Scanning Electron Microscopy
Elizabeth R. Fischer, Elizabeth R. Fischer, Bryan T. Hansen, Bryan T. Hansen, Vinod Nair, Vinod Nair, Forrest H. Hoyt, Forrest H. Hoyt, Cindi L. Schwartz, Cindi L. Schwartz, David W. Dorward, David W. Dorward
critical point drying
cryo-SEM quantum dots
EM specimen preparation
immune-labeling
microwave-processing
scanning electron microscopy
specimen fracturing
sputter coating
Abstract
Scanning electron microscopy (SEM) remains distinct in its ability to allow topographical visualization of structures. Key elements to consider for successful examination of biological specimens include appropriate preparative and imaging techniques. Chemical processing induces structural artifacts during specimen preparation, and several factors need to be considered when selecting fixation protocols to reduce these effects while retaining structures of interest. Particular care for proper dehydration of specimens is essential to minimize shrinkage and is necessary for placement under the high-vacuum environment required for routine operation of standard SEMs. Choice of substrate for mounting and coating specimens can reduce artifacts known as charging, and a basic understanding of microscope settings can optimize parameters to achieve desired results. This article describes fundamental techniques and tips for routine specimen preparation for a variety of biological specimens, preservation of labile or fragile structures, immune-labeling strategies, and microscope imaging parameters for optimal examination by SEM. © 2024 The Authors. Current Protocols published by Wiley Periodicals LLC.
Basic Protocol 1 : Chemical preparative techniques for preservation of biological specimens for examination by SEM
Alternate Protocol 1 : Practical considerations for the preparation of soft tissues
Alternate Protocol 2 : Removal of debris from the exoskeleton of invertebrates
Alternate Protocol 3 : Fixation of colonies grown on agar plates
Alternate Protocol 4 : Stabilization of polysaccharide structures with alcian blue and lysine
Alternate Protocol 5 : Preparation of non-adherent particulates in solution for SEM
Support Protocol 1 : Application of thin layer of adhesive on substrate to improve adherence
Support Protocol 2 : Poly-L-lysine coating specimen substrates for improved adherence
Support Protocol 3 : Microwave processing of biological specimens for examination by SEM
Basic Protocol 2 : Critical point drying of specimens
Alternate Protocol 6 : Chemical alternative to critical point drying
Basic Protocol 3 : Sputter coating
Alternate Protocol 7 : Improved bulk conductivity through “OTOTO”
Basic Protocol 4 : Immune-labeling strategies
Alternate Protocol 8 : Immune-labeling internal antigens with small gold probes
Alternate protocol 9 : Quantum dot or fluoronanogold preparations for correlative techniques
Basic Protocol 5 : Exposure of internal structures by mechanical fracturing
Basic Protocol 6 : Exposure of internal structures of tissues by fracturing with liquid nitrogen
Basic Protocol 7 : Anaglyph production from stereo pairs to produce 3D images
INTRODUCTION
With the increasing number of advanced imaging tools available, the utility of conventional imaging techniques is often overlooked. In fact, the ability to visualize structures with the high resolution achieved by using electron microscopes provides the foundation for developing valid conclusions about functional relationships. Despite advances in other types of light microscopy (LM), atomic force microscopy (AFM), and transmission electron microscopy (TEM), scanning electron microscopy (SEM) remains distinct in its ability to examine dimensional topography and the distribution of exposed features. The ultimate resolution achieved is controlled by optimizing both specimen preparation and instrumental parameters.
Preservation of biological structures in a manner that prevents decay under the high vacuum necessary for a mean free path of travel for the electron beam is a primary concern. Biological specimens are generally composed of nonconductive, thermally sensitive, fragile material, which if not stabilized, results in specimen damage and imaging artifacts. Specimens can be prepared through chemical and physical (e.g., cryopreservation) methods or both. Although cryopreservation may be the preferred method for optimal near native state preservation, it requires expensive instrumentation, advanced skills, and is not necessary to address many scientific questions. In general, preparation of specimens for examination by SEM requires stabilization of structures as described in Basic Protocol 1, then dehydration of specimens as described in Basic Protocol 2 and, finally, ensuring adequate conductivity as discussed in Basic Protocol 3, or their respective alternatives.
Specifically, Basic Protocol 1 provides some procedures and tips for chemical preservation of animal tissues and cells, bacteria, viruses, and macromolecular specimens such as amyloid fibrils or lipid droplets. Basic Protocol 2 provides options for specimen mounts and critical point or chemical drying, and Basic Protocol 3 addresses the rationale for metal coating and alternatives. Additional techniques for immune-labeling strategies with special considerations for correlative techniques are outlined in Basic Protocol 4, and “affordable” specimen fracture is discussed in Basic Protocols 5 and 6. Basic Protocol 7 describes stereo pair and anaglyph generation to produce 3-dimensional (3D) images. The Commentary section discusses basic theory and application for both preparative and imaging techniques of biological specimens for SEM and offers a troubleshooting guide for common problems.
There is no one method or condition that ensures successful preparation and imaging for all biological specimens; thus, the user should be willing to experiment with preparative and imaging techniques and technologies to achieve the desired results. The content of this article is not meant to be exhaustive, but rather offers a starting point for biological specimen preparation and imaging by SEM.
SAFETY CONSIDERATIONS
Preparation of biological specimens for electron microscopy includes biological, chemical, and mechanical risks to the user. It is imperative to understand the requirements for proper protection to minimize exposure in handling infectious agents, hazardous chemicals, and operation of high voltage instrumentation. Pathogen-specific information can be found in CDC guidelines and should be handled according to risk (Burnett et al., 2009). Consult your local biosafety officer if needed. Common fixatives and embedding resins are highly toxic, carcinogenic, oxidative, volatile, or radioactive; thus, it is imperative to understand the proper handling, use, and disposal according to approved safety and environmental regulations. General requirements include performing protocols in an appropriate fume hood while wearing personal protective equipment (PPE) including laboratory coats, appropriate gloves, and eye protection. Latex gloves are considered inadequate for most chemicals utilized for EM preparative techniques, and nitrile gloves are generally more suitable, although selection should be determined based on specific chemicals used (Lunn & Lawler, 2011; Meisenhelder & Semba 2006). The associated instruments and technologies also pose potential risks related to high voltage, radiation, pinching hazards, liquid nitrogen use, and high pressure (critical point dryer). Proper operation of instrumentation requires an understanding of the risks and precautions, and heeding advice according to manufacturers’ specifications.
Basic Protocol 1: CHEMICAL PREPARATIVE TECHNIQUES FOR PRESERVATION OF BIOLOGICAL SPECIMENS FOR EXAMINATION BY SEM
The following sections describe general protocols for the preservation of prokaryotic and eukaryotic cells grown as a monolayer or in suspension. Alternate protocols will include preparation for bulk materials, particulate specimens, and suggestions for additional cleaning steps and preservation of friable structures. In general, samples must be processed to dryness. Bulk, granular, and powdered materials may not require processing. Biological specimens will typically be treated with protein and lipid cross-linking reagents, such as glutaraldehyde (GA) and osmium tetroxide (OsO4) respectively, dehydrated with an organic solvent, such as ethanol or acetone, and critical point dried through carbon dioxide. Sample containers with appropriate-sized porosity and chemical tolerance should be used for this purpose. Examples include polystyrene 24-well tissue culture plates (except with use of acetone or propylene oxide), microcentrifuge tubes, glass vials, and stainless steel and polytetrafluoroethylene (PTFE or Teflon) baskets with matching lids, which are available from various EM supply providers.
Adherent cells can be grown on a variety of substrates including silicon chips, ACLAR, membrane filters, Thermanox, or glass coverslips. In recent years, silicon chips have become popular mounting substrates for SEM imaging of widely varied types of samples. They offer convenient dimensions for use with most SEM sample stubs, a virtually featureless surface with minimal background electron emission, and better charge dispersal properties than similar substrates such as glass or plastic polymers. Some cell types show preference for a specific substrate and substrates can be treated, for example with poly-L-lysine to aid in cell adhesion. Experiments requiring use of polarized cells may require cultures to be grown on Transwell membrane filters.
Sample preparation for EM inherently introduces structural changes in specimens that can lead to artifacts or loss of structure, which can be reduced with optimized preparative techniques. The following methodologies provide a general protocol suitable for most eukaryotic and bacterial cells and alternate protocols to meet specific circumstances.
Materials
-
Eukaryotic cells grown in suspension, or on solid substrate, e.g.:
- Thermanox coverslips
- Silicon chips
- ACLAR film
- Transwell membrane filters
- ACLAR typically comes in sheets that can be cut or punched to desired size. Items available from Ted Pella, cat. nos. 10501-10, 16003, Electron Microscopy Sciences, cat. nos. 50425, 50426, 72296, and other sources.
-
Physiologically appropriate buffer, e.g.:
- Hanks’ buffered saline solution (HBSS) (Thermo Fisher Scientific, cat. no. 14025076)
- 1× phosphate-buffered saline (PBS) (Thermo Fisher Scientific, cat. no. J61196.AP)
- We use Gibco HBSS and can also be found through other sources.
-
Primary fixatives (see recipe):
- 2.5% glutaraldehyde (GA) and/or 4% paraformaldehyde (PFA) in 0.1 M sodium cacodylate (CAC) or phosphate buffer (PB) (see recipe), pH 6.8 to 7.4
-
Rinsing buffer:
- 0.1 M CAC, pH 7.2 or,
- PB (see recipe)
- CAC can be purchased as a powder or pre-made from a variety of microscopy sources, including Ted Pella, Electron Microscopy Sciences, Poly Sciences, etc. We typically use the powder form from Ted Pella, cat. no. 18851.
-
Osmium or reduced osmium tetroxide, secondary fixative (see recipe):
- 1% OsO4 in dH2O or,
- Reduced osmium with potassium ferrocyanide [0.5% OsO4/0.8% K4Fe(CN)6] in dH2O or 0.1 M CAC
-
H2O, distilled (dH2O)
-
Dehydrating agent, typically ethanol or acetone
-
Fine-tipped forceps
-
Microcentrifuge tubes
-
Microcentrifuge
-
Scissors
-
Mounting substrate (e.g., silicon chip, coverslip, or membrane filter)
-
Container for processing (e.g., 24-well plate, microcentrifuge tube, PTFE basket)
-
Water/ethanol-proof marking pen
-
Parafilm or tape, optional
NOTE : All procedures are carried out at room temperature unless noted otherwise.
Fix adherent or non-adherent cells
1.Remove the number of silicon chips, coverslips, or Transwell membrane filters required for the experiment with fine-tipped forceps. Trim the coverslips using scissors on two sides so they can be later placed in the container for critical point drying, if applicable, as shown in Figure 1.

2a. Adherent cells : Place one sterile coverslip, silicon chip (shiny side up; Fig. 2A), or membrane filter per well in 24-well plate. Label the plate cover with sample designation as desired using a water/ethanol-proof marking pen. Plate the adherent cells at an approximate density of 1–2 × 105/ml under sterile conditions and conduct experiment.
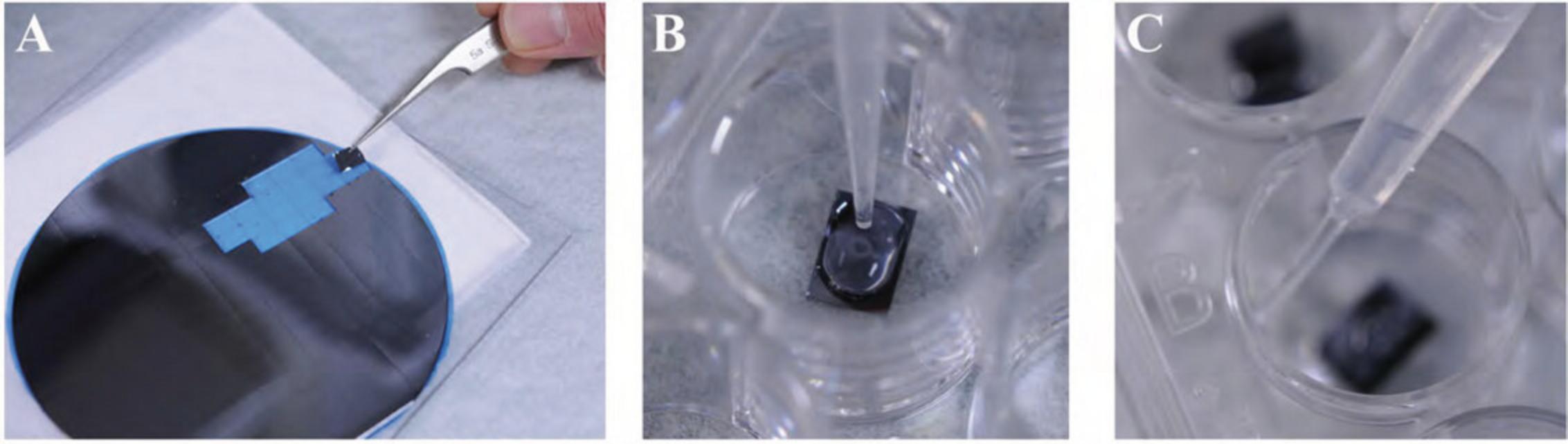
2b. Non-adherent cells : After the experiment, wash the cells in a microcentrifuge tube with an appropriate physiological buffer (e.g., PBS or HBSS) by gently centrifuging (greater than ∼500,000 cells per sample) for 5 to 10 min at 1000 to 2000 × g , 22°C. Remove the supernatant and gently resuspend in 50 µl buffer. (Often when the supernatant is removed, the pellet remains in a small volume of liquid adequate for resuspension by simply flicking the tube with fingertip before adding the buffer.) Pipet the suspension (Fig. 2B) and allow to settle for 20 to 30 min and then proceed to step 4 as for adherent cells.
3.To fix the cells, remove most of the medium but not all to avoid drying of sample, which adversely affects ultrastructure. Briefly wash the cells with 0.5 ml (or enough to completely cover the specimen) of physiologically appropriate buffer (e.g., PBS or HBSS).
4.In a fume hood, quickly but gently add ∼0.5 ml primary fixative, typically 2.5% GA in 0.1 M CAC or PB, by dispersing a slow stream of the liquid as shown in Figure 2C. Be careful to keep the coverslip or chip from floating, making sure the specimen remains completely immersed.
5.Fix the specimen at room temperature by completely immersing in primary fixative [e.g., 2.5% glutaraldehyde in 0.1 M sodium cacodylate or 0.1 M phosphate buffer at appropriate pH (∼6.8 to 7.4)] for 30 to 60 min (fixing at 4°C may improve preservation of some specimens).
6.Manually remove the fixative using a pipette leaving a small puddle of liquid over the sample to prevent drying of the specimen. Wash three times, each time for 2 min with ∼0.5 ml rinsing buffer.
7.Post-fix with ∼0.5 ml secondary fixative (e.g., osmium mixture) for 30 to 60 min at room temperature.
8.Rinse once for 2 min with ∼0.5 ml rinsing buffer, and then twice, each time for 2 min with ∼0.5 ml dH2O.
9.Dehydrate with a graded ethanol series by subsequent exchanges of the following dilutions in distilled water as follows:
- 25% ethanol, 1 × 5 min for delicate specimens
- 50% ethanol, 1 × 5 min
- 75% ethanol, 1 × 5 min (specimen can be stored overnight at 4°C at this step)
- 95% ethanol, 1 × 5 min
- 100% anhydrous ethanol 3 × 10 min (less time may be required for monolayers).
10.Proceed to Basic Protocol 2 for drying options of specimens and then Basic Protocol 3 for coating options.
Alternate Protocol 1: PRACTICAL CONSIDERATIONS FOR THE PREPARATION OF SOFT TISSUES
Morphological changes occur immediately upon sacrifice or excision of tissue from an animal. Tissues should quickly be immersed in fixative and cut into pieces small enough for adequate penetration by fixatives. Typically, specimens should be no wider than 1 mm in at least one dimension, although this can vary depending on the density or porosity of a particular tissue. Tissue pieces in general require extended time for each of the steps in Basic Protocols 1 and 2.The combination of 4% PFA and 2.5% GA to the primary fixative and additional impregnation with heavy metals can help stabilize and improve bulk conductivity of the specimen.
Additional Materials (also see Basic Protocol 1)
-
Excised tissues
-
1% tannic acid (TA) in dH2O
- We use Thermo Fisher Scientific, cat. no. 36410, Electron Microscopy Sciences, cat. no. 21700, but can be found from other sources.
