Isolation and Micromass Culturing of Primary Chicken Chondroprogenitor Cells for Cartilage Regeneration
Roland Takács, Roland Takács, Tamás Juhász, Tamás Juhász, Csaba Matta, Csaba Matta, Éva Katona, Éva Katona, Csilla Somogyi, Csilla Somogyi, Judit Vágó, Judit Vágó, Tibor Hajdú, Tibor Hajdú, Krisztina Bíróné Barna, Krisztina Bíróné Barna, Péter Nagy, Péter Nagy, Róza Zákány, Róza Zákány
Abstract
Much of the skeletal system develops by endochondral ossification, a process that takes place in early fetal life. This makes the early stages of chondrogenesis, i.e., when chondroprogenitor mesenchymal cells differentiate to chondroblasts, challenging to study in vivo. In vitro methods for the study of chondrogenic differentiation have been available for some time. There is currently high interest in developing fine-tuned methodology that would allow chondrogenic cells to rebuild articular cartilage and restore joint functionality. The micromass culture system that relies on embryonic limb bud-derived chondroprogenitor cells is a popular method for the study of the signaling pathways that control the formation and maturation of cartilage. In this protocol, we describe a technique fine-tuned in our laboratory for culturing limb bud-derived mesenchymal cells from early-stage chick embryos in high density (Basic Protocol 1). We also provide a fine-tuned method for high-efficiency transient transfection of cells before plating using electroporation (Basic Protocol 2). In addition, protocols for histochemical detection of cartilage extracellular matrix using dimethyl methylene blue, Alcian blue, and safranin O are also provided (Basic Protocol 3 and Alternate Protocols 1 and 2, respectively). Finally, a step-by-step guide on a cell viability/proliferation assay using MTT reagent is also described (Basic Protocol 4). © 2023 The Authors. Current Protocols published by Wiley Periodicals LLC.
Basic Protocol 1 : Micromass culture of chick embryonic limb bud-derived cells
Basic Protocol 2 : Transfection of cells with siRNA constructs using electroporation prior to micromass culturing
Basic Protocol 3 : Qualitative and quantitative assessment of cartilage matrix production using dimethyl methylene blue staining and image analysis
Alternate Protocol 1 : Qualitative assessment of cartilage matrix production using Alcian blue staining
Alternate Protocol 2 : Qualitative assessment of cartilage matrix production using safranin O staining
Basic Protocol 4 : Measurement of mitochondrial activity with the MTT assay
INTRODUCTION
In synovial joints, the articulating bone surfaces are covered by a layer of hyaline cartilage offering smooth and frictionless movement during locomotion. The dominant cell type in articular cartilage is the chondrocyte, which secretes an abundant extracellular matrix (ECM) with a unique architecture consisting of a high amount of water and a dense mixture of macromolecules, such as type II collagen, aggrecan, other matrix constituents (e.g., hyaluronan). Unlike the other connective tissue types, cartilage is avascular and receives oxygen and nutrients by diffusion. For articular cartilage, its main source is synovial fluid. Mature articular chondrocytes are therefore characterized by a low metabolic rate and anaerobic energy metabolism (Goldring, 2006).
The loss of articular cartilage due to trauma, degeneration or inflammation is one of the most prevalent challenges of orthopedics, as this can develop into osteoarthritis (OA). The clinical symptoms of OA include pain, stiffness, and restricted motion of joints, impairing the physical activity and wellbeing of the patient. Given that mature articular cartilage has very limited capacity for repair, the chondrocyte is one of the main targets of regenerative medicine to enhance pathways potentially restoring the cartilage ECM (Solanki et al., 2021). Articular cartilage develops early during ontogenesis, making it challenging to study the process in detail in a laboratory setting (Cancedda et al., 2000). A better understanding of the molecular mechanisms which lead to the differentiation of hyaline cartilage in the limb mesenchyme may help us develop more efficient regenerative approaches and enhance the healing response of articular cartilage.
A well-accepted in vitro model to recapitulate embryonic chondrogenesis is the high density micromass cell culture established from embryonic limb buds (Ahrens et al., 1977; Takacs et al., 2023). Limb bud-derived chondrogenic progenitor cells aggregate and then spontaneously differentiate into chondroblasts and chondrocytes, which produce hyaline cartilage ECM in micromass cell cultures in vitro (Fig. 1. steps of chondrogenesis). A high initial in vitro seeding density (1.5-2 × 107 cells/ml) of the chondroprogenitor cells is indispensable to mimic the condensation phase of early skeletogenesis that happens in vivo. The high seeding density creates a microenvironment which supports chondrogenesis in the micromass cultures and prevents differentiation of the other progenitor cells, such as epithelial or muscle progenitors. In chicken embryo-derived micromass cultures, overt chondrogenesis is completed in 6 days in culture (Takacs et al., 2023).
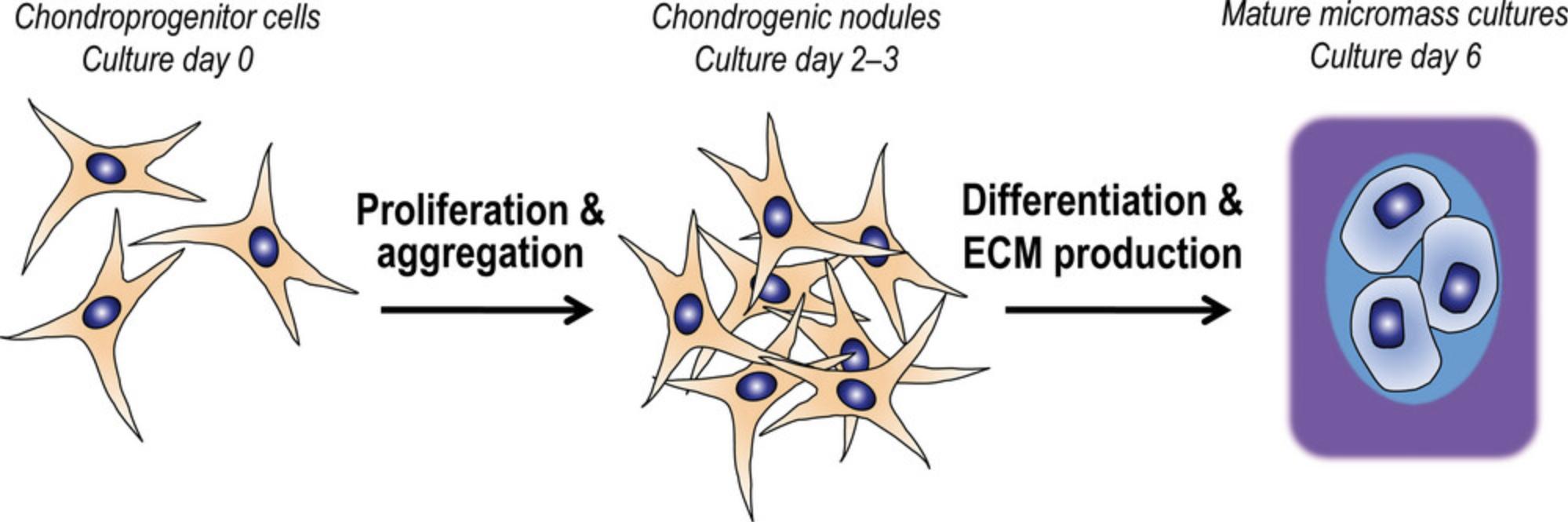
This method provides an opportunity to apply various chemical, physical, or biological interventions at different stages of primary chondrogenic differentiation. Staining of the differentiating or mature micromass cell cultures using dyes (such as dimethyl methylene blue, Alcian blue, and safranin O) that visualize the cartilage ECM, which is particularly rich in highly negatively charged glycosaminoglycans by metachromasia, is a cost-effective method for estimating the efficacy of chondrogenesis and ECM production. Generating samples for mRNA and protein-based expression analyses from the micromass cultures is relatively straightforward (Koff et al., 1988).
Here, we report a refined protocol on setting up chondrifying micromass cultures of embryonic limb bud-derived chondrogenic progenitor cells obtained from early-stage (Hamburger–Hamilton developmental stage 23–24; Hamburger & Hamilton, 1951) chicken embryos used in our laboratory (Basic Protocol 1). In addition, we provide a refined approach on the transient transfection of cells with siRNA constructs using electroporation prior to micromass culturing (Basic Protocol 2). Confirming cartilage ECM production by simple metachromatic staining procedures is necessary for evaluating the efficacy of chondrogenesis; we therefore supply a protocol for the qualitative and quantitative assessment of chondrogenic differentiation and cartilage matrix production using dimethyl methylene blue (DMMB) staining and image analysis (Basic Protocol 3). If DMMB staining is not the preferred choice, we provide protocols for the qualitative assessment of cartilage matrix production using Alcian blue 8GX (Alternate Protocol 1) and safranin O staining (Alternate Protocol 2). Finally, a step-by-step guide on how to determine mitochondrial activity in cells of chondrifying micromass cultures with the MTT assay is also included (Basic Protocol 4).
STRATEGIC PLANNING
Here we provide a list of considerations in order to carefully prepare for the execution of the protocols.
