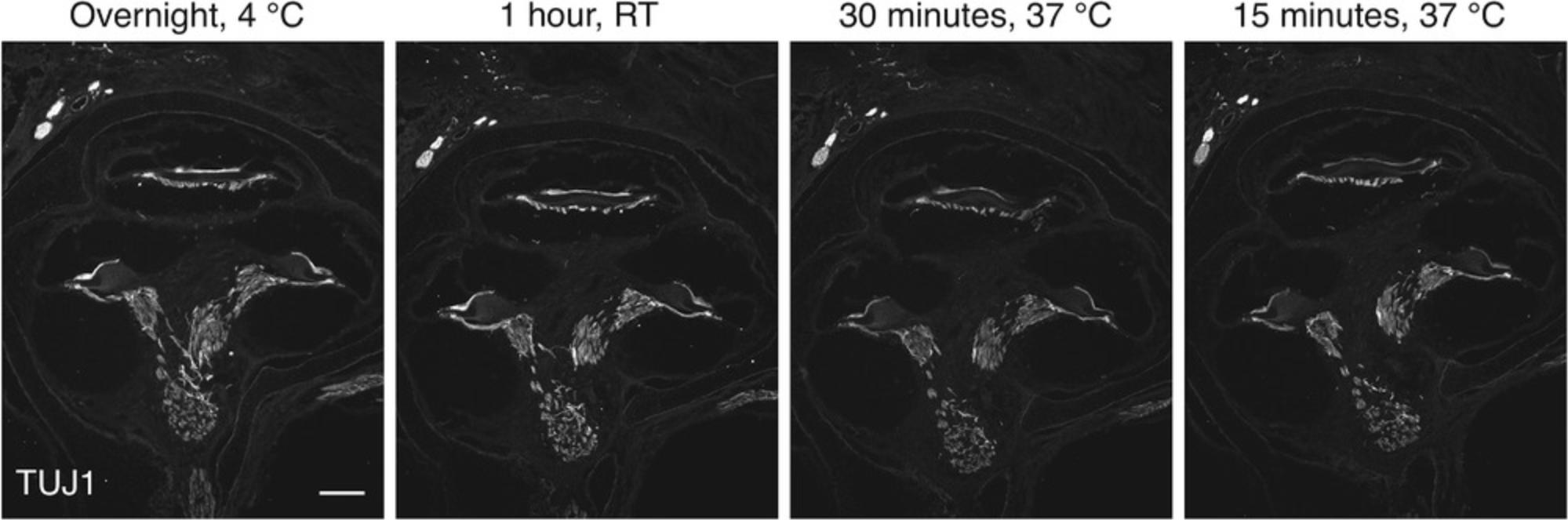
To demonstrate the applicability of the double-blocking protocol in the protein detection using mouse cochlear sections, we used a commercially available primary mouse antibody (TUJ1 clone) to detect β3-tubulin, a neural marker expressed in spiral ganglion neurons and nerve fibers (Radde-Gallwitz et al., 2004). We applied various incubation times and temperatures. In all experimental settings, β3-tubulin staining resulted in cytoplasmic labeling of the auditory nerve fibers and spiral ganglion neurons, consistent with its known expression pattern (Fig. 6). Interestingly, the traditional incubation conditions (overnight at 4°C) produced identical results as the incubation for 1 hr at RT. Furthermore, a similar staining pattern appeared after incubation at 37°C for 30 min on an orbital shaker with incubating function (Fig. 4D), and even for as short as 15 min. The findings suggest that the double-blocking protocol can be reliably used to localize antigens in mouse cochlear cryosections using primary mouse antibodies and immunofluorescence detection. The protocol is flexible and amenable to individual modification of incubation times and steps. Thus, it can be considerably shortened to account for the time taken by the extra blocking step.
Time Considerations
The presented protocol takes 4 days from animal sacrifice to microscopic analysis. There is some flexibility to this protocol concerning the number of required days. The protocol duration can be extended to 5 days if it is not convenient to start the immunostaining on the same day as the cryosectioning. In the event of time pressure, deadlines, or other necessary reasons, the protocol can also be reduced to 3 days, if cryosectioning is started only a few hours after cryoembedding (on the same day).