-
1% to 2% uranyl acetate (UA) in dH2O
- We typically use powder form from Electron Microscopy Sciences, cat. no. 22400.
-
Dissecting tools (e.g., scalpel, micro-scissors, or razor blade)
-
Dish for dissection
1.Place excised tissues in an appropriate container such as a microcentrifuge tube, glass vial, or plate filled with primary fixative (e.g., 4% PFA/2.5% GA in 0.1 M CAC or PB) for a minimum of 1 to 2 hr at room temperature.
2.Wash the small tissue pieces three times, each time for 5 min with ∼0.5 ml rinsing buffer.
3.Post-fix the tissue by completely immersing in ∼0.5 ml reduced OsO4 [e.g., 0.5% to 1% OsO4/0.8% K4Fe(CN)6 in 0.1 M CAC, pH 6.8 to 7.4] for 1 hr.
4.Wash the specimen three times, each time for 5 min with ∼0.5 ml rinsing buffer (e.g., 0.1 M CAC or PB, pH 6.8 to 7.4).
5.Stain with ∼0.5 ml of 1% TA in dH2O for 1 hr.
6.Wash once for 5 min with ∼0.5 ml rinsing buffer and then twice for 5 min each time with ∼0.5 ml dH2O.
7.Stain with ∼0.5 ml of 1% UA in dH2O 1 hr at room temperature or overnight at 4°C.
8.Wash three times for 5 min each time with ∼0.5 ml dH2O.
9.Dehydrate as in Basic Protocol 1 (step 9) with a graded ethanol or acetone series.
10.Proceed to Basic Protocols 2 and 3 for drying, coating, and mounting specimens for SEM analysis.
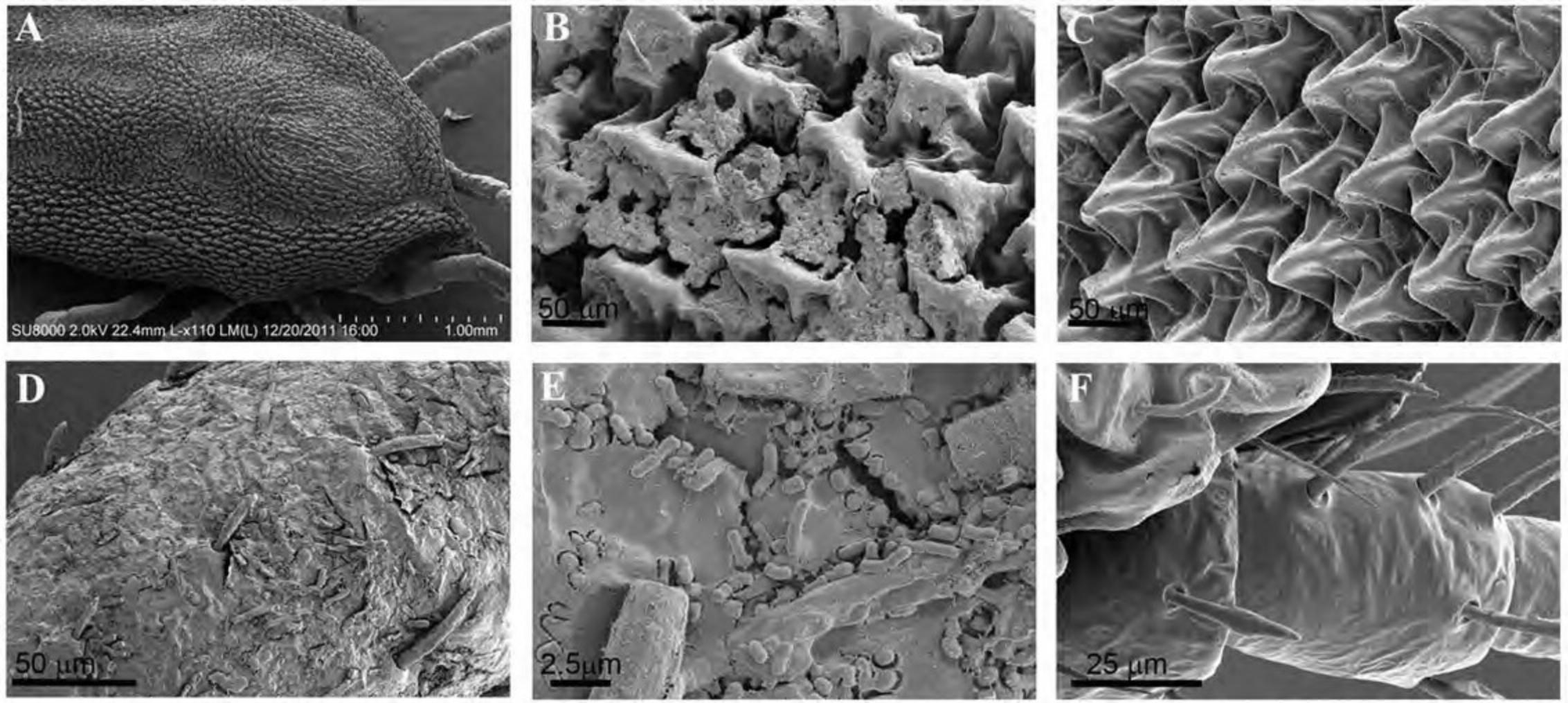
Alternate Protocol 2: REMOVAL OF DEBRIS FROM THE EXOSKELETON OF INVERTEBRATES
The terrestrial lifestyle of insects and arachnids (such as ticks and mites) often results in debris covering most of the exoskeleton, obscuring structural details. Washing with buffers proves insufficient for adequate removal of contaminants, whereas treatment with solvents and sonication has been shown effective for use on hard ticks as described by Dixon et al. (2000). Alternatively, if solvent treatment damages a particular specimen, Corwin's technique offers an alternative that includes the application of an adhesive and careful mechanical removal of the debris (Corwin, 1979). Although the results of this technique are excellent, it can be technically challenging.
The following procedure demonstrates the removal of debris from the surface of a soft tick using the Dixon method.
Additional Materials (also see Basic Protocol 1)
-
Tick, mite, or another arthropod
-
Ethanol
-
Acetone
-
Xylene
-
1.5-ml glass vials with tightly sealed caps or 1.5-ml microcentrifuge tubes or appropriate container
-
Sonicator
1.Place the tick in a 1.5-ml glass vial, microcentrifuge tube, or appropriate container filled with 70% ethanol for ∼1 hr at room temperature.
2.Manually remove any tissue still attached to the tick mouth area with fine-point forceps.
3.Place the ticks in individual, sealable glass vials.
4.Dehydrate twice, each time with ∼0.5 ml 100% ethanol for 1 hr at room temperature.
5.Replace the ethanol with 1.5 to 5 ml dry acetone (to fill the container) and leave overnight at room temperature or at 4°C, ensuring the glass vial is well sealed.
6.Replace the acetone with xylene and sonicate for 15 to 30 min.
7.Wash two times with ∼0.5 ml acetone, each time for 1 hr at room temperature.
8.Proceed to critical point drying, mounting, and coating options in Basic Protocols 2 and 3.
Alternate Protocol 3: FIXATION OF COLONIES GROWN ON AGAR PLATES
Many free-living and pathogenic bacteria are known to produce extracellular bio-products that aggregate and form components such as s-layers and biofilm matrices. As with the bacteria themselves, such components exhibit considerable biochemical diversity. Often such layers and matrices are poorly cross-linked when preserved with standard aldehyde primary fixatives and are lost during further processing. If the biochemical nature of the extracellular material is known, fixatives can be chosen to optimize preservation. If unknown, it may be necessary to determine the best fixative empirically by testing multiple cross-linking reagents. Minimizing immersion and agitation of samples in fluids during processing is also helpful. The following protocol is intended for bacterial colonies on excised pieces of agar-based solid medium. The protocol involves prefixing the samples with osmium tetroxide vapors and allowing fixatives to diffuse through the substrate to reach the colonies. The methods described can be modified as necessary for bacteria on other types of substrata.
Additional Materials (also see Basic Protocols 1 and 2)
-
Colonies grown on agar plates
-
Scalpel or razor blade
-
25-mm to 90-mm glass or plastic Petri dish
1.Excise blocks of agar with colonies of interest with scalpel, razor blade, or other dissecting tool (pieces of ∼5-mm across or in diameter and 2- to 3-mm thick work well).
2.Place blocks, colony side upward, into a glass or plastic Petri dish.
3.Pipet 0.5 ml of 1% OsO4 in dH2O into the Petri dish avoiding contact with the sample blocks. Replace the cover on the Petri dish, seal the dish with Parafilm, and let stand for 1 hr at room temperature.
4.Remove the blocks from the Petri dish and place in wells of a 24-well tissue culture plate or other convenient vessel. To each sample well, add a volume of primary fixative sufficient to wet the agar block without allowing the fluid or meniscus to reach the colonies. This will be ∼0.5 ml depending on the thickness of the agar block. Cover and let stand for 1 to 2 hr at room temperature.
5.Aspirate the fixative and gently replace with a rinsing buffer that now covers the colonies for all subsequent steps. Let stand for 30 min at room temperature. Repeat the step for a second wash to remove the primary fixative.
6.Post-fix for 1 to 2 hr using 1% OsO4 in 0.1 M PB, or other appropriate buffers such as 0.1 M CAC.
7.Wash the samples twice as in step 5 with 0.5 to 1 ml dH2O.
8.Dehydrate the samples using graded ethanol series (see Basic Protocol 1, step 9), but allow 1 to 2 hr per step for thorough diffusion through the agar blocks and colonies.
9.See Basic Protocol 2 for critical point drying (steps 1 to 7).
10.Carefully remove the agar blocks from the critical point dryer sample container.
11.Proceed to Basic Protocol 3.
Alternate Protocol 4: STABILIZATION OF POLYSACCHARIDE STRUCTURES WITH ALCIAN BLUE AND LYSINE
Polysaccharides, often a constituent of extracellular matrices, are typically lost during conventional EM processing. Fassel et al. showed improved retention of these structures through the addition of alcian blue and lysine to the primary fixative (Fassel et al., 1997). The addition of ruthenium red to the primary fixative has also been shown to improve retention of these friable structures (Luft, 1971). Below is an example of bacterial colonies grown on agar plates and fixed with alcian blue/lysine additives for retention of glycoprotein-rich components on the biofilm produced by Yersinia pestis.
Additional Materials (also see Basic Protocol 1)
- Colonies grown on agar plates or in suspension
- Alcian blue/lysine fixative (see recipe)
1.Gently press coverslip or silicon chip on bacterial colonies on agar plate or settle suspension as described in Basic Protocol 1 for non-adherent cells and place the coverslip/chip in a 24-well plate or other suitable container.
2.After a brief rinse with ∼0.5 ml physiologically appropriate buffer, fix with alcian blue/lysine mixture for 2 hr at room temperature.
3.Rinse three times, each time for 5 min with ∼0.5 ml of 0.1 M CAC, pH 7.2.
4.Post-fix with ∼0.5 ml of 1% OsO4/0.8% K4Fe(CN)6 in 0.1 M CAC, pH 7.2, for 1 hr at room temperature.
5.Rinse once for 5 min with ∼0.5 ml of 0.1 M CAC, pH 7.2, and two times for 5 min with ∼0.5 ml dH2O.
6.Dehydrate with graded ethanol series, starting with 25%, 50%, 75%, and 95% ethanol and three exchanges of anhydrous 100% ethanol prior to critical point drying and sputter coating procedures outlined in Basic Protocols 2 and 3.
Alternate Protocol 5: PREPARATION OF NON-ADHERENT PARTICULATES IN SOLUTION FOR SEM
In many cases, particulate or powdery substances can simply be air dried on a silicon chip or coverslip before sputter coating when structures would not be expected to collapse after water or volatile liquid is removed from the sample. (Basic Protocol 3).
Materials
- Particulates/macromolecules in water or volatile salt solution at desired concentration
- Appropriate mounting substrate
1.Apply 50 µl droplets to the appropriate mounting substrate and allow to air dry completely.
Support Protocol 1: APPLICATION OF THIN LAYER OF ADHESIVE ON SUBSTRATE TO IMPROVE ADHERENCE
As with glass and plastic, not all samples adhere readily to the surface of silicon chips. Below are relatively easy methods for improving adherence to such surfaces by precoating the surfaces with a very thin adhesive layer.
Additional Materials (also see Basic Protocol 1)
-
Acetone
-
Carbon conductive mounting tabs (available from microscopy suppliers, including Ted Pella, Electron Microscopy Sciences, and Ladd Research)
-
Microcentrifuge tubes, acetone-resistant such as polypropylene (PP) or high-density polyethylene (HDPE)
-
Tweezers
-
Petri dish lid or similar cover
1.Immerse four conductive carbon mounting tabs, into 1 ml acetone in a solvent-resistant tube. Agitate by manually shaking the container for 1 to 2 min. Remove the carbon tabs with tweezers.
2.Place the desired number of silicon chips into the acetone/adhesive mixture. Up to ten or so chips can be added to a microcentrifuge tube. Allow 1 to 2 min for coating, inverting the tube periodically.
3.Remove each chip individually with tweezers and place on a clean surface, desired side up. Cover the chip with a Petri dish lid or similar cover to prevent dust accumulation on the chip.
4.Sprinkle particulate samples onto chips to the desired spatial density or apply and fix as described in Basic Protocol 1 for non-adherent specimens (step 1b).
5.Proceed to Basic Protocols 2 and 3 for proper drying and coating techniques.

Support Protocol 2: POLY-L-LYSINE COATING SPECIMEN SUBSTRATES FOR IMPROVED ADHERENCE
For similar results, coverslips or silicon chips can be precoated with a protein or lectin layer to which cells can attach and be stabilized by protein-protein interactions during fixation. Although this works quite well for many non-adherent cell types, the layer itself can be quite visible in the electron microscope, and for small particles, may interfere with visualization of the particle of interest.
Additional Materials (also see Basic Protocol 1)
-
0.1% poly-L-lysine in solution (without sodium azide) (e.g., Electron Microscopy Sciences, cat. no. 19320)
-
Small glass beaker
-
Coverslip holder for drying
1.Dip the coverslip or chip into a small glass beaker filled with 0.1% poly-L-lysine.
2.Air dry the coverslip or chip completely by suspending in a coverslip rack or placing on a clean surface.
3.Proceed to Basic Protocol 1, step 2a for attachment of non-adherent specimens.
Support Protocol 3: MICROWAVE PROCESSING OF BIOLOGICAL SPECIMENS FOR EXAMINATION BY CONVENTIONAL SEM
Microwave-assisted fixation for EM application has become increasingly popular over the last decade with advancements in technology and refinement of the technique. Advantages include reduction in sample preparation time, and for some specimens, improved structural integrity. The following protocol was adapted from techniques developed by others (Giberson & Demaree, 2002). This protocol can be used for TEM or SEM specimen preparation, and reagents can be modified according to preferred protocol, including immunology.
Additional Materials (also see Basic Protocol 1 and Alternate Protocol 1)
-
Specimens on SEM appropriate substrate (e.g., silicon chips, coverslips, etc.) in fixative
-
Appropriate buffer
-
Suitable container (e.g., 24 cell well tissue culture plate)
-
Laboratory wattage-controllable microwave oven with load cooler, vented into a fume hood
- The wattage control with coldspot is required to prevent overheating of specimens. We use the Ted Pella Pelco BioWave. See Internet Resources for more information on how it functions.
NOTE : Do not fill liquid solutions over 1 cm in height to prevent inadequate microwave oven penetration.
1.Rinse the specimen on appropriate substrate in a suitable container twice, each time with 0.5 ml of the appropriate buffer for 45 s in a microwave oven at 250 W/24°C, without vacuum.
2.Post-fix by adding 0.5 ml of 0.5% OsO4/0.8% K4Fe(CN)6 in buffer two times (2 min on-2 min off-2 min on cycle) at 80 W/24°C, under vacuum (at 20 in. Hg).
3.Rinse the specimen twice, each time with 0.5 ml of the appropriate buffer for 45 s in microwave oven at 250 W/24°C, without vacuum.
4.Stain with ∼0.5 ml of 1% TA in dH2O, two times (2 min on-2 min off-2 min on cycle) at 80 W/24°C, under vacuum (at 20 in. Hg).
5.Rinse twice with ∼0.5 ml dH2O, each time for 45 s in a microwave oven at 80 W/24°C, without vacuum.