Consider the full timeline of the experiment (Fig. 2). Keep in mind that a single experiment takes 6 days to complete. Plan the time of setting up the micromass cultures in a way that none of the critical days, in terms of workload (e.g., day 6), fall on a weekend. Obtain fertilized eggs no more than 7 days ahead of the experiment. Keep them in a cool place (∼18°C), away from direct sunlight. Choose a reliable poultry farm to obtain fertilized chicken eggs. They should use a single breed, e.g., the Ross hybrid breed. Given the key importance of embryos to be in the right developmental stage (Hamburger–Hamilton stages 23–24), a very precise timing of incubation in the egg hatcher is required. Although this depends on the egg hatcher model, it may be necessary to start the incubation late at night. For this, obtaining a plug-in timer or a programmable egg hatcher is recommended. During the incubation in the egg hatcher (∼4.5 days), maintaining appropriate humidity level (∼90%) is important. Make sure to refill water into the egg hatcher to supply humidity for extended periods (e.g., over the weekend). Alternatively, manually top up water every second day (depending on the egg hatcher model). Designate a dark room (or a dark corner of a room) for candling the eggs. Depending on the experimental plan (and the number of embryos to be processed), at least two staff members are required for setting up the micromass cultures. One person is tasked with cracking the eggs and fishing out the embryos while the other person is dissecting the limb buds under a microscope. In case a high number of embryos need to be processed (e.g., over ∼50), involvement of three staff members is recommended. Egg cracking and limb bud dissection should not take more than 1 hour to avoid unnecessary stress to the chondroprogenitor cells. Check local ethical guidelines regarding work of early-stage chicken embryos. Before cracking, disinfect eggs by wiping them generously with 70% ethanol. Once embryos are removed from the eggs, they should be kept in a sterile environment (in a laminar flow cabinet). Note that eggs are usually sourced from poultry farms. Please check whether any decontamination procedures are in place (e.g., treatment with ozone) to reduce the risk of infections (e.g., contagious poultry diseases that may be transmitted through eggs) of cell cultures in vitro.
NOTE : All protocols involving animals must be reviewed and approved by the appropriate Animal Care and Use Committee and must follow regulations for the care and use of laboratory animals.
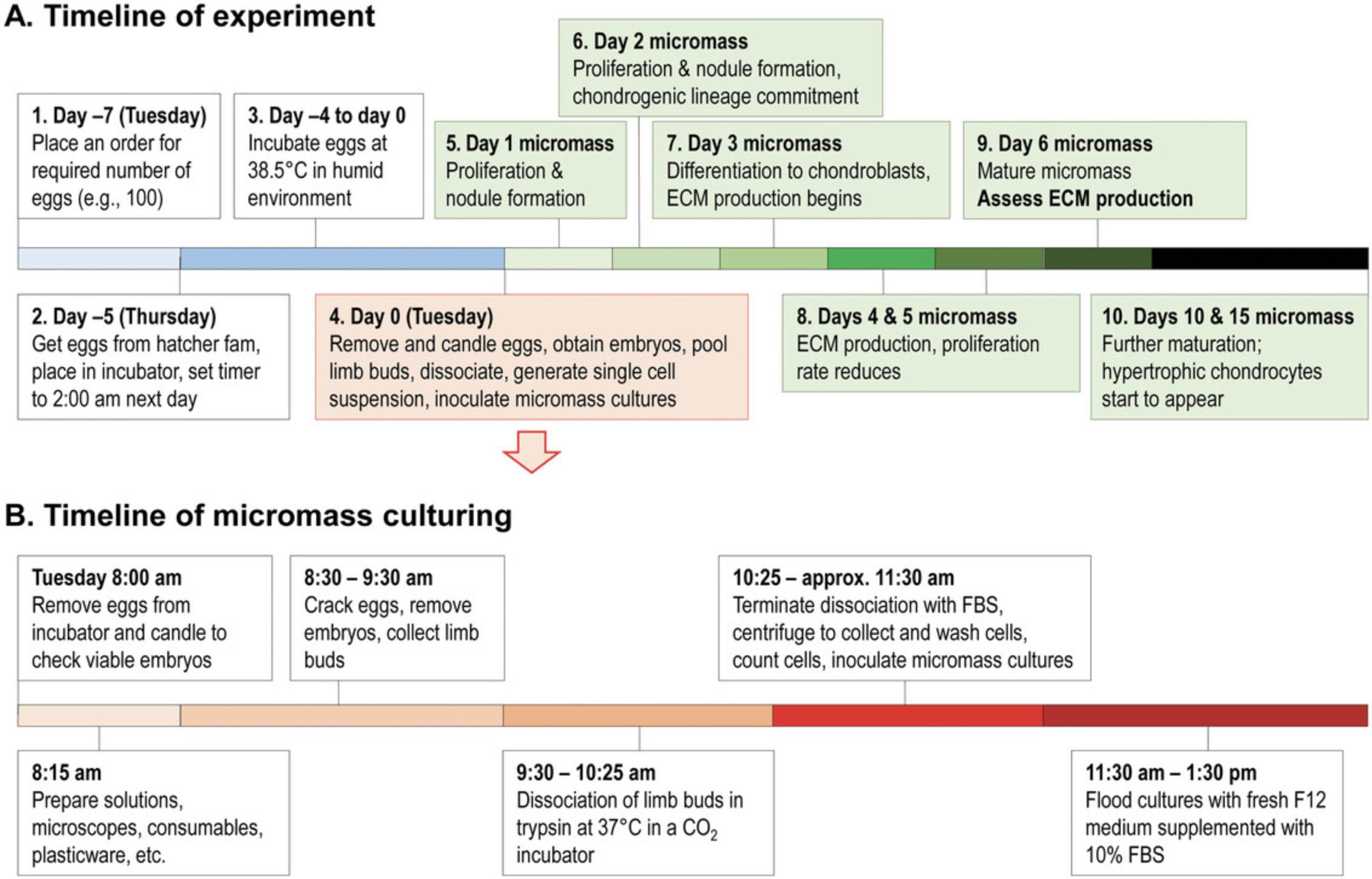
Basic Protocol 1: MICROMASS CULTURE OF CHICK EMBRYONIC LIMB BUD-DERIVED CELLS
Embryonic limb bud-derived chondrifying micromass cell cultures are used as an in vitro experimental model for hyaline cartilage formation. The chondroprogenitor cells in micromass cultures first proliferate and then differentiate into matrix-producing chondroblasts during the first 3 days of culture, and a well detectable hyaline cartilage-specific ECM forms by the sixth day of culture. To set up the chondrifying cultures, the distal parts of the fore and hind limbs of early-stage (Hamburger–Hamilton stages 23–24; ∼4.5 day-old; Hamburger & Hamilton, 1951) chicken embryos are dissected and pooled, the limb buds are dissociated using trypsin, the cell density is adjusted to 1.5 × 107, and micromass cultures of required size are established.
Materials
-
Fertilized eggs
-
96% ethanol, diluted to 70% (v/v) (VWR, cat. no. EM1.59010.2500)
-
Calcium and magnesium free phosphate buffered saline (CMF-PBS) (see recipe)
-
Gentamycin solution (Sigma-Aldrich, cat. no. G1397), or equivalent
-
Trypsin-EDTA solution (see recipe)
-
Fetal bovine serum (FBS), qualified (Thermo Fisher Scientific, cat. no. 26140079)
-
Ham′s Nutrient Mixture F12 (Sigma-Aldrich, cat. no. 51651C), or equivalent
-
0.4% trypan blue solution (Sigma-Aldrich, cat. no. 93595)
-
5000 U/ml penicillin, 5 mg/ml streptomycin solution (Sigma-Aldrich, cat. no. P4458), or equivalent
-
L-glutamine (Euroclone, cat. no. ECB3000D)
-
L-ascorbic acid (vitamin C) (Sigma-Aldrich, cat. no. A4544)
-
Personal protective equipment (PPE) including nitrile gloves
-
Egg hatcher, Brinsea Ova-Easy Advance Hatcher Series II (manufacturer no. MJ4923A), or equivalent
-
Egg candling device, Brinsea OvaView Standard Egg Candling Lamp (MacEoin General Merchants Ltd. UK), or equivalent
-
Stereomicroscope, Leica S6 E LED 2500 (Leica Microsystems), or equivalent
-
Laminar flow cabinet, FlowFAST H (Faster S.R.L), or equivalent
-
0.2-mm/115-mm micro forceps, curved (SUBAN Instruments, cat. no. PC-855-11)
-
0.2-mm/110-mm micro forceps, straight (SUBAN Instruments, cat. no. PC-858-11)
-
Filter spoon (BaltaLab, cat. no. TL85.1)
-
Crystallizing dishes, Pyrex (Merck, cat. no. SLW1470/02D)
-
10-mm soda lime glass Petri culture dishes, Pyrex Vista (Thermo Fisher Scientific, cat. no. 07-770-214)
-
30-ml weighing bottle, Duran (Thermo Fisher Scientific, cat. no. 11740944)
-
Incubator, BINDER model BD 23, or equivalent
-
Pipettes and pipette tips (5-ml, 1000-µl, 200-µl, and 10-µl)
-
50-ml centrifuge tubes (Corning, cat. no. 430290)
-
Benchtop centrifuge, Eppendorf 5804/5804 R, or equivalent
-
Vacuum aspirator, Aspire laboratory aspirator, or equivalent
-
20-µm nylon net filter 50-ml Steriflip centrifuge tube (Millipore, cat. no. SCNY00020)
-
Luna automated cell counter (Logos Biosystems), or equivalent
-
35-mm cell culture petri dishes (Eppendorf, cat. no. 0030700112) and/or 24-well cell culture plate (Eppendorf, cat. no. 0030722116)
1.Place the desired number of eggs in the egg hatcher and adjust the temperature setting to 38.5°C.
2.After the 104 hr incubation time, switch off the hatcher and remove the eggs. Use 70% (v/v) ethanol to wipe clean the surface of the eggs. Immediately afterwards, in a dimly lit room, use the egg candling device to assess the presence and position of the embryo within the egg (Fig. 3A; Video file 1).