The duration of the immunofluorescence protocol can also be shortened according to the incubation time of the primary antibody. For most primary antibodies, an incubation at RT for 1 hr produces a signal comparable to that obtained after overnight incubation at 4°C. If a shorter incubation is desired, an incubation time of 30 min (and even as short as 15 min) is possible, with the incubation temperature raised to 37°C (Fig. 6).
Generally, an experienced researcher can perform immunostaining of 12 slides or more in parallel. Immunostaining using more than 20 slides in a single experiment is typically inconvenient and counterproductive unless some steps are performed in an automated fashion.
Acknowledgment
The authors would like to thank Prof. Victor Tarabykin (Charité Institute of Cell Biology and Neurobiology), for kindly providing access to the cryostat. Open access funding enabled and organized by Projekt DEAL. This study was financially supported by intramural research funding of Charité - Universitätsmedizin Berlin to AJS.
Conflict of Interest Statement
The authors declare no potential conflict of interest.
Author Contributions
Mohamed Bassiouni : Conceptualization; data curation; formal analysis; investigation; methodology; visualization; writing-original draft; writing-review & editing. Katharina Stölzel : Formal analysis; project administration; validation; writing-review & editing. Alina Smorodchenko : Formal analysis; investigation; methodology; validation; writing-review & editing. Heidi Olze : Formal analysis; project administration; resources; supervision; writing-review & editing. Agnieszka J. Szczepek : Conceptualization; data curation; formal analysis; funding acquisition; methodology; project administration; resources; supervision; validation; writing-original draft; writing-review & editing.
Literature Cited
- Akil, O., & Lustig, L. R. (2013). Mouse cochlear whole mount immunofluorescence. Bio Protocol , 3(5), e332. doi: 10.21769/BioProtoc.332.
- Bako, P., Bassiouni, M., Eckhard, A., Gerlinger, I., Frick, C., Löwenheim, H., & Müller, M. (2015). Methyl methacrylate embedding to study the morphology and immunohistochemistry of adult guinea pig and mouse cochleae. Journal of Neuroscience Methods , 254, 86–93. doi: 10.1016/j.jneumeth.2015.07.017.
- Bassiouni, M., Dos Santos, A., Avci, H. X., Lowenheim, H., & Muller, M. (2016). Bmi1 loss in the organ of corti results in p16ink4a upregulation and reduced cell proliferation of otic progenitors in vitro. Plos One , 11, e0164579. doi: 10.1371/journal.pone.0164579.
- Eckhard, A. H., O'Malley, J. T., Nadol, J. B., Jr., & Adams, J. C. (2019). Mechanical compression of coverslipped tissue sections during heat-induced antigen retrieval prevents section detachment and preserves tissue morphology. Journal of Histochemistry and Cytochemistry , 67, 441–452. doi: 10.1369/0022155419826940.
- Fang, Q. J., Wu, F., Chai, R., & Sha, S. H. (2019). Cochlear surface preparation in the adult mouse. Journal of visualized experiments: JoVE , doi: 10.3791/60299.
- Goodpaster, T., & Randolph-Habecker, J. (2014). A flexible mouse-on-mouse immunohistochemical staining technique adaptable to biotin-free reagents, immunofluorescence, and multiple antibody staining. Journal of Histochemistry and Cytochemistry , 62, 197–204. doi: 10.1369/0022155413511620.
- Haque, K. D., Pandey, A. K., Kelley, M. W., & Puligilla, C. (2015). Culture of embryonic mouse cochlear explants and gene transfer by electroporation. Journal of visualized experiments: JoVE , 52260.
- Hierck, B. P., Iperen, L. V., Gittenberger-De Groot, A. C., & Poelmann, R. E. (1994). Modified indirect immunodetection allows study of murine tissue with mouse monoclonal antibodies. Journal of Histochemistry and Cytochemistry , 42, 1499–1502. doi: 10.1177/42.11.7930532.