6.Stain with ∼0.5 ml of 1% UA in dH2O, two times (2 min on-2 min off-2 min on cycle) at 80 W/24°C, under vacuum (at 20 in. Hg).
7.Rinse twice with ∼0.5 ml dH2O, each time for 45 s in a microwave oven at 80 W/24°C, without vacuum.
8.Dehydrate with graded ethanol or acetone series as described in Basic Protocol 1 (step 9), using 50%, 75%, 95% ethanol, and 3 × 100% anhydrous ethanol exchanges for 45 s each in the microwave oven at 250 W/24°C, without vacuum.
9.Proceed to critical point drying (Basic Protocol 2) and then coating (Basic Protocol 3).
Basic Protocol 2: CRITICAL POINT DRYING SPECIMENS
Biological specimens are composed largely of water. Although environmental SEMs and microscopes equipped with cryo-stages do not require water removal from specimens, the conventional SEM still does.
If allowed to simply air dry, most biological structures would shrink, collapse, and break due to the surface tension of the water leaving the specimen. Solvent dehydration followed by critical point drying (CPD) is the most common method for removing water from biological specimens for SEM processing although chemical alternatives can also be used. As the name implies, the specimen is heated to the critical point of the transition agent where at specific temperatures and pressures the gas and liquid phases are indistinguishable. At this phase, the agent can be slowly released as a gas, and the artifacts associated with shrinkage minimized. The critical point for water and ethanol are high (∼374°C at 217 atmospheres and 241°C at 60 atmospheres, respectively), but other agents such as carbon dioxide (CO2) are in a range tolerable for most specimens (31.1°C at 73 atmospheres). The following section provides a brief overview of critical point drying. Alternate Protocol 6 describes a chemical alternative.
Materials
-
100% dry ethanol
-
Silicon chip, pretrimmed coverslip, or Transwell membrane (see Basic Protocol 1)
-
Critical point dryer (CPD)
-
Specimen container
-
Solvent-resistant container, e.g., polyethylene (PE) specimen cup
-
Fine-tipped forceps
-
Scalpel
-
CO2 siphon tank
1.Place the critical point dryer specimen container/holder in a solvent-resistant container such as a PE specimen cup and fill with 100% dry ethanol to the top of the holder.
2.With fine-tipped forceps, quickly remove and place the chip or pretrimmed coverslip in the slot of the CPD holder, being careful to avoid damaging and drying specimen as shown in Figure 6.

3.Remove Transwell membrane filter inserts by immersing the filter end in a dish containing 100% ethanol (to avoid drying) and carefully cutting around the rim of the filter with a scalpel as shown in Figure 7A to C. Once cut, pick up the filter with fine-tipped forceps and place in the CPD container (as described in step 2).

4.Insert the specimen holder into the chamber of the CPD instrument, filled per manufacturers’ recommendations with 100% ethanol.

5.When the chamber has reached 10°C, the transition agent (e.g., CO2 from a siphon tank) can be administered as indicated by the manufacturer. The objective is to replace all the ethanol with liquid CO2 prior to heating to the critical point under pressure.
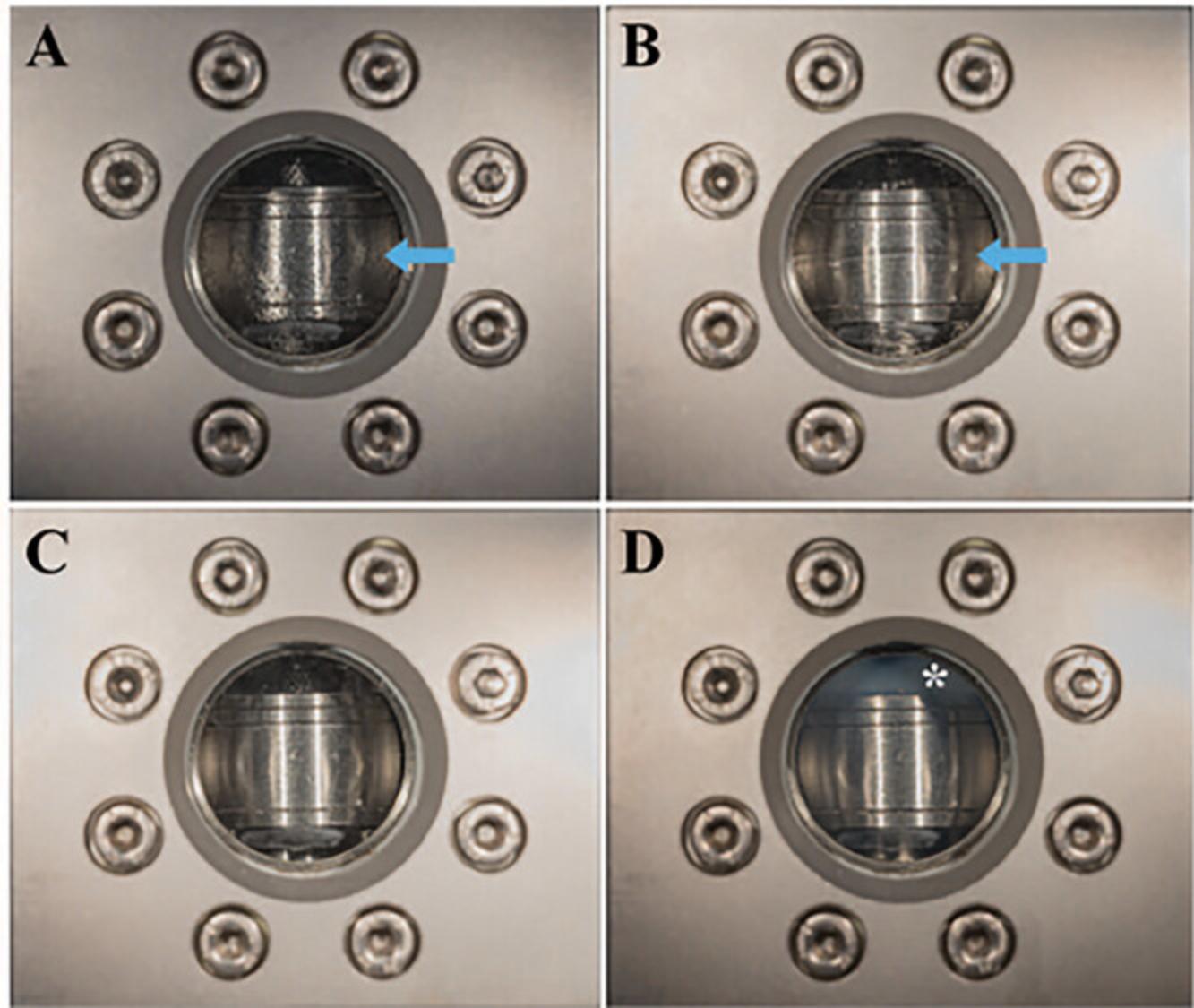
6.When the ethanol has been fully exchanged with the liquid CO2 in the CPD chamber, the CPD heater is turned on. As the CO2 reaches its critical point the liquid CO2 is converted to gaseous CO2 minimizing the effects of drying the sample (Fig. 9D).
7.After the CPD heating temperature stabilizes, the gas is slowly bled off using a needle valve. Again, if the gas is released too quickly, shrinkage artifacts will be noticeable such as cracks in cellular membranes.
Alternate Protocol 6: CHEMICAL ALTERNATIVE TO CRITICAL POINT DRYING
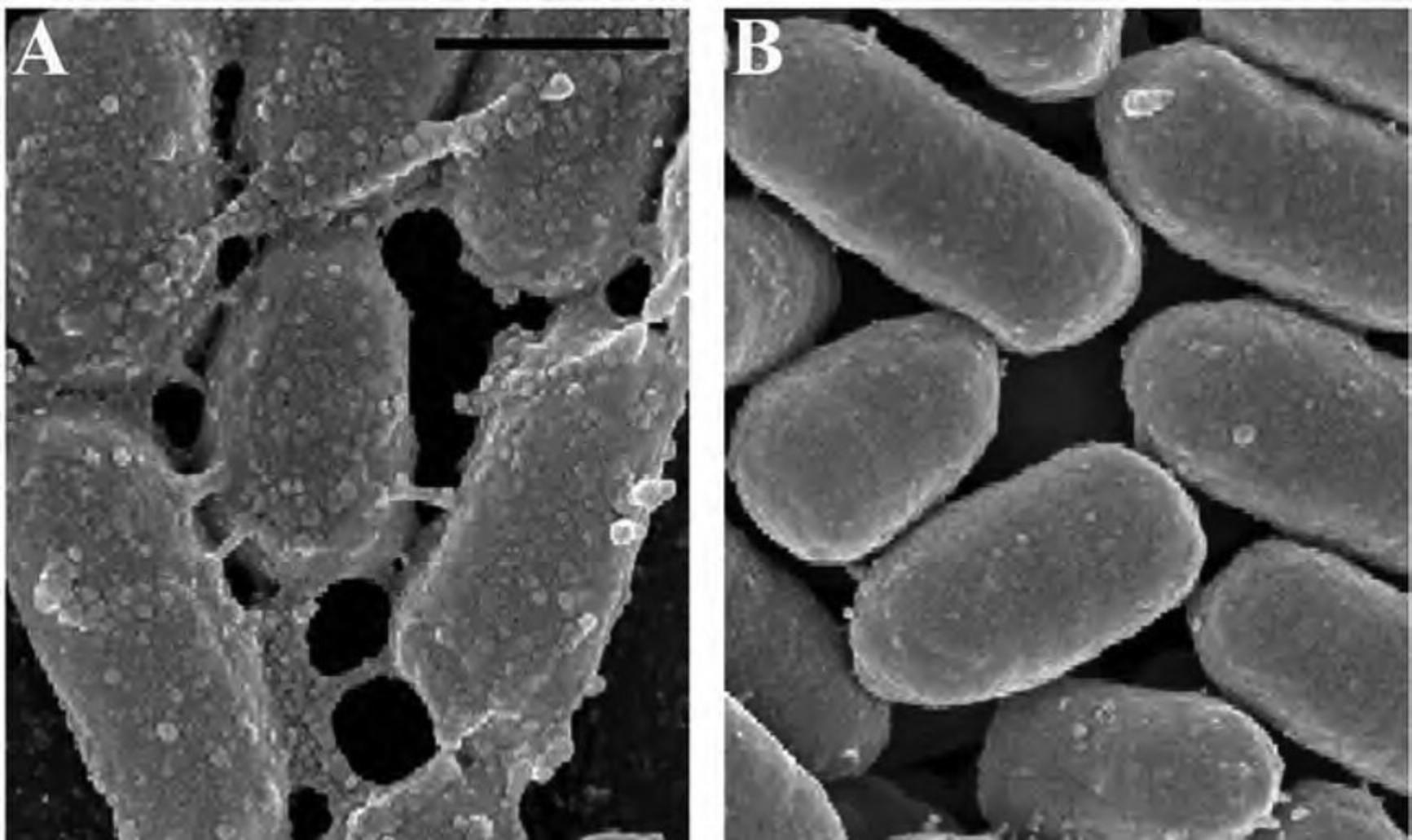
Critical point drying (CPD) is the most common and universal method for full dehydration of biological specimens, although it may be practical in some instances to use a chemical alternative. If a CPD is unavailable, chemical drying by using a dehydrant such as hexamethyldisilazane (HMDS) offers a less expensive and relatively quick alternative. In other cases, specimens may not fit into the dryer, or may be unable to tolerate the turbulence generated in the CPD. Other groups have examined chemical drying techniques for a variety of cell types For many specimens, the results can be equivalent (Braet et al., 1997; Lee & Chow, 2012; Nation, 1983). The manner in which HMDS dehydrates without compromising the underlying structure is poorly understood. It is speculated that HMDS cross-links proteins, strengthening the biological sample, enabling it to resist collapsing as HMDS evaporates out of the specimen (Nation, 1983). The following compares the ultrastructure of HeLa cells after preparation with either a CPD or HMDS.
Additional Materials (also see Basic Protocol 1)
-
Specimen adhered to chip or coverslip
-
Hexamethyldisilazane (HMDS)
-
Laboratory microwave oven with controllable wattage, optional
-
Filter paper
CAUTION : HMDS is a highly volatile and flammable liquid. It is corrosive to skin, mucous membranes, and eyes and should be used only in a chemical fume hood in well-ventilated areas with proper PPE and waste handling.
1.Fix the specimen adhered to silicon chip or coverslips with 2.5% GA in 0.1 M CAC buffer for 30 to 60 min at room temperature.
2.Wash the cells three times, each time for 5 min with 0.5 ml (enough to completely cover the specimen) 0.1 M CAC buffer.
3.Post-fix the cells with ∼0.5 ml of 1% OsO4/0.8% K4Fe(CN)6 in 0.1 M CAC, pH 7.4, for 30 to 60 min at room temperature or alternatively in a laboratory microwave oven (see Support Protocol 3).
4.Wash once for 2 min with 0.5 ml of 0.1 M CAC buffer and twice, for 2 min each time with 0.5 ml dH2O (ensuring the specimen is completely covered with fluid).
5.Dehydrate once in 95% ethanol for 1 min, followed by three times for 5 min each time in 100% ethanol.
6.Dehydrate by fully immersing the specimen in 1:1 (HMDS:ethanol) for 5 to 15 min at room temperature.
7.Dehydrate twice, each time for 5 to 15 min with 0.5 ml 100% HMDS at room temperature or in a laboratory power-controllable microwave oven at 250 W/24°C, for 2 × 5 min, no vacuum.
8.Wick away excess liquid with filter paper and allow a minimum of 4 hr for drying before proceeding to the next step.
9.Proceed to sputter coating in Basic Protocol 3.
Basic Protocol 3: SPUTTER COATING
When bombarded with electrons, biological specimens ineffectually dissipate the resulting charge, which can cause imaging artifacts. After fixing and drying specimens as described in Basic Protocols 1 and 2, coating specimens with a thin layer of a conductive metal minimizes damage to specimens and improves topographical contrast for improved imaging by SEM using secondary electron detection. In general, there are two grades of sputter coating units available: low vacuum systems, which are adequate for low magnification/low-resolution work, and high vacuum systems, which are necessary for high-resolution imaging. Choice of metal should be carefully considered depending on the application. Gold, gold/palladium or platinum alloys, or platinum (Au, Au/Pd, Au/Pt, and Pt, respectively) are common choices for low-resolution imaging. Iridium (Ir), tungsten (W), or carbon (C), provide better coating for high-resolution imaging. When visualizing gold or other electron-dense moieties, low-atomic number elements such as chromium (Cr) are required to allow distinct backscattered electron (BSE) detection. In all cases, application of a minimal layer is important to avoid obscuring structural details. Although many of the concepts are universal for all coating methods, the protocol described below is specifically outlined for an ion-beam sputtering device. Other types of sputter coaters are discussed in the commentary section.
Materials
-
Appropriately fixed and dried specimen (see Basic Protocols 1 and 2)
-
Microscope-specific stage mount (e.g., aluminum stubs from Ted Pella, cat. no. 16111 or other sources)
-
SEM specimen storage box
-
SEM stub tweezers
-
Conductive double-sided adhesive tape and/or conductive paint
-
Fine-tip tweezers
-
Sputter coater
-
Desiccator
1.Place the microscope-specific aluminum stub in the SEM specimen storage container using SEM stub tweezers.
2.Apply double-sided conductive tape to the stub, and remove the protective layer as shown in Figure 11.