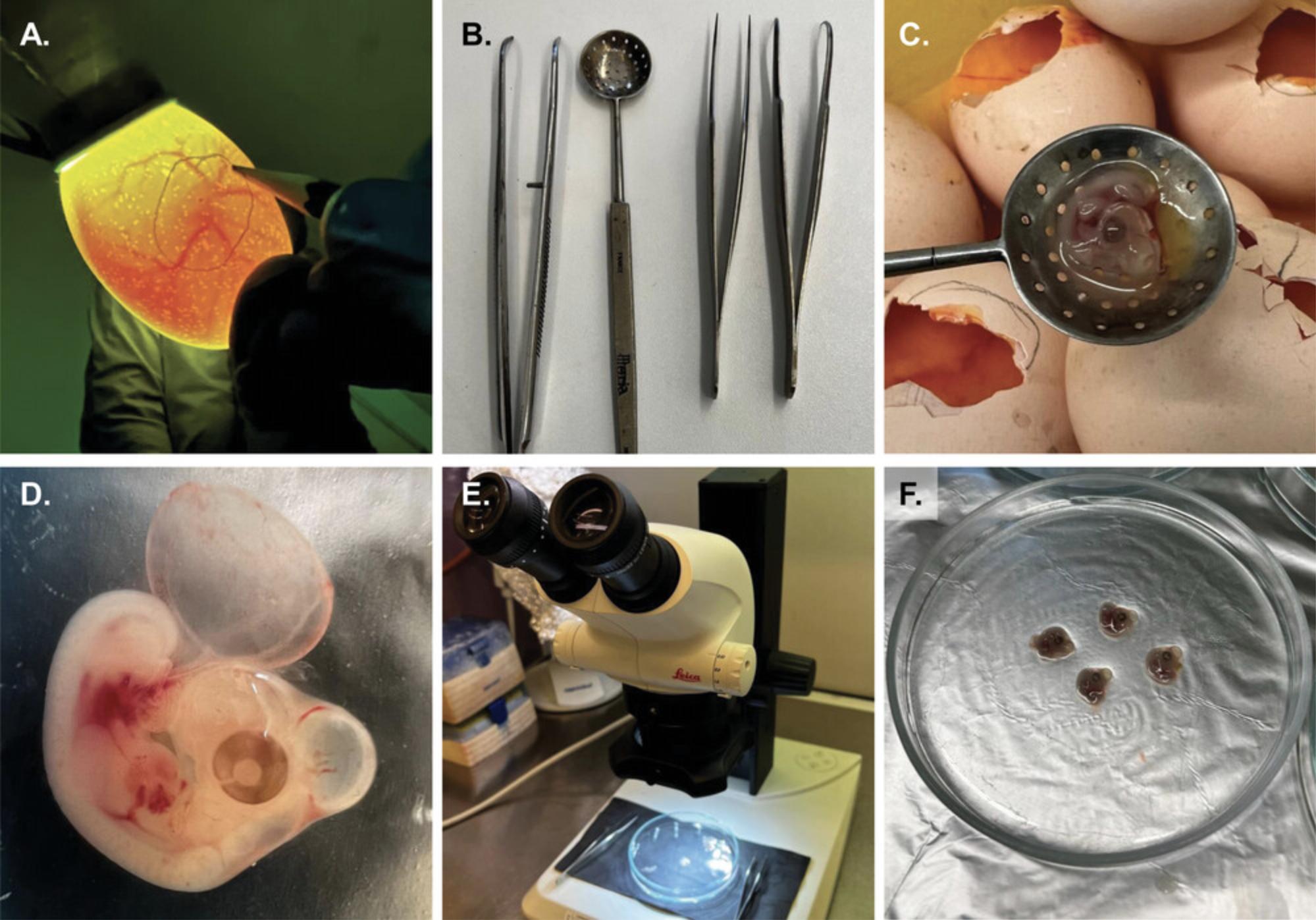
3.Use a pair of curved micro forceps to crack the eggshell immediately next to the circular marking that indicates the position of the embryo. Remove enough of the shell to access the embryo with a filter spoon (Fig. 3B) and use it to remove the embryo (Fig. 3C; Video file 2).
4.Immediately place the embryos (Fig. 3D) into glass crystallizing dishes or 10-mm Petri dishes containing 10 ml prewarmed (37°C) CMF-PBS supplemented with gentamycin (0.2 mg/ml) and pool ∼10 embryos.
5.Transfer the desired number of embryos (between 5 and 10) into separate Petri dishes containing 10 ml prewarmed (37°C) gentamycin-supplemented CMF-PBS and place the Petri dish under the stereomicroscope (Fig. 3E-F; Video file 3).
6.Use an operating microscope and two pairs of fine forceps (a straight and a curved set) to remove distal portions of all four limb buds from each embryo (Video file 4). Collect removed limb buds in a crystallizing dish that also contains prewarmed (37°C) gentamycin-enriched CMF-PBS (∼5 ml).
7.Once all limb buds from all embryos have been gathered, remove unwanted tissue debris (e.g., epithelial membranes, inappropriate parts of the embryo) from the Petri dish with the aid of a stereomicroscope. Remove the CMF-PBS by careful pipetting and transfer the limb buds to 15 ml trypsin-EDTA solution preincubated at 37°C in a 30-ml weighing bottle with a glass lid (Video file 5). Incubate 55 min at 37°C in a CO2 incubator.
8.Carefully dissociate the limb buds using a 5-ml pipette by careful pipetting several times. Terminate the enzymatic dissociation of limb buds by adding an equal volume of fetal bovine serum (Video file 6). Mix well and transfer the solution to a 50-ml centrifuge tube, and centrifuge 5 min at 150 × g , room temperature, to pellet the cells.
9.Carefully remove supernatant with a vacuum aspirator and resuspend pellet in 30 ml Ham's F12 culture medium supplemented with 10% FBS.
10.Centrifuge cell solution 10 min at ∼150 × g , room temperature. Resuspend cells in an adequate volume of Ham's F12 culture medium supplemented with 10% FBS.
11.Filter the cell suspension through a 20-µm nylon net filter (Video file 7).
12.Determine the cell concentration of the suspension, preferably using an automated cell counter (Video file 8) and adjust the final volume with the same medium to achieve the desired concentration of 1.5 × 107 cells/ml.
13.Inoculate droplets (of various size ranging from 15 µl to 100 µl) into 35-mm cell culturing dishes or 24-well plates that fit the purpose of the experiment (Video file 9). Allow cells to attach to the surface for 2 hr in a CO2 incubator (37°C, 5% CO2 and 90% humidity).
14.Flood cultures with medium (Ham's F12 supplemented with 10% FBS, 0.5 mM L-glutamine, and 1% penicillin/streptomycin solution) after the 2 hr attachment period. Make sure to add L-ascorbic acid (vitamin C) at 50 mg/L final concentration to the culture medium at this stage. Change the culture medium every other day for the duration of the experiment.
Basic Protocol 2: TRANSFECTION OF CELLS WITH siRNA CONSTRUCTS USING ELECTROPORATION PRIOR TO MICROMASS CULTURING
High efficiency transfection of foreign nucleic acids into this model is particularly challenging. Transfections reagents that are gentle on limb bud cells (such as Lipofectamine 2000) usually result in low (∼25%) transfection efficacy, whereas other methods (such as Amaxa nucleofector technology) can achieve much higher efficiency (up to 97%) but at the same time can induce high rates of apoptosis (Bobick et al., 2014). Due to the necessity of plating droplets at a high initial cell density, the best window of opportunity for this intervention is while cells are in suspension (following step 11 of Basic Protocol 1). Below, we describe a modified version of the method described by Bobick et al. (2014). This method consists of square wave pulse electroporation of cells resuspended in a protective sucrose buffer containing siRNA. It results in a comparatively high transfection efficiency without significant harm on cell viability, and chondrogenic differentiation potential. Following electroporation, cells are pooled, resuspended in Ham's F12 culture medium, counted, and inoculated into cell culture dishes or plates that fit the purpose of the experiment.
Additional Materials (also see Basic Protocol 1)
-
Modified sucrose electroporation buffer (see recipe)
-
Pre- and/or custom-designed siRNAs, including controls (Silencer Select, Thermo Fisher Scientific)
-
2.0-mm gap sterile electroporation cuvette (VWR, cat. no. 732-1136)
-
Square wave electroporation system (BTX model ECM 830)
-
Ice
-
1.5-ml Eppendorf tubes
1.Generate a single cell suspension of chondrogenic progenitor cells and determine cell density according to steps 1-12 of Basic Protocol 1.
2.Take the required amount of cell suspension, centrifuge 5 min at 150 × g , room temperature, and remove supernatant with a vacuum aspirator.
3.Resuspend cell pellet in cold (4°C) modified sucrose electroporation buffer.
4.Add 2 µg siRNA to every 250 µl of cell suspension, mix well by pipetting, and transfer the entire volume to a 2.0-mm gap sterile electroporation cuvette.
5.Incubate cuvettes containing the solution at 4°C for 5 min then administer pulses with the electroporation system according to the following settings: three 400 V pulses, 150 s in length, at 100 ms intervals (Fig. 4A-C).