- Landegger, L. D., Dilwali, S., & Stankovic, K. M. (2017). Neonatal Murine cochlear explant technique as an in vitro screening tool in hearing research. Journal of visualized experiments: JoVE , (124), 55704.
- Lu, Q. L., & Partridge, T. A. (1998). A new blocking method for application of murine monoclonal antibody to mouse tissue sections. Journal of Histochemistry and Cytochemistry , 46, 977–984. doi: 10.1177/002215549804600813.
- Montgomery, S. C., & Cox, B. C. (2016). Whole Mount dissection and immunofluorescence of the adult mouse cochlea. Journal of visualized experiments: JoVE , (107), 53561.
- Mulvaney, J. F., & Dabdoub, A. (2014). Long-term time lapse imaging of mouse cochlear explants. Journal of visualized experiments: JoVE , e52101.
- Nielsen, B., Borup-Christensen, P., Erb, K., Jensenius, J. C., & Husby, S. (1987). A method for the blocking of endogenous immunoglobulin on frozen tissue sections in the screening of human hybridoma antibody in culture supernatants. Hybridoma , 6, 103–109. doi: 10.1089/hyb.1987.6.103.
- Ogier, J. M., Burt, R. A., Drury, H. R., Lim, R., & Nayagam, B. A. (2019). Organotypic culture of neonatal Murine inner ear explants. Frontiers in Cellular Neuroscience , 13, 170. doi: 10.3389/fncel.2019.00170.
- Parker, M., Brugeaud, A., & Edge, A. S. (2010). Primary culture and plasmid electroporation of the murine organ of Corti’, Journal of visualized experiments: JoVE , (36), 1685.
- Radde-Gallwitz, K., Pan, L., Gan, L., Lin, X., Segil, N., & Chen, P. (2004). Expression of Islet1 marks the sensory and neuronal lineages in the mammalian inner ear. Journal of Comparative Neurology , 477, 412–421. doi: 10.1002/cne.20257.
- Sakamoto, A., Kuroda, Y., Kanzaki, S., & Matsuo, K. (2017). Dissection of the Auditory Bulla in postnatal mice: Isolation of the middle ear bones and histological analysis. Journal of visualized experiments: JoVE , (119), 55054.
- Santi, P. A., Aldaya, R., Brown, A., Johnson, S., Stromback, T., Cureoglu, S., & Rask-Andersen, H. (2016). Scanning electron microscopic examination of the extracellular matrix in the decellularized mouse and human cochlea. Journal of the Association for Research in Otolaryngology , 17, 159–171. doi: 10.1007/s10162-016-0562-z.
- Santi, P. A., & Johnson, S. B. (2013). Decellularized ear tissues as scaffolds for stem cell differentiation. Journal of the Association for Research in Otolaryngology , 14, 3–15. doi: 10.1007/s10162-012-0355-y.
- Santi, P. A., Rapson, I., & Voie, A. (2008). Development of the mouse cochlea database (MCD). Hearing Research , 243, 11–17. doi: 10.1016/j.heares.2008.04.014.
- Tse, J., & Goldfarb, S. (1988). Immunohistochemical demonstration of estrophilin in mouse tissues using a biotinylated monoclonal antibody. Journal of Histochemistry and Cytochemistry , 36, 1527–1531. doi: 10.1177/36.12.3057073.
- Tung, V. W., Di Marco, S., Lim, R., Brichta, A. M., & Camp, A. J. (2013). An isolated semi-intact preparation of the mouse vestibular sensory epithelium for electrophysiology and high-resolution two-photon microscopy. Journal of visualized experiments: JoVE , e50471.
- Yamashita, S., & Korach, K. S. (1989). A modified immunohistochemical procedure for the detection of estrogen receptor in mouse tissues. Histochemistry , 90, 325–330. doi: 10.1007/BF00508308.
Citing Literature
Number of times cited according to CrossRef: 1
- Mohamed Bassiouni, Alina Smorodchenko, Heidi Olze, Agnieszka J. Szczepek, Identification and Characterization of TMEM119-Positive Cells in the Postnatal and Adult Murine Cochlea, Brain Sciences, 10.3390/brainsci13030516, 13 , 3, (516), (2023).