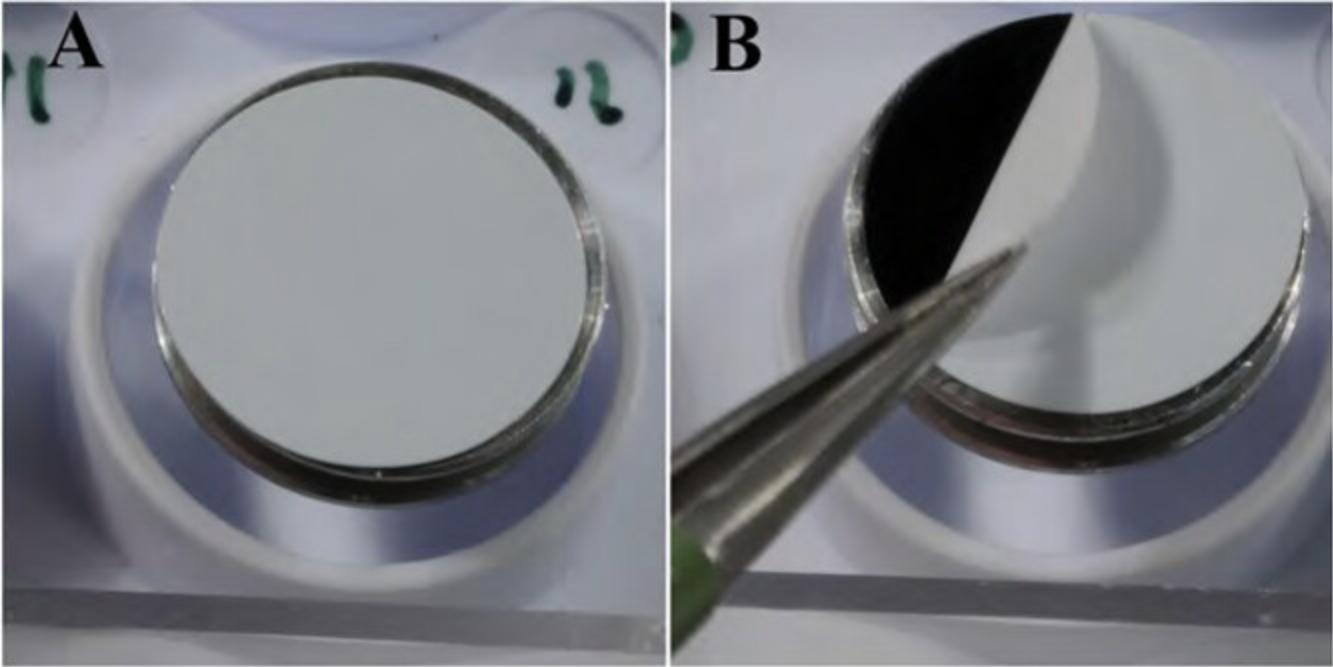
3.Place the appropriately fixed and dried specimen (see Basic Protocols 1 and 2) where desired on the stub.

4.After placement of the specimen, contact between the specimen substrate and stage mount can be improved by applying a thin layer of conductive paint around the specimen as shown in Figure 13.Allow sufficient drying time prior to placement under vacuum conditions in the sputter coater (e.g., 4 hr or overnight in a desiccator).


5.Place the dried mount in the sputter coater and proceed as advised by the manufacturer. Basic steps include:
-
Achieving sufficient vacuum.
-
Out-gas argon or gas line before applying voltage.
-
Making sure the correct target is in place.
-
Setting tilt and rotation conditions (e.g., usually ± 90° tilt and 360° rotation according to desired result).
-
Applying voltage.
-
Opening the gas valves to specified current, usually in milliamps.
-
Coating to desired thickness or time and turning off the power supply.
-
Releasing the vacuum.
-
Removing the sample, placing the stub in SEM storage box, and preferably storing in a desiccator until ready for examination.
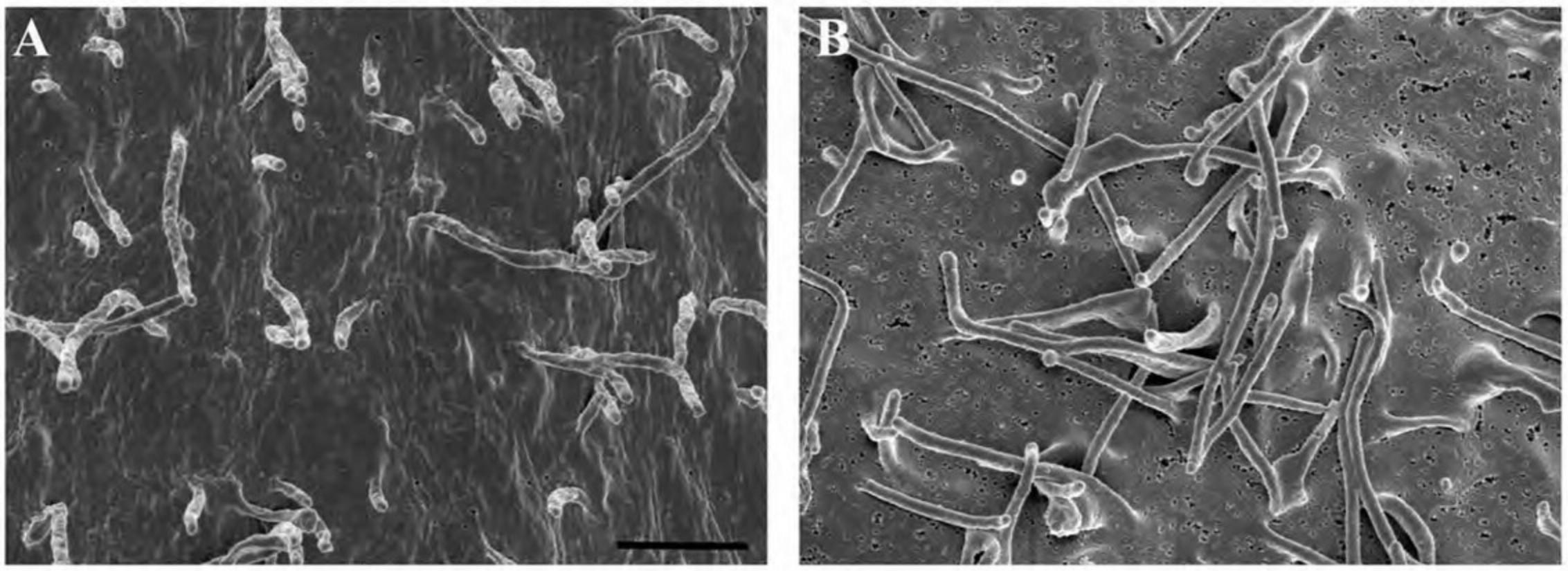
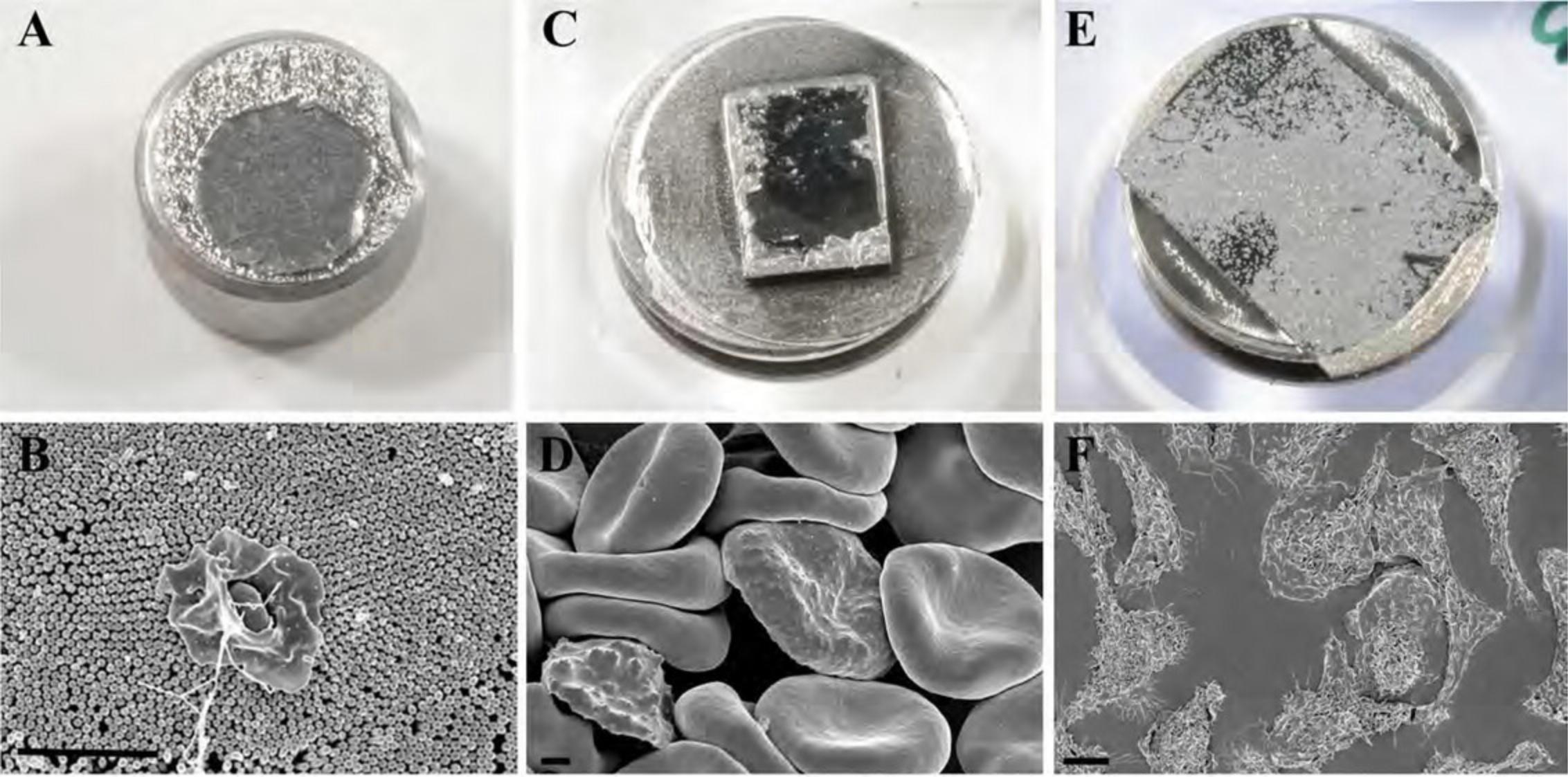
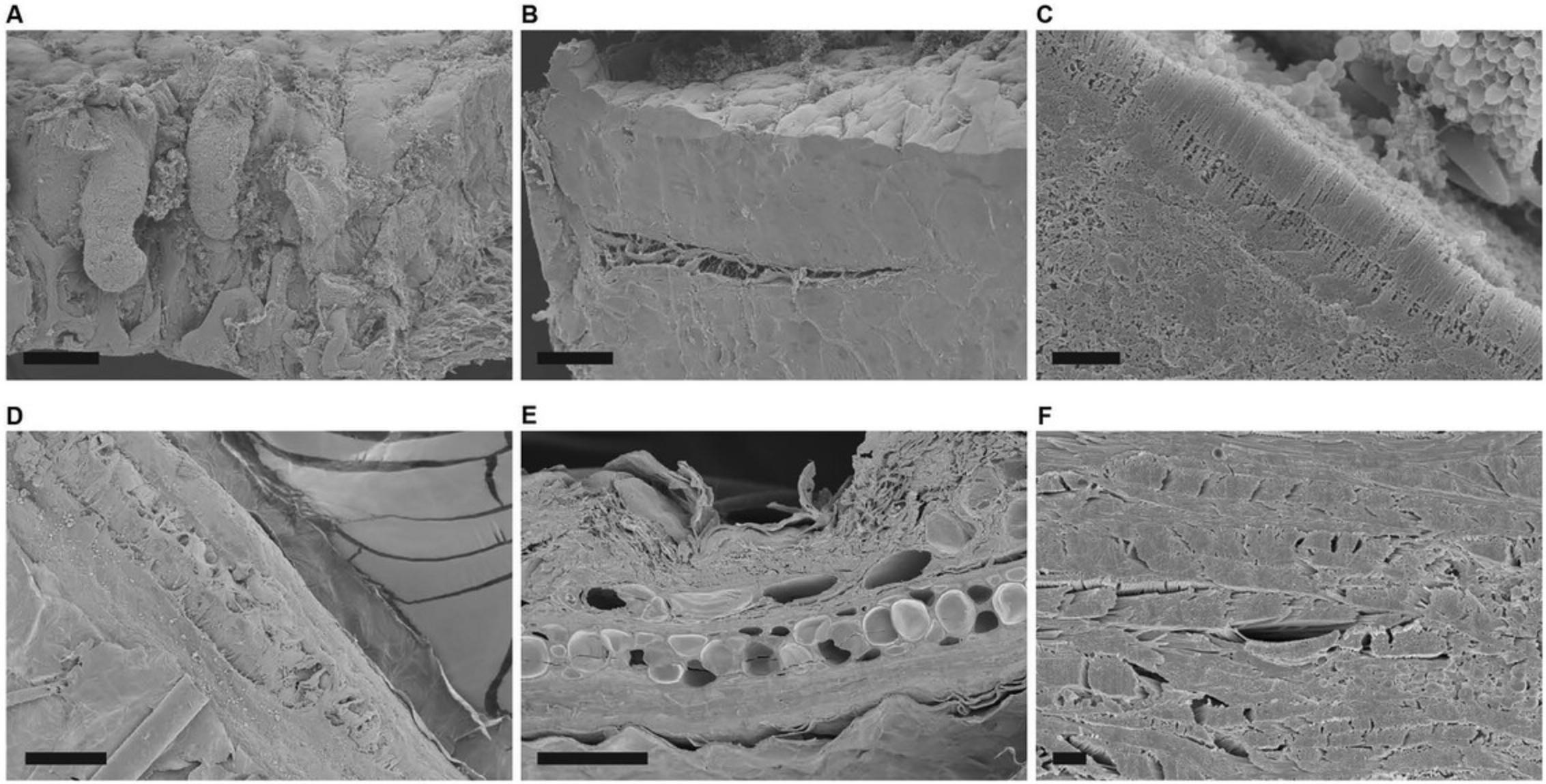
Figure15shows the appearance of specimens after coating on membrane filters (A, B), non-adherent cells on untreated silicon chips (C, D), and adherent cells on an ACLAR coverslip (E, F).
Specimens with rough or highly irregular surfaces may require thicker application of metals, or an increase in overall bulk conductivity or both, as described in the next section.
Alternate Protocol 7: IMPROVED BULK CONDUCTIVITY THROUGH “OTOTO”
Biological specimens are notoriously nonconductive and require special preparations either through coating with a thin layer of metal and/or increasing the bulk conductivity of a specimen by impregnation with heavy metals to prevent or reduce damage and imaging artifacts for examination in an SEM. In some instances, it may be beneficial to minimize or obviate the need for sputter coating for better visualization of minute topographical details or improved visualization of gold particles used for immune-labeling. Successive incubations alternating OsO4 - TA - OsO4 - TA - OsO4 can introduce enough conductivity into many specimens making samples electrostatically neutral for direct examination in the SEM without further coating requirements. Saturated thiocarbohydrazide (TCH) can be substituted for tannic acid (TA). In this process, coined OTOTO, TA and TCH act as mordants for better adhesion of OsO4, laminating layers of metal on membranes in particular.
Materials
- See Basic Protocols 1, 2, and 3
1.Wash and fix the specimens on a suitable mounting substrate as outlined in Basic Protocol 1, steps 1 to 7.
2.Rinse three times, for 2 min each with appropriate rinsing buffer as described in Basic Protocol 1, step 7.
3.Stain for 30 to 60 min at room temperature with ∼0.5 ml of 1% TA or saturated TCH in dH2O.
4.Rinse three times, each time for 2 min with ∼0.5 ml rinsing buffer.
5.Repeat steps 2 and 3 until the following pattern has been achieved: OsO4 - TA - OsO4 - TA - OsO4.
6.Proceed to washing and dehydration as described Basic Protocol 1 steps 8 to 9, and proper drying as described in Basic Protocol 2, steps 1 to 7.
7.Mount the dried specimen on aluminum stub with conductive paint and allow thorough drying (e.g., overnight) prior to examination in the SEM as discussed in Basic Protocol 3 (see steps 1 to 5).
Basic Protocol 4: IMMUNE-LABELING STRATEGIES
The ability to perform immunology and label specific antigens with an electron-dense probe provides a powerful tool for identification and site-specific localization for proteins of interest. Although it is beyond the scope of this article to fully review immunological theory, there are some important considerations when labeling for visualization by SEM. Labeling for electron microscopy can be challenging in that preservation of structure and retention of antigen are often mutually exclusive. Labeling for SEM is generally performed on surface-exposed antigens, with the advantage that immunology can be performed after light fixation with PFA, which typically does not affect antigenicity, followed by fixation with GA and OsO4, which will improve ultrastructure after immune-labeling. For viewing internally labeled structures, it is possible to perform labeling after a mild fixation, followed by cell fracture as discussed in Alternate Protocol 8.
Additional information can be found in the Commentary section including proper controls, signal-to-noise, and selection of gold probe size.