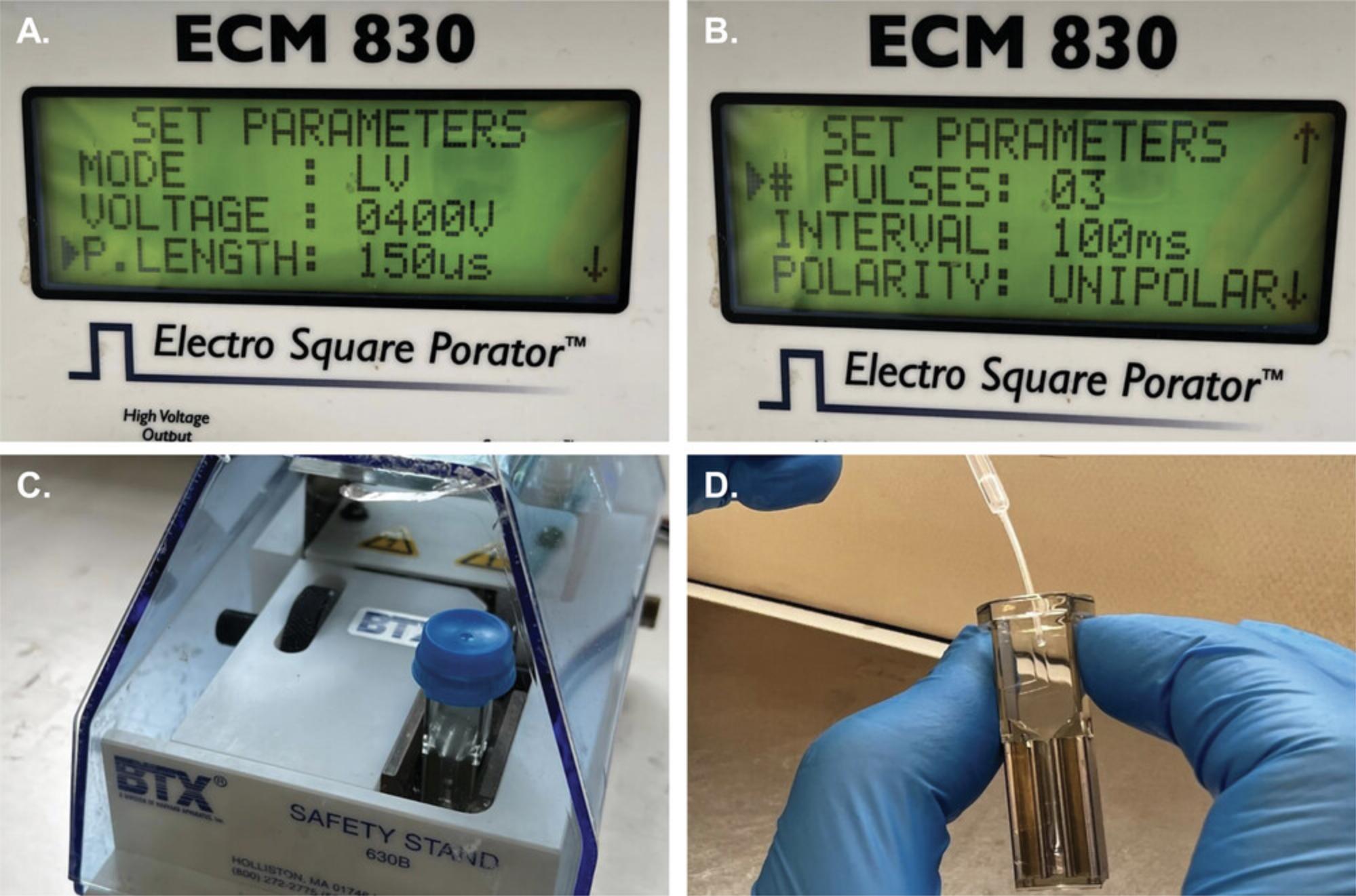
6.Incubate cells following electroporation at 4°C for 10 min, then at room temperature for 5 min, and at 37°C for 5 min to allow cells to sink to the bottom of the cuvette.
7.Pool the lower four-fifths of the suspension from each cuvette that belongs to the same experimental group (Fig. 4D) in 1.5-ml Eppendorf tubes, and pellet cells by centrifugation 5 min at 150 × g , room temperature.
8.Continue with step 12 of Basic Protocol 1.
Basic Protocol 3: QUALITATIVE AND QUANTITATIVE ASSESSMENT OF CARTILAGE MATRIX PRODUCTION USING DIMETHYL METHYLENE BLUE STAINING AND IMAGE ANALYSIS
1,9-dimethyl methylene blue is a cationic dye which preferentially binds to negatively charged sulfated glycosaminoglycans (sGAG). This staining technique is widely used in cartilage research (Templeton, 1988). The dye shows purple metachromasia in the presence of polyanionic sGAG. The pH must be kept at pH 1.5 to avoid erroneous estimation of sGAG content in newly produced cartilage ECM due to interference caused by polyanions such as hyaluronan, DNA, or RNA (Zheng & Levenston, 2015).
Additional Materials (also see Basic Protocol 1)
-
Chondrogenic cell suspension samples (see Basic Protocol 1, step 13)
-
Phosphate buffered saline (PBS) tablets (Thermo Fisher Scientific Inc., cat. no. 003002), or equivalent
-
Ethanol-formalin fixative (see recipe)
-
80%, 70%, and 50% (v/v) ethanol
-
3% (v/v) acetic acid
-
0.1% (w/v) DMMB solution (see recipe)
-
Distilled H2O
-
12-mm microscope cover glasses, heat-sterilized for 60 min at 180°C before use (VWR, cat. no. 631-1577)
-
Chemical hood
-
Aquatex mounting medium (Merck, cat. no. 1.08562.0050)
-
Microscope slides, 76 × 26 mm (VWR, cat. no. 631-1553)
-
Plastic Pasteur pipette (VWR, cat. no. 612-1684)
-
Hooked needle
-
Forceps with curved or flat tips
-
Water absorbent paper strips
-
Computer and software (see Internet Resources)
NOTE : Each step is performed at room temperature in a 24-well plate. Use ∼500 µl of solution per well at each step. Handle liquids using plastic Pasteur pipettes. To aspirate solvents, a vacuum aspirator may be used.
CAUTION : Formaldehyde, the aqueous solution (40%) of which is formalin, is a known carcinogen. Formaldehyde is a sensitizing agent that can cause an immune response upon initial exposure. Subsequent exposure may cause severe allergic reactions of the skin, eyes, and respiratory tract. Long-term or repeated exposure to low levels in the air or on the skin can cause asthma-like respiratory problems and skin irritation. Acute exposure can be highly irritating to the eyes, nose, and throat. Because of the serious potential hazards, precautions must be taken to eliminate or reduce the potential risk for exposure, e.g., by wearing appropriate PPE, and adhering to safe use practices.
1.Wash samples (established from 30-µl droplets of the chondrogenic cell suspension; see Basic Protocol 1, step 13) cultured on 12-mm cover glasses in a 24-well plate twice in PBS prepared from the tablets at room temperature.
2.Fix samples in ethanol-formalin fixative solution for 30 min at room temperature.
3.To rehydrate the samples, wash cultures 3 times, 10 min each in 80% (v/v) ethanol, then in 70% (v/v) ethanol for 5 min, then in 50% (v/v) ethanol for 2 min, and rinse in distilled H2O for 2 min.
4.Wash cultures in 3% (v/v) acetic acid for 3 min to reduce the pH.
5.Stain the samples in 0.1% (w/v) DMMB solution for 5 min
6.Aspirate the DMMB solution from the wells and add 3% (v/v) acetic acid to the samples to remove unbound/excess dye. Aspirate the acetic acid solution immediately.
7.Wash cover glasses three times in distilled H2O. Keep the samples in distilled H2O until mounting.
8.Mount samples using a Pasteur pipette by placing one droplet of Aquatex mounting medium (∼50 µl) on a microscope slide, then carefully lower the cover glass onto the droplet with the stained cultures facing downward.
9.Let the specimens air-dry for 24 hr before examining under a microscope.
Quantitative evaluation of microscopic images
10.Install the metachromasiaIndex program on your computer. The application was written in MATLAB, and there are several options for running it on your system:
-
If you have MATLAB installed on your computer, you can download it from one of the web pages provided (see Internet Resources). Start MATLAB and type metachromasiaIndex at the command prompt.
-
If you do not have MATLAB installed on your computer, you can download and install MATLAB Runtime from the webpage of Mathworks (see Internet Resources). Choose R2022a corresponding to your operating system and download the compressed folder containing the executable version of the program (see Internet Resources). After unzipping the file, double-click the metachromasiaIndex.exe file to start the program.
We have introduced three different indices for quantifying metachromasia based on the color of digital microscopic images. Since previous quantitative measures of metachromasia are based on comparison of optical densities at two different wavelengths (Hattori et al., 1996) or at two different polarization angles (Heuft & Bohm, 1978; Romhanyi, 1963), they are not easily applied under conditions available in ordinary cell biological laboratories. Since the novel indices are based on quantifying color, a brief introduction to the RGB color model is required. An arbitrary color can be defined by a number triplet with the first, second and third values specifying the intensity of the red, green, and blue colors, respectively. Color intensities are usually specified on a scale between 0-255.This color model, named RGB, can be represented by a cube in which the three spatial coordinates correspond to the intensity of either the red, green, or blue colors. Consequently, every different spatial position in the cube corresponds to a certain color (Fig. 5A).