Materials
-
Cells
-
Fixative: 2% to 4% EM-grade paraformaldehyde (PFA) in 0.1 M PB, pH 6.8 to 7.4
-
Rinsing buffer (see Basic Protocol 1)
-
Blocking buffer (see recipe), e.g., serum-free bovine serum albumin (BSA) in 0.1 M Tris buffer, PB, or PBS
-
Primary antibodies or anti-serum (e.g., rabbit or mouse anti-sera)
-
Secondary antibodies (e.g., goat anti-rabbit or mouse) conjugated to electron dense probe (e.g., 10 to 40 nm colloidal gold)
-
PBS (Invitrogen, cat. no. 10010-023)
-
Silicon chips
-
24-well plates
-
Fine-tipped tweezers, optional
1.Grow cells on the shiny side of a silicon chip in a 24-well plate.
2.Remove most of the liquid but not all to avoid drying of sample, which adversely affects the ultrastructure. In a hood, quickly but gently add ∼0.5 ml of 2% to 4% EM-grade PFA fixative in 0.1 M PB, pH 6.8 to 7.4, by dispensing a slow stream on the side of the well, avoiding direct pressure on the sample. Be careful to prevent the chips from floating (may need to press down on a corner using fine-tipped tweezers).
3.Let the specimen incubate in the fixative for 20 to 30 min at room temperature, then wash twice, each time for 5 min in 0.5 ml rinsing buffer (PB or CAC).
4.Block with 0.5 ml blocking buffer (e.g., 1% to 3% BSA/PBS, pH buffered to approximately neutral) for 10 min at room temperature.
5.Add primary antibody 1:100 in blocking buffer (e.g., 1% to 3% BSA/PBS) for 1 hr at room temperature.
6.Rinse twice, each time for 5 min with 0.5 ml blocking buffer (e.g., 1% to 3% BSA/PBS).
7.Add secondary antibody (e.g., 1:20 colloidal gold) in blocking buffer (e.g., 1% to 3% BSA/PBS) for 30 to 60 min at room temperature.
8.Rinse three times, each time for 5 min with ∼0.5 ml PBS.
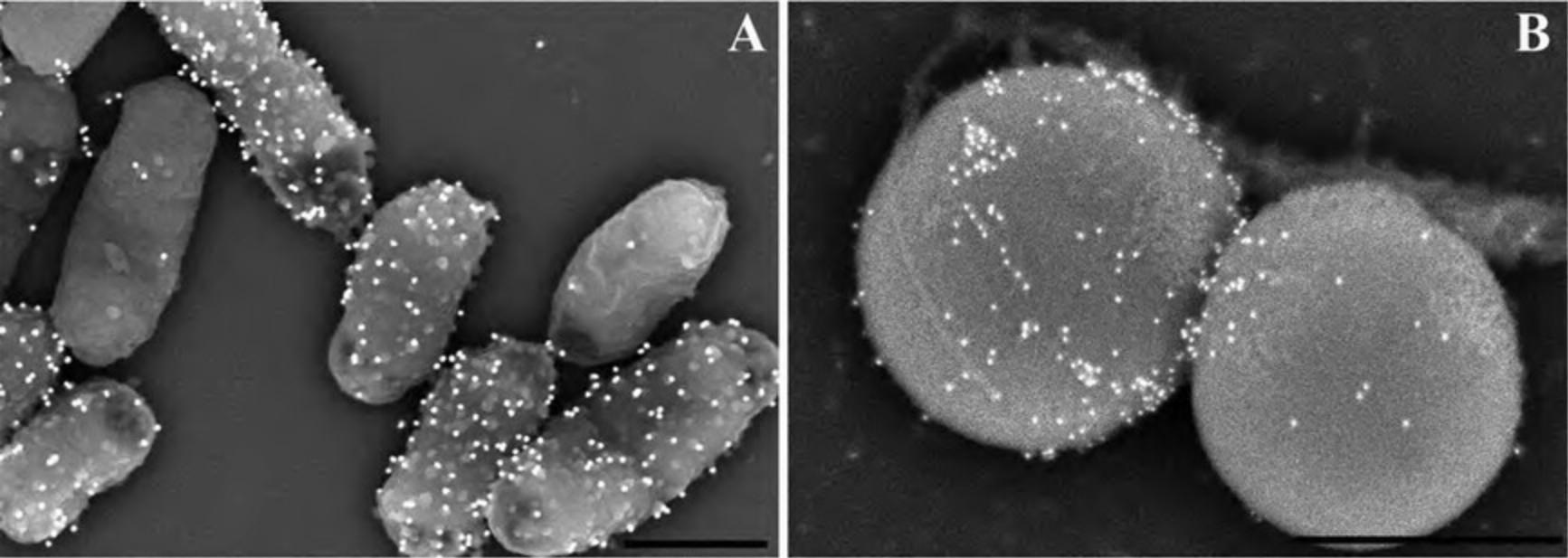
Alternate Protocol 8: IMMUNE-LABELING INTERNAL ANTIGENS WITH SMALL GOLD PROBES
Intracellular immune-labeling requires additional considerations for accessing the antigens without excessive destruction of the specimen and subsequent fracture to reveal the structures of interest. Triton-X and methanol, common permeabilizing agents for LM, severely compromise membranes. Use of saponin, a mild detergent, allows diffusion of antibodies and small gold probes that can be subsequently enhanced with gold or silver for visualization by SEM. The following protocol has been adapted from an enzymatic method using horseradish peroxidase and 3,3′-diaminobenzidine (DAB) (Brown & Farquhar, 1989).
Additional Materials (also see Basic Protocols 1 and 4)
-
2% to 4% paraformaldehyde (PFA)
-
2.5% glutaraldehyde (GA) stock solution (see “Primary fixatives” recipe)
-
Periodate-lysine-PFA (PLP) fixative (see recipe)
-
Glycine
-
Saponin
- Prepare fresh from powder daily as making from a stock solution loses effectivity. Typically, we use Sigma-Aldrich cat. no. S7900.
-
Small diameter (<1.5 nm) gold conjugates (Nanoprobes Nanogold or Aurion Ultrasmall gold)
-
Nanoprobes or Aurion's R-Gent silver enhancement kit
Perform light primary fixation
1.Plate cells on Thermanox coverslips or silicon chips in 24-well plates. Infect or treat as experimentally desired.
2.Rinse twice, each time for 5 min with ∼0.5 ml Hanks’ buffered saline solution (HBSS).
3.Fix with PLP fixative + 2% to 4% PFA + 0% to 0.25% GA (titrate aldehydes for each antigen/antibody, if necessary, as the glutaraldehyde may adversely affect antigenicity) for 2 hr at room temperature.
4.Rinse twice, each time for 5 min with ∼0.5 ml PBS.
5.Incubate the specimen for 5 min at room temperature in ∼0.5 ml of 50 mM glycine in PBS (pH 7.4).
Permeabilize and immune-label
6.Permeabilize in ∼0.5 ml PBS + 0.01% saponin (titrate saponin; range = 0.005 to 0.05) for 5 min at room temperature.
7.Block with ∼0.5 ml of 1% BSA/PBS for 10 min at room temperature.
8.Add primary antibody 1:100 (antibody concentration may need to be titrated) in 1% BSA/PBS + 0.01% saponin for 1 hr at room temperature.
9.Rinse twice, each time for 5 min with ∼0.5 ml of 1% BSA/PBS.
10.Add secondary antibody 1:20 (Nanogold or Ultrasmall gold) in 1% BSA/PBS + 0.01% saponin for 1 hr at room temperature.
11.Rinse three times, each time for 5 min with ∼0.5 ml PBS.
12.Fix with ∼0.5 ml of 2.5% GA in 0.1 M PB for 1 hr at room temperature.
Gold or silver enhancement step
13.Wash the coverslips three times, each time for 5 min with ∼0.5 ml CAC or PBS and then five times, each time for 5 min with 0.5 ml dH2O.
14.Apply the enhancement mixture for 15 to 30 min per the manufacturer's instructions.
15.Wash five times, each time for 5 min with ∼0.5 ml dH2O.
16.Post-fix with 0.5 ml of 1% OsO4 in dH2O for a minimal amount of time (e.g., 15 min at room temperature for a monolayer).
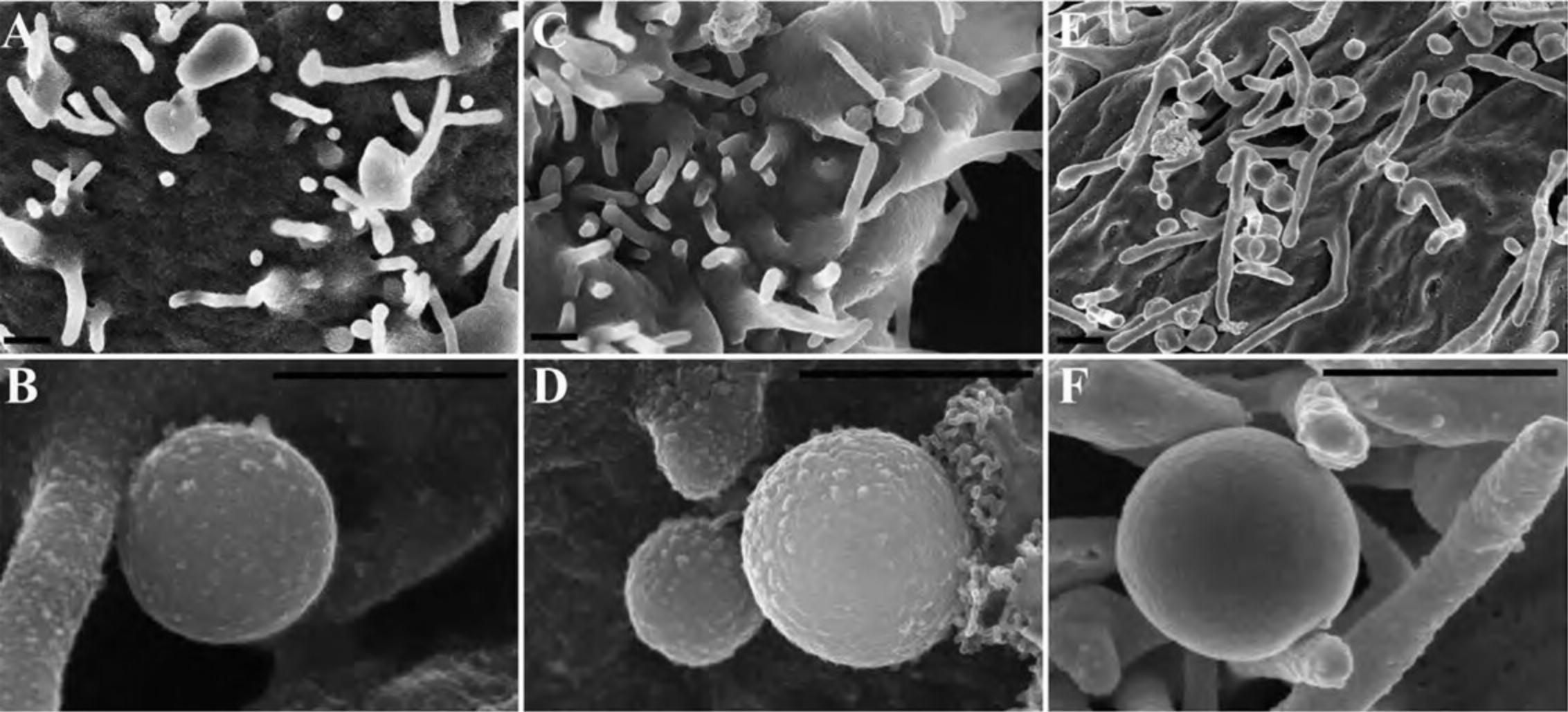
17.Proceed as above for immune-labeled specimens using steps for OTOTO (Alternate Protocol 7) and then CPD (Basic Protocol 3 or its alternative). Following CPD, fracture cells to view internal structures as shown in Figure 18, and if OTOTO is not used, sputter coat with 15 to 20 Å of Cr as described in Basic Protocol 3.
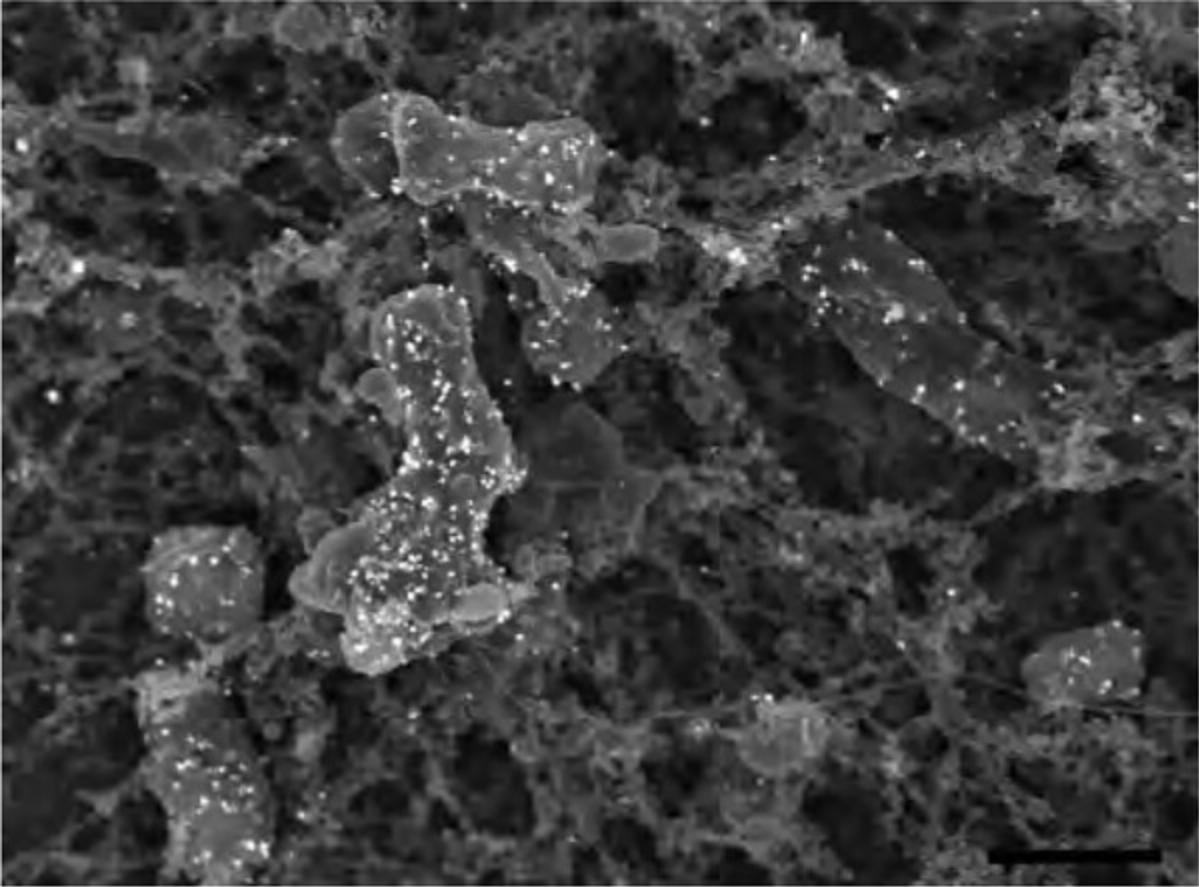
Alternate Protocol 9: QUANTUM DOT OR FLUORONANOGOLD PREPARATION FOR CORRELATIVE TECHNIQUES
During recent years, fluorescent nanocrystals known as quantum dots (Q-dots) have been adapted for specific biological labeling of a wide variety of molecules. Q-dots are readily detectable on standard fluorescent microscopes with little or no loss of photoluminescence over time. Furthermore, Q-dots are sufficiently electron dense for detection by TEM. Because emission wavelength is directly related to size, different size classes with distinct molecular targets can be applied to single samples, allowing identification of multiple targets simultaneously by either fluorescent or ultrastructural analysis. Using conductive fixation methods, carbon coating, and low electron beam potential, Q-dots can be detected on cells by SEM with significant spatial resolution, providing a useful tool for correlative techniques when identifying rare or transient events (DeLeo & Otto, 2008).
Additional Materials (also see Basic Protocols 1 and 4)
- Samples adhered to silicon chip or coverslip
- 4% paraformaldehyde (PFA)/0.1% glutaraldehyde (GA) in PBS
- Blocking buffer (see recipe), e.g., 2% (w/v) globulin-free BSA in PBS
- Secondary antibody conjugate (e.g., goat anti-rabbit 655-nm quantum dots; Invitrogen or eBioscience)
1.Wash the samples adhered to a silicon chip or coverslip twice, each time for 1 min with an appropriate buffer, such as PBS or HBSS, to remove residual medium components.
2.Fix with ∼0.5 ml 4% PFA/0.1% GA in PBS for 15 to 30 min at room temperature.
3.Wash twice, each time for 15 min with buffer (e.g., PBS or HBSS).
4.Block with ∼0.5 ml of 2% globulin-free BSA in PBS (a blocking buffer) for 15 min at room temperature.
5.Replace the blocking buffer with diluted primary antibody (e.g., 1:100) in blocking buffer 30 min at room temperature or overnight at 4°C.
6.Wash twice, each time for 15 min with ∼0.5 ml blocking buffer.
7.Replace the blocking buffer with diluted secondary antibody (e.g., Q-dot conjugate at 1:50) in blocking buffer for 1 hr at room temperature.
8.Wash at least twice, each time for 15 min with 0.5 ml blocking buffer, and then twice, each time for 2 min with ∼0.5 ml PBS to remove excess protein from blocking buffer.
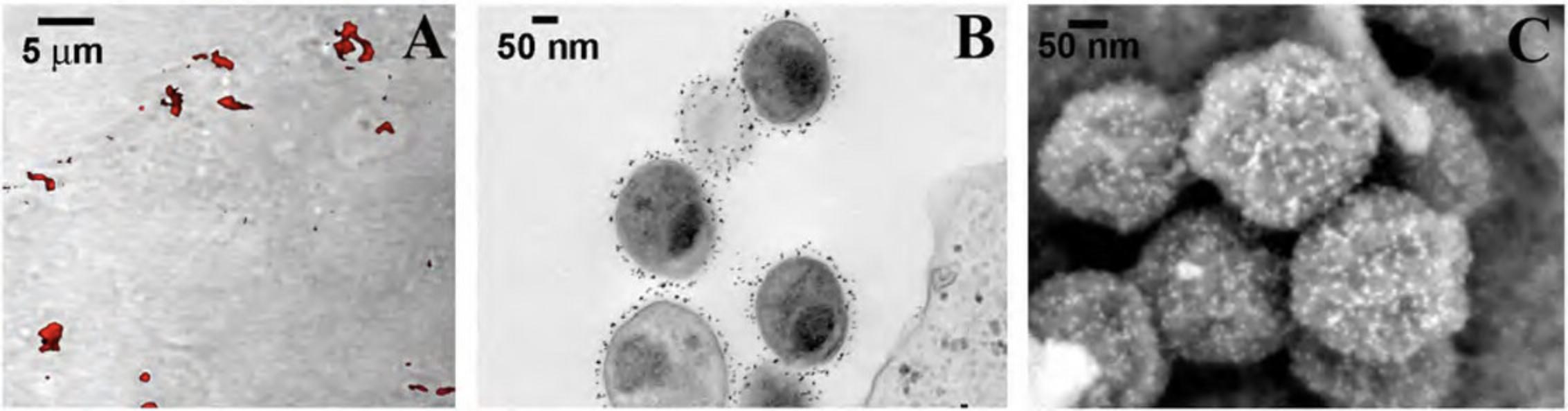
Basic Protocol 5: EXPOSURE OF INTERNAL STRUCTURES BY MECHANICAL FRACTURING
Visualization of internal structures by SEM provides useful information for topographical analysis of the relationship between structures not evident by TEM. Freeze-fracture techniques for cryo-SEM or replica generation, and Focused Ion Beam technologies provide exceptional methodologies but require expensive instrumentation and advanced skills. The “affordable” technique described below does not require either and is suitable for many specimens.
Materials
-
Fixed and dried specimen (e.g., see Basic Protocols 1 and 2)
-
Mild adhesive tape (e.g., Scotch tape) or syringe or micro-dissection scissors
-
Eyelash brush
-
Additional reagents and equipment for mounting the specimen on SEM stub (Basic Protocol 3)
1.After the specimen is appropriately fixed and dried (see Basic Protocols 1 and 2 or alternatives), mount on SEM stub as described in Basic Protocol 3.
2.Prior to sputter coating (Basic Protocol 3), lightly touch mild adhesive tape (Fig. 20) to the surface of the specimen to remove surface membranes or the outer cell wall.

3.Alternatively, a syringe tip or scissors can be used to disrupt larger tissues, and the exposed areas should be lightly manipulated with an eyelash brush to the desired location and pressed around the edges gently for good contact on the adhesive mount.
4.Proceed to sputter coating steps as desired in Basic Protocol 3.
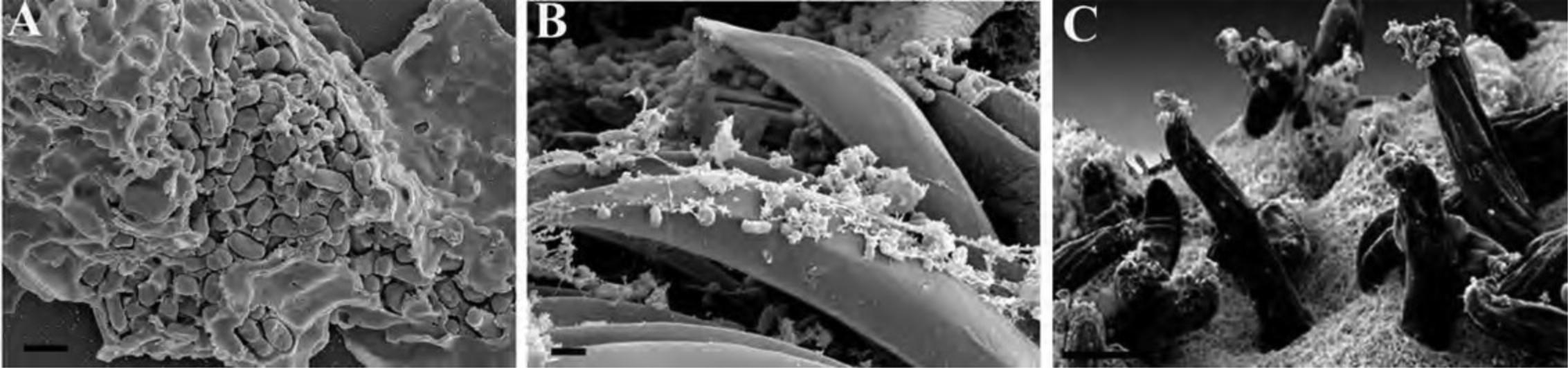
Basic Protocol 6: EXPOSURE OF INTERNAL STRUCTURES OF TISSUES BY FRACTURING WITH LIQUID NITROGEN
When imaging tissues, the edges cut by a razor blade often show mechanical damage, such as tears, compression, and pulling away of intracellular material from the surface of the cut. One can try to freeze-fracture and cleave a plane across the tissue; however, this requires expensive equipment. Instead, properly dehydrated tissue can be dropped into liquid nitrogen and fractured using a razor blade into pieces with smooth edges suitable for scanning electron microscopy (SEM) (Specian & Neutra, 1981). For tissues, it is preferrable to use a combination of microwave processing as outlined in Support Protocol 3 with special considerations as outlined in Alternate Protocol 1.
Materials
-
Fixed tissue specimens (chunks with largest dimensions no greater than ∼1 mm) prepared up to dehydration as in Support Protocol 3
-
100% ethanol
-
Liquid nitrogen
-
Thick metal plate, at least 5-mm thick
-
Styrofoam box
-
Aluminum weighing dish
-
Forceps
-
Pliers
-
Single-sided razor blades
-
Additional reagents and equipment for critical point drying and mounting the specimen on SEM stub (Basic Protocols 2 and 3)
1.Dehydrate with 100% dry ethanol in a graded series by subsequent exchanges as discussed in Support Protocol 3, step 8.Then, perform an additional two 100% anhydrous ethanol exchanges (five 100% exchanges total).
2.Place the thick metal plate in a Styrofoam box that is just larger in length and width than the plate. Wearing appropriate eye and skin protection, cool the metal plate completely with liquid nitrogen. Place the aluminum weighing dish on the metal plate and fill most of the depth with liquid nitrogen such that the level inside the weighing dish is just below the lip. (Fig. 22A)
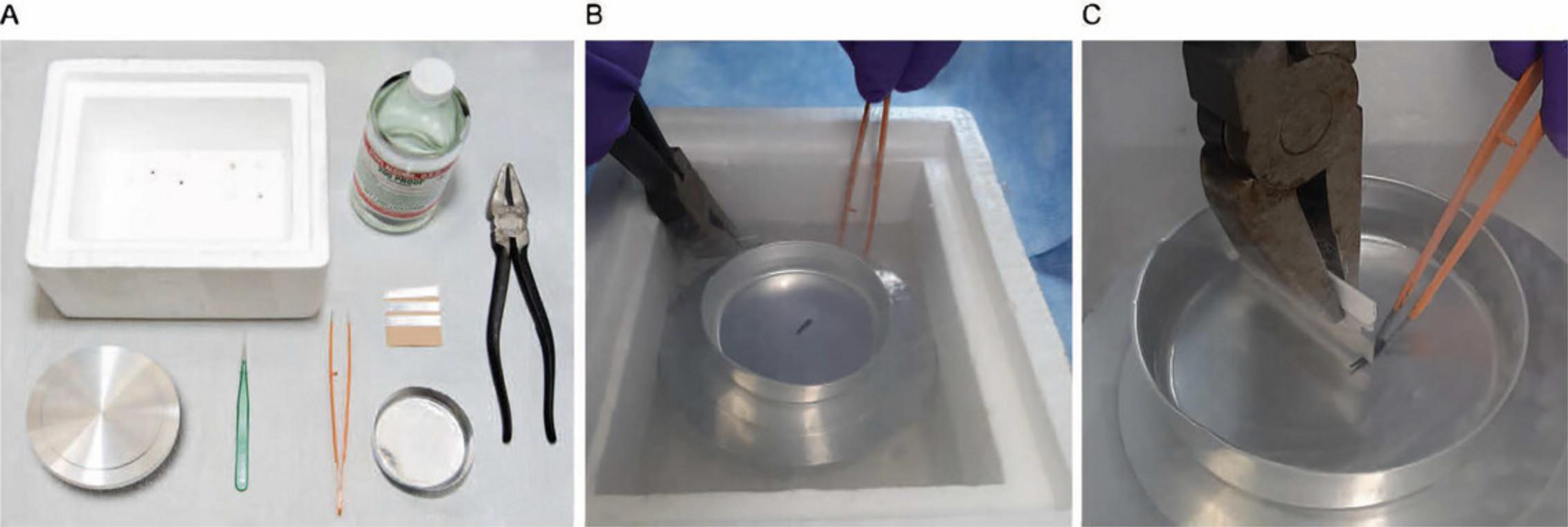
3.Use forceps to drop your tissue chunk into the aluminum weighing dish. The tissue will dance around until it is completely frozen. Do not hold it in place with your forceps as they will also need to cool and will slow down the cooling process of your tissue chunk.
4.Use the pliers to grasp a fresh, single-sided razor blade and cool the edge in liquid nitrogen away from the tissue chunk. (Fig. 22B)
5.Using pre-cooled forceps, grasp the chunk of tissue and then press the edge of the razor blade on the center of the tissue chunk, directing the force perpendicular to the top surface of the tissue chunk. It will crack into pieces. (Fig. 22C)
6.With the pre-cooled forceps, pick up your pieces and drop them back into the 100% dry ethanol.
7.If you have more tissue chunks, especially different kinds of tissue or different treatments, pick up the aluminum weighing dish and quickly dump the liquid nitrogen (and tiny tissue pieces you could not pick up with forceps), then place back in liquid nitrogen to repeat on each subsequent tissue chunk.
8.Critical point dry your tissue pieces as in Basic Protocol 2.
9.Tissue pieces can be mounted on carbon sticky tape and sputter coated as in Basic Protocol 3.Use a dissecting scope to carefully place the fractured surfaces facing up (towards the detectors when in the microscope). Tissue pieces will sometimes need longer sputter coating times.
Basic Protocol 7: ANAGLYPH PRODUCTION FROM STEREO PAIRS TO PRODUCE 3D IMAGES
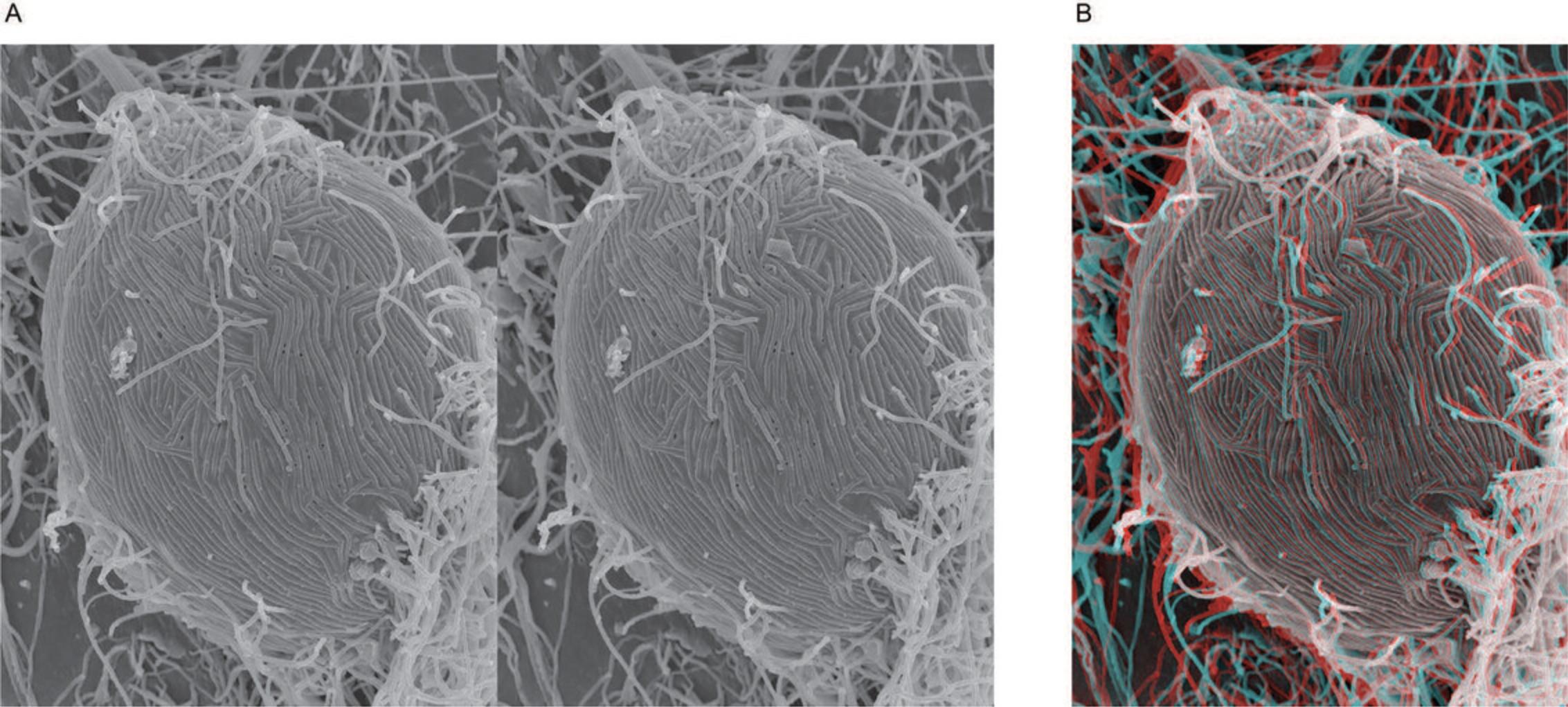
Stereo pairs can be viewed with red/blue 3D glasses by producing anaglyphs with a computer imaging program capable of separating channels in RGB files, such as Adobe Photoshop, as demonstrated below. The following anaglyph of an Ebola–virus infected Vero cell was produced following established methods (Mannle, see Internet Resources).
Materials
- Stereo pair
- Computer with imaging program, e.g., Adobe Photoshop
- Red/blue 3D glasses
NOTE : PC control key short cuts are utilized in the example below; Mac users should use the command key.