The first metachromasia index is described by the following equation:
where d is the distance between the points representing the color of the most purple, most metachromatic area in an experiment and the pixel under investigation (Fig. 5B). 255⋅√3 is the main diagonal in the RGB cube, i.e., the largest possible distance in the cube. The second metachromasia index is defined according to the following equation:
where dmax is the distance between the most purple, most metachromatic color and the color of the gray background in the RGB cube. In the third index, d1 is the distance between the projection of the color of the pixel under investigation on the line connecting the most purple and the gray colors in the RGB cube:
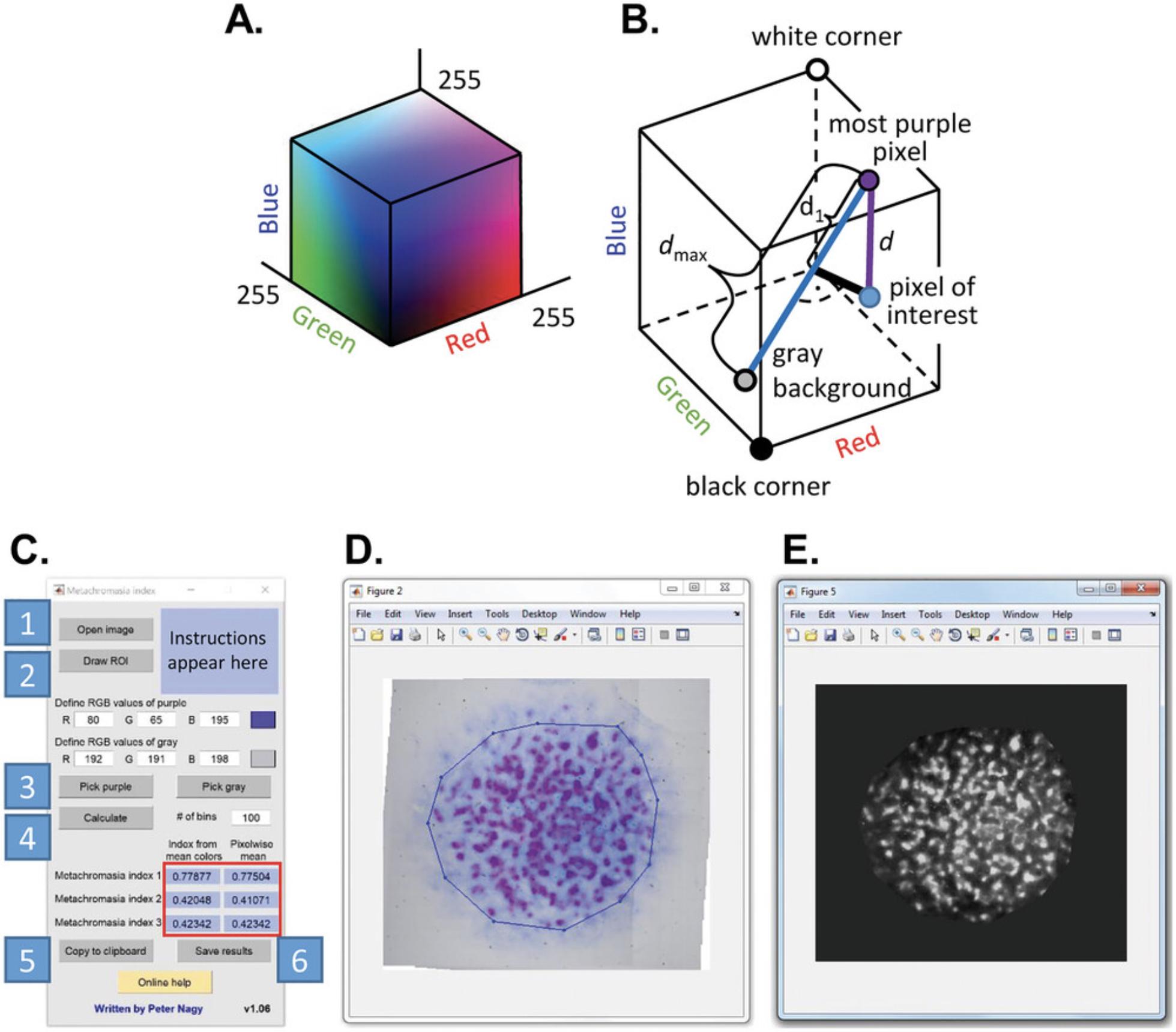
11.Follow these steps to evaluate the images (Fig. 5C):
-
Click on ‘Open image’ to import a photomicrograph of the entire micromass culture.
-
Circumscribe the area you would like to evaluate (region of interest, ROI) by clicking on ‘Draw ROI.’ You can draw polygon regions by clicking on corners of the polygon and finishing by double-clicking. You can also draw square- or rectangle-shaped ROIs by holding down the SHIFT or CTRL keys, respectively, while dragging your mouse. You can delete an ROI by right clicking on one of its vertices. A vertex can be moved by left clicking and dragging. You can leave the ROI drawing cycle by a double click. If you draw multiple ROIs, their union constitutes the evaluated area. Such an ROI is shown in Figure5D.
-
Identify the most purple (most metachromatic) color, and the gray color (the background) by clicking on ‘Pick purple’ and ‘Pick gray,’ respectively. You can also manually specify these colors by entering values (0-255) in the text boxes under ‘Define RGB values of purple’ and ‘Define RGB values of gray’. The specified colors are displayed in the box on the right. When evaluating an experiment from a single batch of micromass cultures, it makes sense to define the RGB values of purple and gray globally, i.e., to use the same RGB values for every image in the experiment.
-
Click on ‘Calculate’ to determine the three different metachromasia indices in the ROI on a pixel-by-pixel basis. This operation generates three images corresponding to the three indices. The means of these pixelwise values are displayed in the blue fields labeled ‘Pixelwise mean’ in the red box. The values under ‘Index from mean colors’ are the metachromasia indices calculated from the mean red, green and blue values in the ROI. A histogram of the pixelwise values of the metachromasia indices is also displayed. By default, the histogram has 100 class intervals, which can be modified by entering a different value into the field ‘# of bins’.
-
The mean values in the blue fields in the red box can be copied to the clipboard by clicking on ‘Copy to clipboard.’
-
The results can be saved by clicking on ‘Save results.’ The pixelwise values of the three indices can be saved in three images, either in TIFF or JPG format. The user must specify a base name that is appended by ‘_1,’ ‘_2’ and ‘_3.’ The three histograms are also saved in a single comma-delimited file.
Alternate Protocol 1: QUALITATIVE ASSESSMENT OF CARTILAGE MATRIX PRODUCTION USING ALCIAN BLUE STAINING
Alcian blue staining technique is an alternative method to detect the sulfated GAGs in the cartilage ECM. The stained tissue parts are cyan or greenish-blue, and this staining effect is called “Alcianophilia.” Alcian blue staining is pH sensitive; at pH 1.0 it stains sulfated GAGs only, but at pH 2.5 it also stains hyaluronan and sialomucins (Green & Pastewka, 1974).
Additional Materials (also see Basic Protocols 1–3)
-
1% (w/v) Alcian blue solution (see recipe)
-
DEPEX mounting medium (Merck, cat. no. 1.00579.0500)
NOTE : Each step is performed at room temperature in a 24-well plate. Use ∼500 µl of solution per well at each step. Handle liquids using plastic Pasteur pipettes. To aspirate solvents, a vacuum aspirator may be used.
1.Wash micromass samples from Basic Protocol 1, step 13 (established from 30-µl droplets) cultured on 12-mm cover glasses in a 24-well plate two times in PBS.
2.Fix samples in ethanol-formalin fixative for 30 min.
3.To rehydrate the samples, wash cultures three times, 10 min each in 80% (v/v) ethanol, once in 70% (v/v) ethanol for 5 min, once in 50% (v/v) ethanol for 2 min, and rinse in distilled H2O.
4.Wash cultures in 3% (v/v) acetic acid for 3 min to reduce the pH.
5.Stain the samples using 1% (w/v) Alcian blue 8GX solution for 5 min.
6.Remove excess dye from the samples and wash 3 times in distilled H2O. Keep the last round of distilled H2O on the samples.
7.Remove cover glasses from the bottom of the wells with the help of a hooked needle and a pair of forceps. Carefully wipe off excess water from the edge of the cover glass and place it on a microscope slide with stained cultures facing upward. Air-dry the specimens for ∼30 min at room temperature.
8.Mount samples by placing one droplet (∼50 µl) of DEPEX mounting medium on a microscope slide, then carefully lower the cover glass onto the droplet with the stained cultures facing down.
9.Let the specimens air-dry for 24 hr before examining under a microscope.
Alternate Protocol 2: QUALITATIVE ASSESSMENT OF CARTILAGE MATRIX PRODUCTION USING SAFRANIN O STAINING
Safranin O is a metachromatic dye which binds to highly polyanionic structures. This dye is widely applied to visualize GAGs and proteoglycans with an orange color (Kiraly et al., 1996; Rosenberg, 1971).
Additional Materials (also see Basic Protocol 1–3 and Alternate Protocol 1)
- 0.1% (w/v) safranin O solution (see recipe)
NOTE : Each step is performed at room temperature in a 24-well plate. Use ∼500 µl of solution per well at each step. Handle liquids using plastic Pasteur pipettes. To aspirate solvents, a vacuum aspirator may be used.
1.Wash micromass samples from Basic Protocol 1, step 13 (established from 30-µl droplets) cultured on 12-mm cover glasses in a 24-well plate two times in PBS.
2.Fix samples in ethanol-formalin fixative solution for 30 min.
3.Rehydrate the samples by incubating the cultures 3 times, 10 min each in 80% (v/v) ethanol, and once in 70% (v/v) ethanol for 5 min.
4.Add 1 ml distilled H2O to the cultures and incubate for 5 min. Aspirate water.
5.Incubate the cultures with 1 ml 0.1% safranin O solution for 15 min.
6.Rinse 5 times in tap water. Keep the last round of water in the wells.
7.Carefully remove the coverslips from the wells using a needle and forceps.
8.While holding the coverslips with the forceps, carefully wipe off excess water from the edge of the cover slip and place it on a microscope slide with stained cultures facing up.
9.Air dry specimens for 30 min.
10.Mount samples by placing one droplet (∼50 µl) of DEPEX mounting medium on a microscope slide, then carefully lower the cover glass onto the droplet with the stained cultures facing down.
11.Let the specimens air-dry for 24 hr before examining under a microscope.
Basic Protocol 4: MEASUREMENT OF MITOCHONDRIAL ACTIVITY WITH THE MTT ASSAY
The MTT assay is a colorimetric method suitable for the analysis of cell viability, cell proliferation, and cytotoxicity. Living cells can reduce the yellow tetrazolium salt in the MTT reagent by mitochondrial oxidoreductase enzymes. This metabolic activity will lead to the formation of purple formazan crystals, which can be dissolved using a solubilization solution. At the end of the reaction, the absorbance of the purple-colored solution at a specific wavelength is measured by a spectrophotometer (van Meerloo et al., 2011).