1.Align stereo pairs in an orientation such that when eyes are crossed, a 3D image is observed. Typically, this is achieved by rotating the image 90° counterclockwise and placing the 0° tilt image on the left, although dependent on microscope stage design. Sometimes, the tilt is already in the orientation illustrated above and rotation is not necessary. Adjust contrast and brightness of both images to desired levels. (Fig. 24)
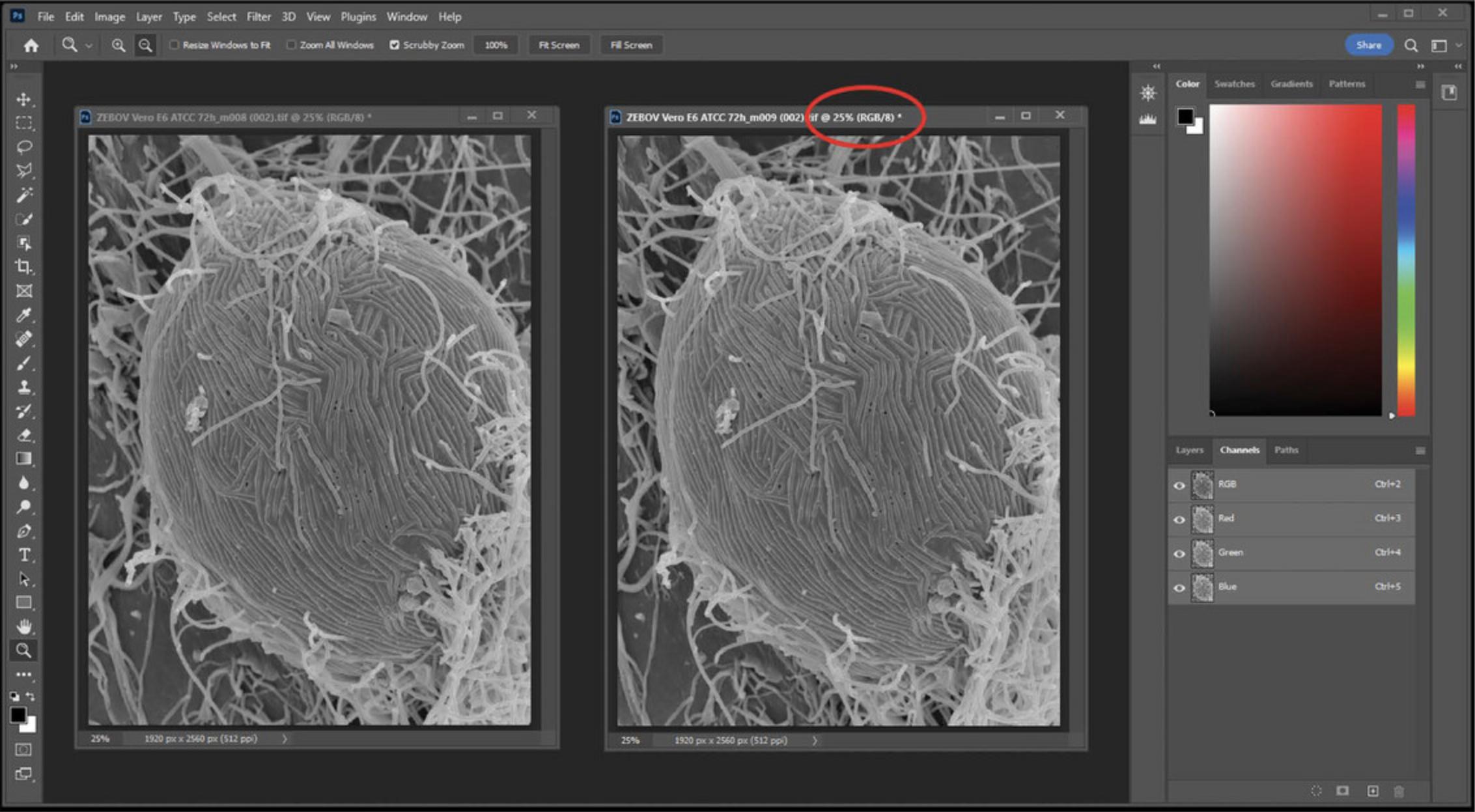
2.Convert both images to RGB to split the red, green, and blue channels (Fig. 24).
3.Click the image on the left; select the red channel, then select all (Ctrl + A), and then cut (Ctrl + X) to remove the red channel from the image. Note that the red channel now appears blank (Fig. 25).
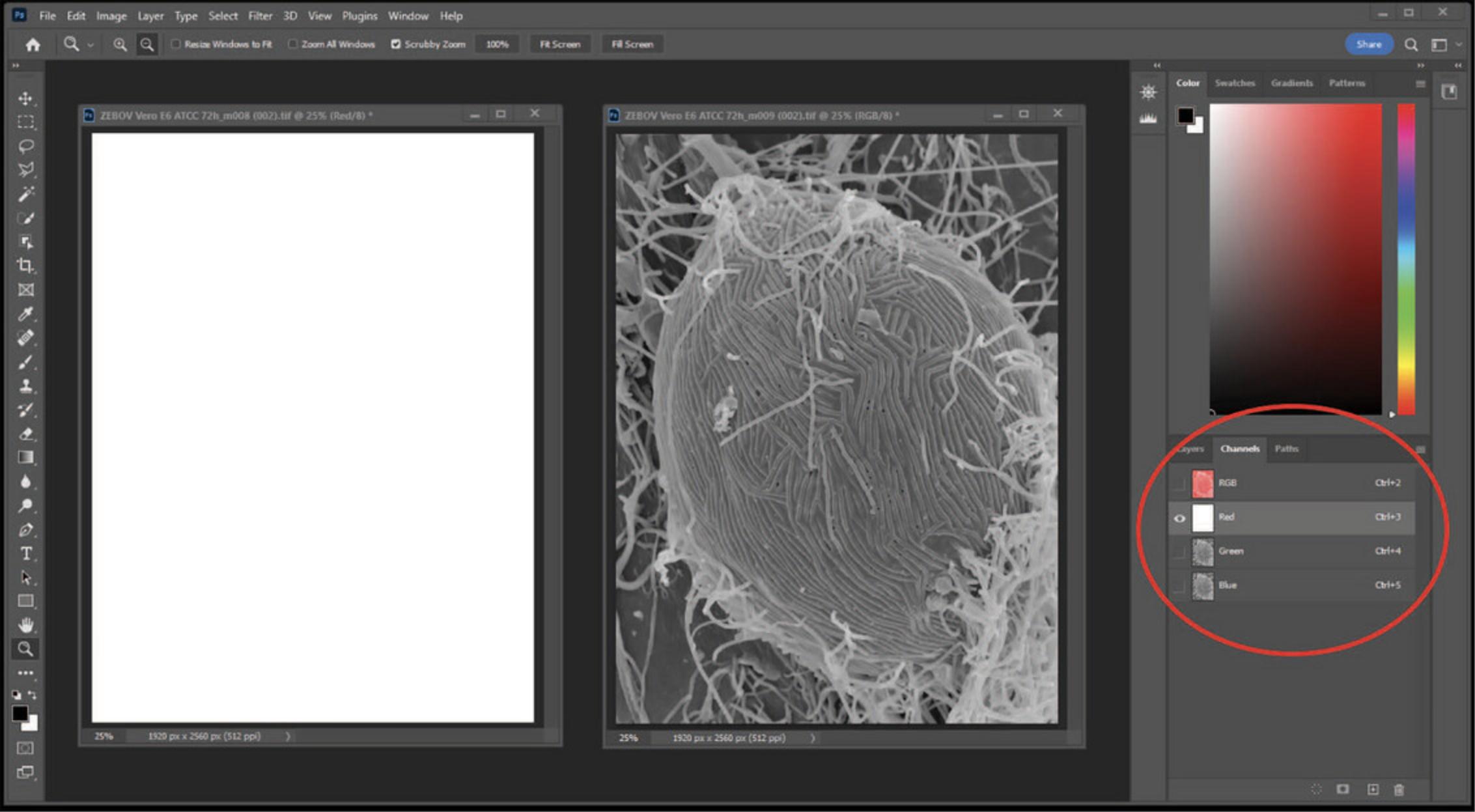
4.Click on the right image, select the red channel, then select all (Ctrl + A), and then copy (Ctrl + C). (Fig. 26)
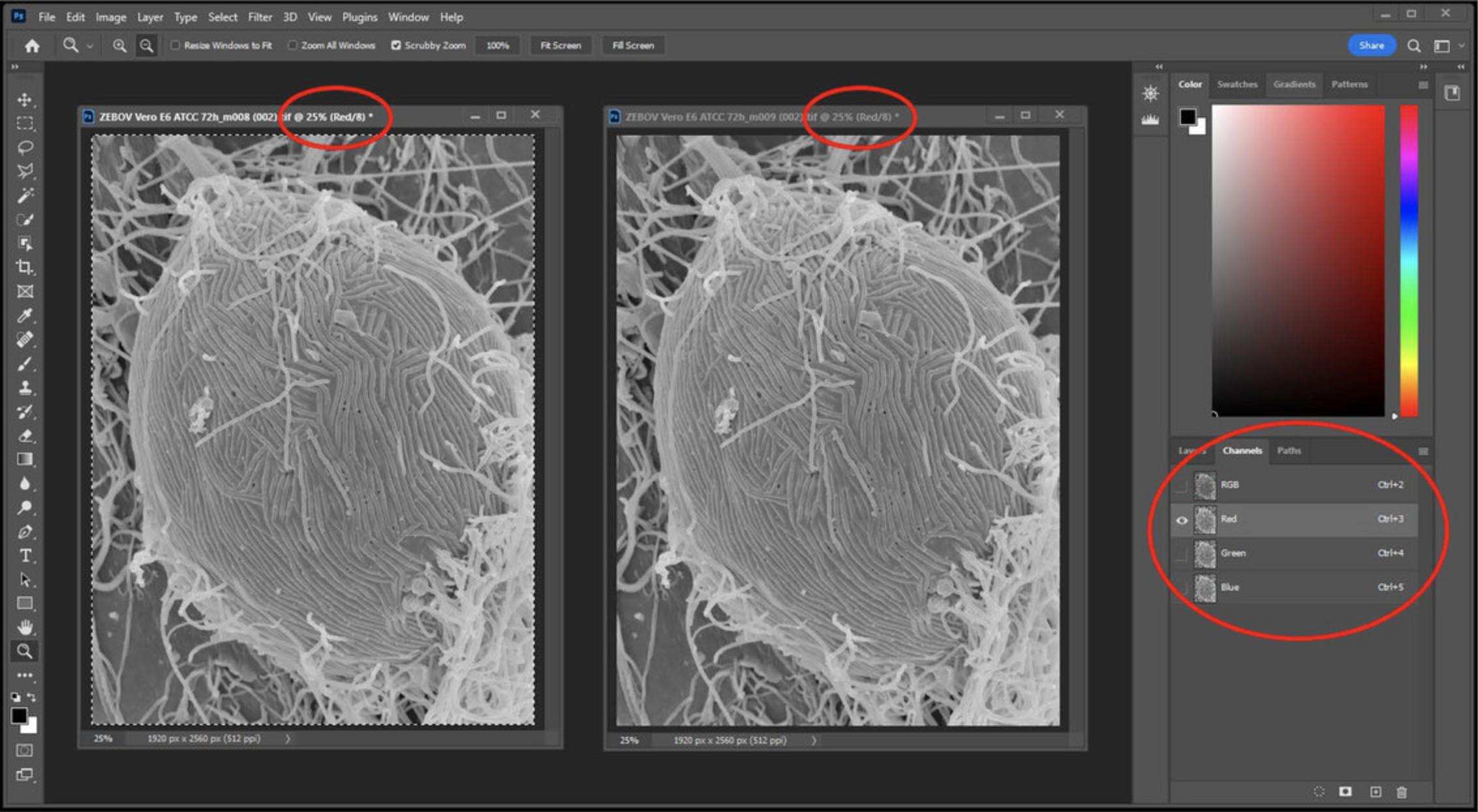
5.Click back on the left image, and then paste the previous selection (red channel from right image) on the left image by pasting the red channel (Ctrl + V) (Fig. 27).
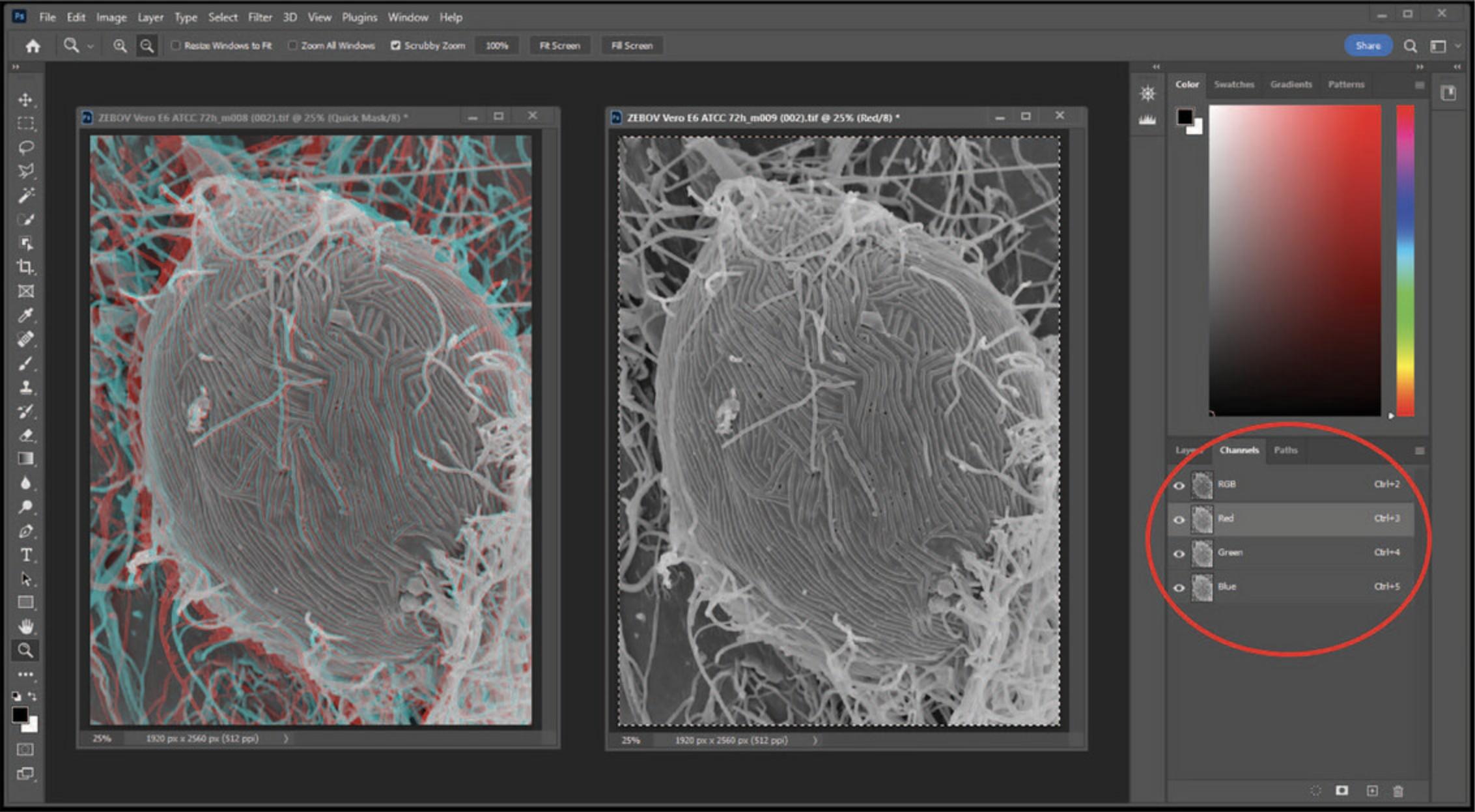
6.Now select the RGB channel on the left window and view the anaglyph using red/blue glasses (Fig. 28).
REAGENTS AND SOLUTIONS
Use deionized, distilled water in all recipes and protocol steps. For common stock solutions, see Current Protocols (2006).