Additional Materials (also see Basic Protocol 1–3)
-
MTT (thiazolyl blue tetrazolium bromide; VWR, cat. no. 0793-1G), or equivalent
-
Isopropanol (Merck, cat. no. 278475), or equivalent
-
Triton X-100 (Reanal Labor, cat. no. 32190-0-98-64), or equivalent
-
Electronic laboratory balance (Kern ABS 120-4), or equivalent
-
Wizard Advanced IR vortex mixer (VELP Scientifica), or equivalent
-
Microtiter plate shaker incubator (Stuart SI505), or equivalent
-
96-well sterile plastic plate (Eppendorf, cat. no. 0030730119), or equivalent
-
Multilabel detection platform (Chameleon microplate reader, Hidex), or equivalent
-
Microsoft Excel, or equivalent
1.On culturing day 0 (see Basic Protocol 1, step 14), inoculate samples (30 µl droplets of the original chondroprogenitor cell suspension) for mitochondrial activity studies into 24-well plates. Handle cultures (i.e., add compounds to block/activate pathways or ion channels, etc.) as and when required by the experiment.
2.On a designated day in culture (depending on the experimental setting), measure 5 mg of MTT reagent into sterile 1.5-ml Eppendorf tubes on an electronic laboratory balance. Dissolve the MTT powder in sterile 1 × PBS. Final concentration of the MTT solution is 5 mg/ml. Vortex the solution until the powder is fully dissolved.
3.Remove the 24-well plate containing live micromass cultures from the cell culture incubator and transfer into the laminar flow cabinet. Add 25 µl of MTT reagent (5 mg/ml) into each well containing 1 ml cell culture medium.
4.Replace the 24-well plate into the CO2 incubator. Incubate cells for 2 hr.
5.During the incubation time, prepare the stock solubilization solution: add 10 ml Triton X-100 to 90 ml isopropanol.
6.At the end of the 2-hour-long incubation, remove the 24-well plate from the CO2 incubator and aspirate the medium from the wells.
7.Add 500 µl of the solubilization solution to each well.
8.Incubate the 24-well plate for ∼10 min at 37°C in a microtiter plate shaker incubator.
9.When formazan crystals are completely solubilized, pipette 100–100 µl of the solubilization solution from each well of the 24-well plate into a new 96-well plate (in triplicates; 100 µl/well). For a blank reading, use solubilization solution (use triplicate).
10.Insert the 96-well plate into the microplate reader. Measure the optical density of the samples at 570 nm.
11.Analyze the optical density readings with the help of Microsoft Excel or other data analysis software.
REAGENTS AND SOLUTIONS
Alcian blue solution 1% (w/v)
- 1 g Alcian blue 8GX (Sigma-Aldrich, cat. no. 05500-5G), or equivalent
- 3 ml glacial acetic acid
- 1 ml concentrated (37%) HCl
- 96 ml distilled H2O
- Filter using a qualitative filter paper (X100 Grade 1288 125 mm; Ahlstrom-Munksjo Munktell, cat. no. 4.206.125) before use
- Store in dark bottles up to 1 year at room temperature
Calcium and magnesium free phosphate buffered saline (CMF-PBS) stock solution (pH 7.2), 10×
- 80 g NaCl
- 3 g KCl
- 1.84 g Na2HPO4 ⋅ 12H2O
- 0.2 g KH2PO4
- 20 g D-glucose
- Bring volume to 1000 ml with deionized H2O
- Adjust to pH 7.2 with HCl or NaOH
- Sterilize using a 0.2-micron filter in a laminar flow hood
- Store up to 3 months at 4°C
Dimethyl methylene blue (DMMB) solution, 0.1% (w/v)
- 0.1 g DMMB (Sigma-Aldrich, cat. no. 341008-1G), or equivalent
- 3 ml glacial (anhydrous) acetic acid
- 97 ml distilled H2O
- Filter using a qualitative filter paper (X100 Grade 1288 125 mm; Ahlstrom-Munksjo Munktell, cat. no. 4.206.125) before use
- Store in dark bottles up to 1 year at room temperature
Ethanol-formalin fixative
- 400 ml absolute ethanol
- 100 ml 37% aqueous formalin
Handle formalin with care using appropriate PPE and prepare in a fume hood.
- Store up to 2 months at 4°C
Modified sucrose electroporation buffer
Add 272 mM sucrose (VWR, cat. no. 27483.294) to Opti-MEM Reduced Serum Medium (Thermo Fisher Scientific, cat. no. 31985070). Sterilize using a 0.2-micron filter. Store up to 30 days at 4°C.
Safranin O solution, 0.1% (w/v)
- 100 ml deionized H2O
- 0.1 g Safranin O Basic Red 2 (Sigma-Aldrich, Merck, cat. no. S2255), or equivalent
- Filter using a qualitative filter paper (X100 Grade 1288 125 mm; Ahlstrom-Munksjo Munktell, cat. no. 4.206.125) before use
- Store in dark bottles up to 1 year at room temperature
Trypsin-EDTA solution
- 0.75 g trypsin from porcine pancreas (Sigma-Aldrich, cat. no. T6567), or equivalent
- 0.3 g EDTA (VWR, cat. no. 20301.290)
- 300 ml CMF-PBS, pH 7.6 (see recipe)
- Sterilize using a 0.2-micron filter before use
- Store in 30 ml aliquots up to 90 days at −20°C
COMMENTARY
Background Information
The original article that described the methodology of establishing chondrogenic micromass cell cultures from the limb buds of early-stage chick embryos was published 50 years ago (Ahrens et al., 1977). The underlying principle is that limb bud mesoderm cells, when retrieved from a specific developmental stage, are cultured in vitro in a sufficiently high seeding density in a microdrop. The cells form aggregates or pre-cartilage nodules during the first 1-2 days and differentiate into chondrocytic cells, which produce an extracellular matrix from culture day 3. A critical cell mass is necessary for the chondroprogenitor cells to commence chondrogenic differentiation and extracellular matrix formation; when the mesenchymal cells are seeded in low-density, they acquire a fibroblastic phenotype and are incapable of chondrogenesis (Handschel et al., 2007).
In vitro cartilage regeneration methods rely on the re-aggregation approach, during which cells are first dissociated and then the dispersed cells are re-aggregated either by self-organization or into cellular spheres in a scaffold-free manner (Handschel et al., 2007). The capacity to spontaneously form regular, spatially organized patterns of cartilage nodules is preserved in the limb bud-derived progenitor cells.
A major advantage of this system lies in its simplicity. It allows for monitoring the temporal pattern of chondrogenesis in vitro (Takacs et al., 2023). It is also a highly adaptable and well-characterized model (Takacs et al., 2023) that has been used for assessing a variety of conditions including the application of soluble factors or mechanical stimuli (Rolfe et al., 2022). However, there are some major disadvantages of this method that are worth noting. In chondrogenic micromass cultures, hyaline cartilage is present in the form of cartilage nodules, interrupted by internodular areas where a mixture of mesenchymal cells, fibroblasts and chondroprogenitors is present, and the peripheral area of the round cell cultures does not contain any cartilage. Thus, micromass cultures cannot be regarded as ‘pure’ cartilage, although the predominant tissue is hyaline cartilage. Another unfavorable feature of this method is that the hyaline cartilage which forms in the cell cultures is primarily transient cartilage, which undergoes chondrocyte hypertrophy; essentially recapitulating the main steps of endochondral ossification in the developing limbs. Gene expression profiling supports rapid chondrogenesis and transition to hypertrophy (Rolfe et al., 2022; Takacs et al., 2023). Cartilage formed in micromass cultures does not exhibit the unique organization of articular hyaline cartilage matrix. Another inherent feature of this model is that there are known differences between chicken and mammalian cartilage (Eyre et al., 1978), which should be considered when applying data generated using this model to mammalian or human chondrogenesis.
In summary, if the inherent limitations of this in vitro chondrogenesis model are considered, the primary micromass culture system still can provide an answer to questions which are unexplored in cartilage biology.
Critical Parameters
This model relies on freshly isolated progenitor cells from the developing limb buds of early-stage chicken embryos. Therefore, there are a couple of critical factors that influence the protocol and to which special attention should be paid. These are listed below.
Consider the number of fertilized eggs to be procured for the experiment. A proportion (usually ∼10%) of eggs will not contain an embryo (or embryogenesis fails in an early period). Given that the eggs are sourced from a poultry farm, they can be covered in mud and farm detritus, and they must undergo a general rinse to clean the eggshell, followed by a generous wipe with 70% ethanol. The decontamination procedures are very important to reduce the risk of infections of the cell cultures in vitro. Adding gentamycin to CMF–PBS and trypsin is a good idea to reduce the risk of infections. The correct developmental stage of the embryo is of key importance (Ahrens et al., 1977). Embryos should be at Hamburger–Hamilton developmental stage 23–24 (Hamburger & Hamilton, 1951). Limb buds obtained from embryos earlier or later than this stage are unsuitable for micromass culturing. Therefore, we provide readers with a detailed morphological description of the external features of chicken embryos (mainly focusing on the maturation of limb buds) between Hamburger–Hamilton developmental stages 19 and 25 (see Supporting Information). The time window for harvesting the limb buds from embryos is ∼60 min, as the cells in the isolated limb buds start losing vitality. Unskilled personnel should only remove and collect limb buds under supervision. It is very important to remove and collect the distal parts of the developing limb buds only, as removing parts of the body wall and embryonic membranes together with the limb bud mesenchyme will detrimentally affect trypsin dissociation and may introduce inappropriate cell populations into the micromass cultures. Double-checking the pooled limb buds for any remaining membranes and inappropriate contaminants (e.g., parts of the tail, heart, or unidentifiable body parts) is a good idea. Do not allow the trypsin digestion of the limb buds to proceed longer than 60 min, even if the limb buds look intact. Excessive digestion will compromise the integrity of the cells and they will die. A critical cell mass is necessary to proceed with chondrogenic differentiation and extracellular matrix formation. Therefore, adjusting the appropriate seeding density (1.5 × 107 cells/ml) is critically important. When plating the cells, avoid air bubbles or irregularly shaped drops, as this will interfere with micromass culture morphology and chondrogenesis. Let the cells attach to the surface of plasticware/glassware for 2 hr. When feeding the cells, add medium to freshly established micromass cultures carefully. Let the culture medium run down the sides of the wells or Petri dishes so as not to disturb the cells. The cell attachments are very tenuous at this point and if the medium is introduced too quickly, the cells will be washed off the plate and die, adversely affecting chondrogenesis. Check the surface of the plasticware before culture. Surface (cell culture) treated plasticware is necessary for proper adhesion of micromasses. In our experience, Nunc, Eppendorf, Thermo, and Greiner brand tissue culture plasticware (6-, 24-well tissue culture plates, as well as 35-mm Petri dishes) appear to work the best. Adding 272 mM sucrose to the electroporation medium provides a cytoprotective environment, which appears critical in achieving high transfection efficiency/low cytotoxicity (Bobick et al., 2014). In our experience, transferring the electroporation cuvettes to a cold environment (i.e., on ice) immediately after pulsing proved to be of cardinal importance for the high survival rate of transfected cells. Providing living cells with enough incubation time following electroporation to sink to the bottom of the cuvette is also of high importance.
Troubleshooting
Table 1 summarizes common problems with the protocols, their causes, and potential solutions.
| Problem | Possible cause | Solution |
|---|---|---|
| Poor chondrogenic differentiation | Embryos are out of developmental stage | Adjust incubation time so that embryos are in the correct stage (Hamburger–Hamilton 23–24) |
| Inappropriate part(s) of embryos collected (e.g., tail, heart, etc.) or contamination with embryonic membranes | Make sure to remove inappropriate embryo parts or pieces of membranes from the weighing bottle before trypsin dissociation | |
| Not appropriate size of limb buds collected (contains proximal part as well) | Make sure to remove the distal part of the developing limb bud only | |
| Limb bud removal and collection step took more than 60 min | Make sure that the process of limb bud removal and collection does not take more than 60 min | |
| Dissociation in trypsin took more than 50 min | Make sure that the dissociation step does not take more than 50 min; to facilitate dissociation, resuspend the solution once or twice during enzymatic dissociation | |
| Not fully adherent cells were washed away during feeding | Let the culture medium run down the sides of the well so as not to disturb the cells | |
| Not all 4 limb buds have been removed (low yield of chondrogenic cells) | Make sure to remove all 4 limb buds of the embryos | |
| Cultures detach from the surface of plasticware/glassware | Glass coverslips were heat-sterilized more than once | Make sure not to heat-sterilize the glass coverslips more than once |
| Inappropriate plasticware (non-cell culture treated) | Make sure to use cell culture treated plasticware | |
| Improper culture conditions | Check the incubator settings (temperature, humidity, CO2 levels, etc.) and the composition of solutions (e.g., media, etc.) | |
| Bacterial/fungal infection | Eggs not properly decontaminated | Decontaminate the eggs by wiping them with ample 70% ethanol |
| Non-sterile equipment/solutions used | Sterilize the equipment/sterile filter the solution(s) | |
| Inappropriate use of PPE | Wear appropriate PPE | |
| Poor metachromatic ECM production | Insufficient seeding density | Make sure to adjust the cell density correctly (to 1.5 × 107 cells/ml) |
| Low amount/no L-ascorbic acid in medium | Add 50 mg/L L-ascorbic acid to the culture medium | |
| Improper/poor metachromatic staining | Incorrect pH of the staining solutions | Adjust the pH of the solutions as recommended |
| Pre-treatment with acetic acid prior to acidic DMMB | Follow the protocol carefully | |
| Dark spots on cultures after staining | Imperfectly dissolved clumps of dye solution | Filter the stain solutions using a filter paper |
| Low transfection efficiency | siRNA amount incorrectly adjusted to expression level of target gene | Test different siRNA amounts until optimal silencing is achieved |
| Compromised siRNA quality | Obtain siRNA of suitable quality | |
| High rate of cell death following electroporation | Cells are not placed on ice immediately after administering the electric pulses | Transfer cuvettes on ice more rapidly |
| Electroporation cuvettes are not cool enough at the time of experiment | Place cuvettes in a refrigerator at 4°C the night before the experiment; do not remove until needed | |
| Too high siRNA concentration | Lower siRNA amount in the transfection solution while managing transfection efficiency | |
| More than the lower four-fifths of the cell solution is collected from the cuvettes | Reduce the ratio of cell solution collected | |
| Living cells were not given enough time to sink to the bottom of the cuvettes | Increase incubation time | |
| MTT-formazan crystals sometimes difficult to solubilize | Too abundant ECM (especially in more mature micromass cultures, i.e., more than 6 days old) | Allow longer solubilization time but do not agitate as detached ECM will make the solution turbid, interfering with optical density readings |
Understanding Results
If the protocol is conducted properly, chondrogenesis proceeds spontaneously in the micromass cultures. Chondrogenesis progresses through the following stages: day of seeding (day 0), proliferation (culture days 1 and 2), chondrogenic differentiation (culture days 3 and 4), matrix production (starting from culture day 4). Around culture day 10, hypertrophic chondrocytes appear within the cultures.
Cell culture morphology nicely displays the dynamic development of micromass cultures (Fig. 6). Chondrogenesis commences in the center of micromass cultures (C) and gradually proceeds towards the periphery (P) as shown on the low-power magnification phase contrast photomicrographs (Fig. 6B and 6C). Proliferating chondroprogenitor cells are shiny, circular cells in Figure 6B. Their number gradually decreases in more mature cultures as they differentiate to chondroblasts. Chondrogenic cells form clusters or nodular regions (N) within the culture. The areas between the nodules constitute the internodular zone (IN), containing a mixture of undifferentiated mesenchymal cells, some chondroprogenitor cells, and fibroblasts (Fig. 6B and 6C). Initially, the peripheral areas (internodular zone) of the round micromass cultures do not contain cartilage matrix (Fig. 6B). Over time, the nodular regions tend to increase in size and number and (especially towards the center of culture) they coalesce, leaving fewer internodular regions (Fig. 6).
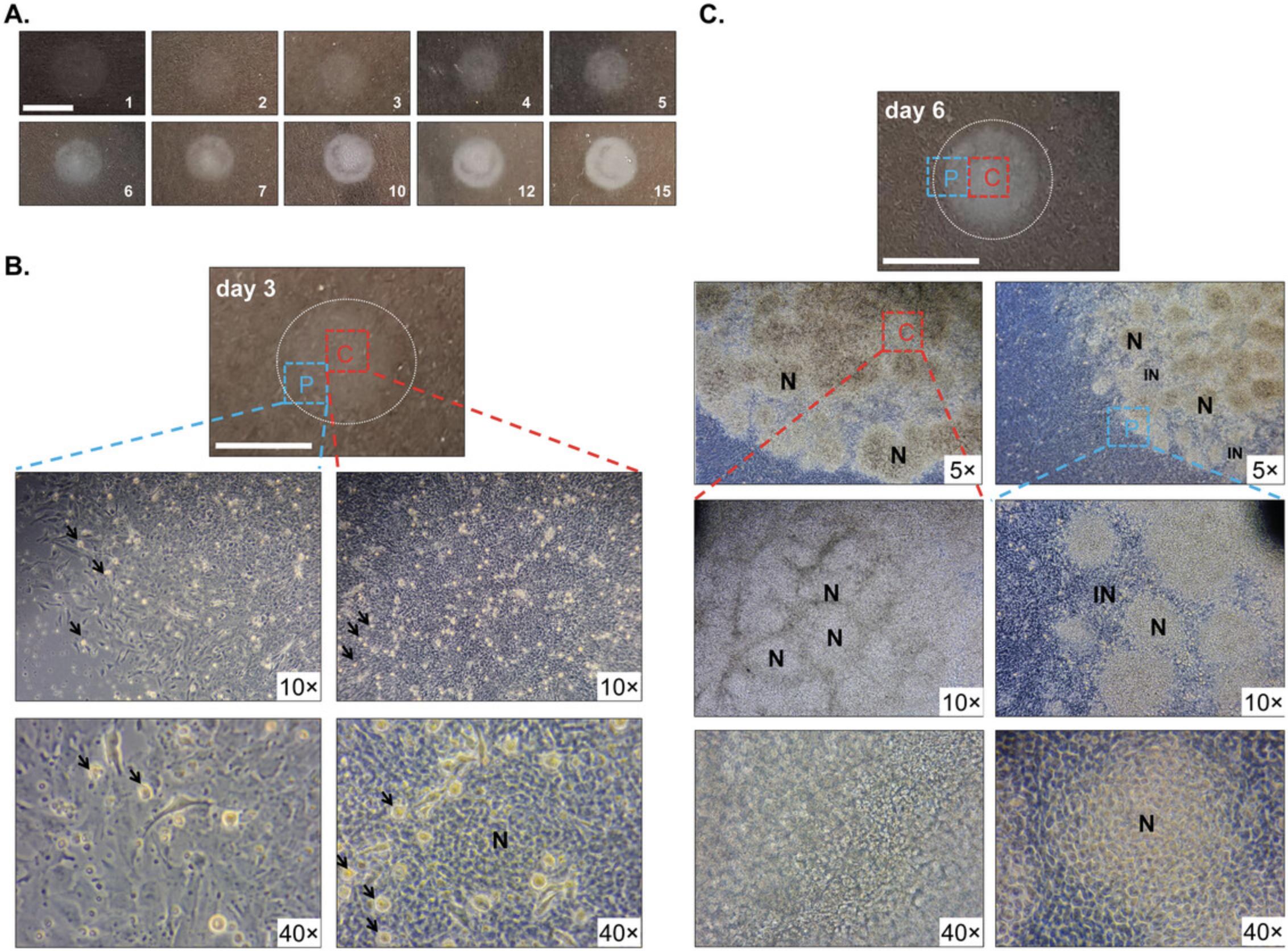
In mature micromass cultures, an abundant, hyaline cartilage-like extracellular matrix is produced by culture day 6 as revealed by staining with the metachromatic dyes DMMB, Alcian blue, and safranin O (Fig. 7A). The method described in Basic Protocol 1 relies on pooling cells derived from forelimbs and hindlimbs. However, it is possible to micromass culture forelimbs and hindlimbs separately, although the forelimb and hindlimb micromass cultures display differences in patterning and size of chondrogenic nodules (Butterfield et al., 2017).
![Details are in the caption following the image Typical outcome of the micromass model (Basic Protocol 1) after 6 days in culture. (A) Metachromatic cartilage matrix areas following staining with dimethyl methylene blue, Alcian blue, and safranin O, showing intense ECM deposition in cartilage nodules. (B) Results of an MTT assay performed every 24 hr in culture, showing an initially intense proliferation, which reaches a plateau in more mature cultures. The chart shows the time course of mitochondrial activity (cell viability) of micromass cultures; data points are average values of 6 biological replicates ± standard deviation (SD). (C) Transfection efficiency in chicken limb bud-derived chondroprogenitor cells using Basic Protocol 2. Normalized average expression data for two genes (EBF1 and ATOH8) are shown (left axis; EBF1 is in the 55<sup>th</sup> percentile, ATOH8 is in the 74<sup>th</sup> percentile ranked according to normalized expression data across all annotated genes in the dataset (BioProject IDs: PRJNA817177 and PRJNA938813; EMBL–EBI accession number: E-MTAB-12770)). Data points are average values of 3 biological replicates ± SD. Relative expression levels following transient silencing are also shown (53% and 85% of non-targeting control, respectively; right axis). Please note that the achieved silencing [as determined by RT-qPCR on culture day 2 (D2)] is dependent on the basal expression level of the target gene.](https://static.yanyin.tech/literature_test/cpz1835-fig-0007-m.jpg)
Open Research
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Supporting Information
| Filename | Description |
|---|---|
| Supplementary-file.pdf984.8 KB | Figures S1-S7 Stage-related external features of early-stage chicken embryos between Hamburger–Hamilton developmental stages 19–25. |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
Literature Cited
- Ahrens, P. B., Solursh, M., & Reiter, R. S. (1977). Stage-related capacity for limb chondrogenesis in cell culture. Developmental Biology , 60(1), 69–82. https://doi.org/10.1016/0012-1606(77)90110-5
- Bobick, B. E., Alexander, P. G., & Tuan, R. S. (2014). High efficiency transfection of embryonic limb mesenchyme with plasmid DNA using square wave pulse electroporation and sucrose buffer. Biotechniques , 56(2), 85–89. https://doi.org/10.2144/000114136
- Butterfield, N. C., Qian, C., & Logan, M. P. O. (2017). Pitx1 determines characteristic hindlimb morphologies in cartilage micromass culture. PLoS ONE , 12(7), e0180453. https://doi.org/10.1371/journal.pone.0180453
- Cancedda, R., Castagnola, P., Cancedda, F. D., Dozin, B., & Quarto, R. (2000). Developmental control of chondrogenesis and osteogenesis. International Journal of Developmental Biology , 44(6), 707–714. https://www.ncbi.nlm.nih.gov/pubmed/11061435
- Current Protocols. (2006). Commonly used reagents. Current Protocols in Microbiology , 00, A.2A.1–A.2A.15. https://doi.org/10.1002/9780471729259.mca02as00
- Eyre, D. R., Brickley-Parsons, D. M., & Glimcher, M. J. (1978). Predominance of type I collagen at the surface of avian articular cartilage. FEBS Letters , 85(2), 259–263. https://doi.org/10.1016/0014-5793(78)80468-2
- Goldring, M. B. (2006). Update on the biology of the chondrocyte and new approaches to treating cartilage diseases. Best Practice & Research. Clinical Rheumatology, 20(5), 1003–1025. https://doi.org/10.1016/j.berh.2006.06.003
- Green, M. R., & Pastewka, J. V. (1974). Simultaneous differential staining by a cationic carbocyanine dye of nucleic acids, proteins and conjugated proteins. II. Carbohydrate and sulfated carbohydrate-containing proteins. Journal of Histochemistry and Cytochemistry , 22(8), 774–781. https://doi.org/10.1177/22.8.774
- Hamburger, V., & Hamilton, H. L. (1951). A series of normal stages in the development of the chick embryo. Journal of Morphology , 88(1), 49–92. https://www.ncbi.nlm.nih.gov/pubmed/24539719
- Handschel, J. G., Depprich, R. A., Kubler, N. R., Wiesmann, H. P., Ommerborn, M., & Meyer, U. (2007). Prospects of micromass culture technology in tissue engineering. Head & Face Medicine, 3, 4. https://doi.org/10.1186/1746-160X-3-4
- Hattori, S., Sakai, K., Watanabe, K., & Fujii, T. (1996). The induction of metachromasia and circular dichroism of Coomassie brilliant blue R-250 with collagen and histone H1 is due to the low content of hydrophobic amino acid residues in these proteins. Journal of Biochemistry , 119(3), 400–406. https://doi.org/10.1093/oxfordjournals.jbchem.a021255
- Heuft, G., & Bohm, N. (1978). [Age-dependency of toluidine-blue dichroism and birefringence of fibers within the costo-chondral junction of human ribs (author's transl)]. Histochemistry , 59(1), 45–53. https://doi.org/10.1007/BF00506476 (Altersabhangigkeit des Toluidinblau-Dichroismus und der Anisotropie der Fasern in der Wachstumszone menschlicher Rippen.)
- Kiraly, K., Lapvetelainen, T., Arokoski, J., Torronen, K., Modis, L., Kiviranta, I., & Helminen, H. J. (1996). Application of selected cationic dyes for the semiquantitative estimation of glycosaminoglycans in histological sections of articular cartilage by microspectrophotometry. Histochemical Journal , 28(8), 577–590. https://doi.org/10.1007/BF02331378
- Koff, S. A., McDowell, G. C., & Byard, M. (1988). Diuretic radionuclide assessment of obstruction in the infant: Guidelines for successful interpretation. Journal of Urology , 140(5 Pt 2), 1167–1168. https://doi.org/10.1016/s0022-5347(17)41991-4
- Rolfe, R. A., Shea, C. A., & Murphy, P. (2022). Geometric analysis of chondrogenic self-organisation of embryonic limb bud cells in micromass culture. Cell and Tissue Research , 388(1), 49–62. https://doi.org/10.1007/s00441-021-03564-y
- Romhanyi, G. (1963). [on the Submicroscopic Structural Principle of the Metachromatic Reaction]. Acta Histochemica , 15, 201–233. https://www.ncbi.nlm.nih.gov/pubmed/14045924 (Uber die submikroskopische strukturelle grundlage der metachromatischen reaktion.)
- Rosenberg, L. (1971). Chemical basis for the histological use of safranin O in the study of articular cartilage. Journal of Bone and Joint Surgery. American , 53(1), 69–82. https://www.ncbi.nlm.nih.gov/pubmed/4250366
- Solanki, K., Shanmugasundaram, S., Shetty, N., & Kim, S. J. (2021). Articular cartilage repair & joint preservation: A review of the current status of biological approach. Journal of Clinical Orthopaedics & Trauma, 22, 101602. https://doi.org/10.1016/j.jcot.2021.101602
- Takacs, R., Vago, J., Poliska, S., Pushparaj, P. N., Ducza, L., Kovacs, P., Jin, E. J., Barrett-Jolley, R., Zakany, R., & Matta, C. (2023). The temporal transcriptomic signature of cartilage formation. Nucleic Acids Research , 51(8), 3590–3617. https://doi.org/10.1093/nar/gkad210
- Templeton, D. M. (1988). The basis and applicability of the dimethylmethylene blue binding assay for sulfated glycosaminoglycans. Connective Tissue Research , 17(1), 23–32. https://doi.org/10.3109/03008208808992791
- van Meerloo, J., Kaspers, G. J., & Cloos, J. (2011). Cell sensitivity assays: The MTT assay. Methods in Molecular Biology , 731, 237–245. https://doi.org/10.1007/978-1-61779-080-5_20
- Zheng, C. H., & Levenston, M. E. (2015). Fact versus artifact: Avoiding erroneous estimates of sulfated glycosaminoglycan content using the dimethylmethylene blue colorimetric assay for tissue-engineered constructs. European Cells & Materials [Electronic Resource], 29, 224–236. discussion 236. https://doi.org/10.22203/ecm.v029a17
Key References
- Ahrens et al. (1977). See above.
This is the original paper describing the embryonic chicken limb bud-derived micromass cultures.
- Hamburger, & Hamilton (1951). See above.
A paper detailing the morphological features of distinct developmental stages of early-stage chicken embryos.
- Takacs et al. (2023). See above.
This paper presents a detailed analysis of the transcriptomic signature during chondrogenesis in this model.
Internet Resources
Source to download the metachromasiaIndex program used in Basic Protocol 3.
Source to download the metachromasiaIndex program used in Basic Protocol 3.
Source to install MATLAB Runtime, a free utility for running MATLAB programs on systems not having MATLAB
Compressed folder containing the executable version of the metachromasiaIndex program to use with MATLAB Runtime.
Citing Literature
Number of times cited according to CrossRef: 1
- Judit Vágó, Csilla Somogyi, Roland Takács, Krisztina Bíróné Barna, Eun‐Jung Jin, Róza Zákány, Csaba Matta, Isolation and Culturing of Primary Murine Chondroprogenitor Cells: A Mammalian Model of Chondrogenesis, Current Protocols, 10.1002/cpz1.1005, 4 , 3, (2024).