Alcian blue/lysine additive in GA/PFA fixative
In a fume hood using nitrile gloves, for 5.5 ml of fixative, add 750 µl of 0.5 M lysine·HCl (e.g., Sigma-Aldrich, cat. no. L0900000) to 1 ml of 10% paraformaldehyde (PFA) (e.g., Electron Microscopy Sciences, cat. no. 15700) in dH2O. Add 2.75 ml of 0.2 M CAC, pH 7.2 (e.g., Ted Pella, cat. no. 18851), and 500 µl of 0.75% (w/v) alcian blue (e.g., Sigma-Aldrich, cat. no. 105234). Mix the solution. Just prior to adding fixative to your specimen (it reacts immediately with the lysine), add 500 µl of 25% glutaraldehyde (GA) (e.g., Electron Microscopy Sciences, cat. no. 16200) in dH2O.
Blocking buffer
To 1 L dH2O, add 2.42 g Tris-acetate (e.g., Fisher Scientific, cat. no. 02-004-508; pH can be adjusted using glacial acetic acid, Fisher Scientific, cat. no. A38-500), 1.3 g sodium azide (NaN3) (e.g., Fisher Scientific, cat. no. 190380050), 9 g NaCl, and mix until all solids have dissolved. Add 0.1% (w/v) BSA (e.g., Sigma-Aldrich, cat. no. A6793) and 1% (v/v) Tween 20 (e.g., Sigma-Aldrich, cat. no. P1379-25ml) to the mixture to complete the solution for use in diluting antibody concentration. The addition of azide impedes bacterial growth, and the buffer can be stored up to 12 months at 4°C.
In some cases, when 1% BSA insufficiently prevents nonspecific binding, an additional 1% to 3% (w/v) BSA, nonfat dry milk, or (v/v) fish gelatin, normal serum from fetal calf, donkey, mouse, sheep, goat, or rabbit can be added to Tris or PBS to make a more effective blocking solution. This can be stored up to 3 months at 4°C. When using PBS, the solution should be monitored for bacterial contamination.
Osmium or reduced osmium tetroxide, secondary fixative
Osmium tetroxide stock solution is available commercially (e.g., Electron Microscopy Sciences, cat. no. 19180), typically as a 4% aqueous solution in 5- or 10-ml glass ampoules sealed under nitrogen gas. Unopened, the stock solution can be kept at 4°C for up to 12 months. After opening, the stock solution should be kept in a container sealed with Parafilm, and used within a few days, diluting with dH2O or CAC buffer to desired concentration. Osmium can be reduced by addition of potassium ferrocyanide from an 8% stock solution (w/v) just prior to use.
Periodate-lysine-paraformaldehyde (PLP) fixative
Solution A (0.1 M lysine-sodium phosphate buffer) :
- Add 1.83 g lysine·HCl (e.g., Sigma-Aldrich, cat. no. L0900000) to 50 ml dH2O
- Add 0.1 M Na2HPO4 until pH 7.4 (∼5 ml)
- Bring up to 100 ml with 0.1 M NaPO4, pH 7.4
Solution B (8% PFA in dH2O stock solution) :
- See “Primary fixatives” recipe below
PLP fixative :
- Prepare fresh on day of experiment. Mix three parts solution A with one part solution B and add sodium periodate (NaIO4) (e.g., Thermo Fisher Scientific, cat. no. 044309.22) to a final concentration of 0.01 M (e.g., 2.13 mg NaIO4/ml of A + B mixture). Add the glutaraldehyde just prior to use. Final concentrations: 2% PFA, 0.01 M periodate, 0.075 M lysine, and 0.075 M PB.
It is also possible to use a final concentration of 4% PFA for better ultrastructural preservation.
GA can be added just prior to fixing, and the concentration should be titrated for each antigen (0% to 0.25%).
Phosphate buffer (PB), 0.1 M
0.2 M monobasic stock solution :
- Add 13.9 g sodium phosphate monobasic to 500 ml of dH2O.
0.2 M dibasic stock solution :
- Add 53.65 g sodium phosphate dibasic heptahydrate (or 28.4 g anhydrous) to 1 L dH2O.
0.1 M PB :
- To make a 0.1 M PB with a pH of ∼7.4, add 57 ml of monobasic stock to 243 ml of dibasic stock, and bring up to final volume of 600 ml with dH2O. For a solution with pH of 7.2, 84 ml of monobasic stock solution can be mixed with 216 ml of dibasic stock solution and brought up to 600 ml as described above. PB buffers can be stored at room temperature or at 0° to 4°C; however, they are susceptible to microbial contaminants, and should be freshly prepared monthly.
The pH can be lowered by increasing the proportion of monobasic volume relative to the proportion of dibasic volume (Hayat, 1993).
Primary fixatives
Glutaraldehyde (GA) and paraformaldehyde (PFA) solutions :
- GA and PFA EM-grade stock solutions are available from electron microscopy supply companies, (e.g., Electron Microscopy Sciences cat. no. 15720 and Ted Pella, cat. nos. 18505 and 1842) and can be purchased as ready-made kits or diluted to desired concentrations using 0.2 M CAC (e.g., Ted Pella, cat. no. 18851) or 0.2 PB (see recipe) and dH2O.
After exposure to air, GA and PFA begin to break down into glutaric and formic acid respectively, causing structural artifacts. Opened vials should be used within a day of opening. Working or stock solutions can be divided into smaller volumes (e.g., 4 to 10 ml), into a freezer-safe labeled container (e.g., PE tube), and sealed with Parafilm. The solutions can be stored up to 6 months in a non self-defrosting –20° to –80°C freezer.
PFA from powder :
- For 8% PFA stock solution, in 150 ml or larger beaker, add 8 g PFA powder (e.g., Fisher Scientific, cat. no. AC416780250) to ∼95 ml dH2O. Heat to 60°C while stirring, and then add 1 N NaOH dropwise until the solution clears. Bring up to 100 ml with dH2O and filter. Keep frozen aliquots in appropriate freezer vials sealed with Parafilm up to 6 months at –20° to –80°C.
COMMENTARY
Background Information
Fixation techniques for SEM
Early in the field of electron microscopy (EM) it was realized that fixation techniques utilized by light microscopists were inadequate for quality preservation of biological specimens at the resolution of EM. Seminal papers by Palade (1951) and Sabatini et al. (1963) describe the benefits of OsO4 and aldehydes, respectively. Although many permutations have evolved, the protocols for chemically fixing biological specimens for electron microscopy still use aldehydes for primary fixation and OsO4 for secondary fixation.
The addition of inorganic compounds to stabilize and add contrast to biological materials by nature induces artifacts. Despite this, steps can be taken to minimize the impact of chemical fixation to answer scientific questions. Ultimately, the specimen, the scientific questions being asked, and the availability of preparative and imaging tools determine the optimal protocol for a given experiment. An organism may require additives to the primary fixative to stabilize delicate structures; a tissue may require additional processing steps if immersion in primary fixative proves inadequate. Other specimens may require removal of components to expose a region of interest either through chemical or mechanical methods. Additional steps to increase the bulk conductivity of a specimen may be critical to prevent charging or improve the ability to visualize gold particles. The pH, temperature, and time are all factors that may need to be adjusted depending on specific requirements of the specimen.
Access to the most advanced instrumentation cannot improve poorly preserved specimens: garbage in = garbage out. Overgrown or “old” cell cultures can be structurally compromised before fixation begins; when trying to make conclusions about pathology, proper controls are essential. Ultrastructural changes occur immediately post-mortem, and tissues should be harvested and placed into fixative as quickly as possible; perfusion may be necessary for optimal results. Great care must be taken to avoid exposure to air during fluid exchanges. Specimens should be fixed with minimal disruption to their optimal environmental conditions, and consideration of preferred pH, salt concentration, and ionic requirements can assist greatly when selecting appropriate buffers for fixatives. Size of the specimen should be evaluated both for adequate penetration of fixative and for size limitations imposed by the microscope stage and lens geometry. The general guideline for adequate penetration of the primary fixative is a maximum of 1 mm in at least one dimension. However, this can vary tremendously depending on the specimen porosity, rigidity, and density. For example, lung tissue contains large void areas for gas exchange; luminal organs such as intestinal pieces may provide a port of entry through tubular space for better access to internal structures. Insects or other specimens with rigid cell walls may require only dehydration and coating for surface imaging. Bulk materials such as protein complexes may simply be air dried on a suitable substrate. Some specimens may be pretreated to remove impeding structures. For instance, an epithelial airway may contain a mucous layer completely obscuring the surface of the cells. It may be necessary to either mechanically or chemically remove such layers.
There are many extensive and comprehensive sources discussing and evaluating the chemistry for most compounds used for EM (Hayat, 1993), and of course familiarity with the literature on similar specimens can provide a starting point.
Critical point drying, specimen mounting, and sputter coating
Proper preparation does not end after the chemical fixation. Specimens need to be thoroughly dried. Most biological specimens require drying with either a critical point dryer, chemical dehydrants, or freeze-drying to avoid collapse. The specimen needs to be mounted on a suitable substrate for meeting microscope stage requirements, and additional considerations may include orientation, adherence properties, and enough surface contact with conductive mount to prevent charging.
Specimens need to be coated or infused with conductive material to prevent radiation and thermal damage to structures. Biological specimens are composed of low atomic number elements. Thus, when the electron beam strikes the sample, electron penetration is deep giving rise to a large interaction volume as the energy is absorbed within the specimen. The scattered electrons come from such a broad range around the point of entry that the resolution is greatly reduced. Deep penetration also contributes to loss of signal within the sample. Nonconductive specimens, when bombarded with electrons, produce artifacts known as charging. This is manifested as image distortion, thermal damage, and dark areas within an image due to repulsion of electrons. To avoid this, samples are coated in a thin layer of a conductive material (usually metal, but sometimes carbon). This decreases the sample interaction with the electron beam to improve signal, although fine structures may be obscured. It can also improve mechanical stability and contrast, which if applied correctly in effect increases resolution by addition of larger atomic elements. This improves the probability of interaction with the electron beam to improve signal, although fine structures may be obscured. Balance between enough and excessive coating must be evaluated and is determined by the desired magnification and resolution. The major types of sputter coaters consist of low (<10–3 Torr) or high vacuum (>10–6 Torr) systems and are briefly discussed below.
Evaporation systems are typically composed of a vacuum chamber connected to pumps able to achieve fairly low pressure. The target source is heated and, as the metal begins to evaporate, falls through the chamber onto the specimen. Since the flow is directional (material evaporates as an expanding void with the deposited portion comprising a range of solid angles from the source), the stage should be able to rotate and tilt for adequate deposition in areas that are not in direct line of sight from the target. The films are generally quite thin, ∼10 Å, but the system is limited to metals with a relatively low melting point, as heat generated within the system can be damaging to a specimen.
Sputter coating with low vacuum systems coats by “chunk” deposition of metals to create large grain films that may be visible at high magnifications, although acceptable for viewing at low- to mid-range magnifications. Magnetron sputter coaters are composed of a vacuum chamber but operate at higher pressure than evaporators. The chamber is evacuated, and the system is then flooded with an inert gas such as argon. Ionization is caused by the voltage applied between the cathode and ground at low pressure. The current is a result of flow of ions between the cathode and ground. The target is composed of a metal usually in the shape of a donut or disc and a magnet. Negative ions are repelled by the cathode and travel toward grounded surfaces (those that pass through the magnetic field spiral and are deflected according to the ionic charge and momentum, and the density and orientation of the magnetic field). They are attracted to the magnet and divert away from the sample, avoiding damage. Positive ions are attracted to the cathode (metal target) and erode the metal, which falls by force of gravity to the bottom of the chamber onto the specimen. In this low vacuum system, there is a short mean path of travel causing metal to move erratically through the chamber, resulting in omni-directional coating of the specimen.
Ion beam sputter coating also erodes metals from a source for deposition on a sample, but in a different manner. In this case, the system is under low pressure, and an electrostatic field is generated within a gun assembly. When argon is introduced into the field, gas ionizes and is directed into a collimated beam towards a target. The specimen should be tilted and rotated to achieve a continuous coating. Ion beam sputtering has an advantage over the magnetron system in being able to produce a very fine film and over the evaporator system in that it does not require the use of low-melting-point metals. Final grain size and thickness of the film can be more accurately assessed by TEM analysis of sections cut from resin blocks on which a film has been deposited.
Immune-labeling for SEM
In general, immune-labeling of antigens is more easily achieved for SEM than for TEM since the labeling is usually done after mild fixation to deactivate and partially stabilize the specimen and subsequently fixed for “good” ultrastructure. This is not meant to imply the protocols are without inherent difficulties and concerns. Titration of primary and secondary antibodies is essential to avoid lack of labeling or excessive background staining; binding efficiency and steric hindrance may limit the number of sites labeled; determining appropriate gold size is critical for optimal imaging at preferred magnifications. Appropriate controls to confirm specificity are required. Too often in the literature a large arrow points at one gold bead with claims of specific labeling despite evidence of gold with similar concentration in the background (or worse, the surrounding area has been cropped out of the picture). Correlative studies are becoming an increasingly important approach for relating structures to specific events or identification of specimens that cannot be labeled with an electron-dense probe and may require modifications for visualization by both light and electron microscopy. Burghardt and Drolesky (2006) provide many useful insights and tips in their TEM protocols that can also be applied to immune-labeling for SEM.
Microscope parameters
When the specimen is finally ready for examination in the microscope there are several parameters controlled by the operator that can greatly affect the quality of an image. Optimization often involves compromise. Improved depth of field usually requires sacrifice in resolution; higher theoretical resolution achieved by higher voltage may be undone by increased depth of beam penetration. Spherical and chromatic aberrations and astigmatism are all factors that can prevent quality imaging. Basic adjustments that can be applied universally will be discussed in the following section.
Critical Parameters and Troubleshooting
Chemical fixation, drying, mounting, and coating specimens
Fixatives are an imperfect solution. They are highly extractive and can induce rearrangement and disruption of cellular and sub-cellular structures. The goal is to minimize deleterious effects and preserve structures representative of their natural state. Tables 1–4 provide a quick reference to address common problems, causes, and possible solutions. Fixative penetration can be slow, and although more apparent in TEM, should still be considered a factor for SEM preparations as it may result in shrunken tissue or poorly fixed internal structures. Tissues require longer exposure time than cell monolayers. GA remains the most utilized primary fixative and is a strong cross-linker of proteins, although dense materials may fix better if fresh PFA is added in conjunction with the GA (Karnovsky, 1965). Although PFA does not cross-link as quickly as GA, it penetrates tissues more rapidly. This may be more critical for TEM preparations but can be useful for SEM preparation of dense specimens. Addition of malachite green (MG) to the primary fixative with subsequent osmication stabilizes particular classes of lipids (Lawton, 1989). Addition of ruthenium red (Luft, 1971) or alcian blue/lysine (Fassel et al., 1997) mixtures to the primary fixative can stabilize polysaccharides that are routinely lost during conventional processing. Generally, room temperature addition of fixative is acceptable although in some cases it may be less extractive to perform the steps at 0° to 4°C.
| Problem | Possible cause | Solution |
|---|---|---|
| Structures appear shrunken | Improper fixation | Alter strength or type of fixative |
| Hypertonic buffer | Adjust the salt concentration | |
| Excessive holes in membranes | Inadequate dehydration | Start dehydration series with lower concentration of ethanol or acetone |
| Specimen exposed to air during fluid exchanges | Avoid unnecessary exposure to air | |
| Avoid complete removal of solutions during exchange; do more exchanges | ||
| Inadequate exchange in critical point dryer | Do more and extended exchanges of fluids in the CPD | |
| Put under vacuum before completely dried | Allow specimen to dry overnight before placing under vacuum | |
| Debris on specimen | Incomplete washing with buffer | Additional wash steps prior to first fixations may be necessary |
| Use of mild detergents or other reagents may be required | ||
| Capsular material appears sloughed off in background | Inadequate fixation | Additives may be required for stabilization (e.g., alcian blue or ruthenium red) |
| Adjust pH or buffer appropriately | ||
| Specimen moves while imaging | Poor contact with mounting substrate | Add conductive paint around specimen to “seal the cracks” |
| Not enough material adhering to substrate | Concentration of specimen too low | Allow longer settling time before fixation |
| Increase initial concentration | ||
| Poor adherence properties | Pretreat mounting substrate with adhesive | |
| Excessive charging of sample (see Table 4) | Poor contact of specimen to mount | Improve contact by firmer placement on conductive adhesive and coat with conductive paint if appropriate |
| Inadequate metal coating | Increase thickness of metal coating | |
| Inadequate bulk conductivity | Increase bulk conductivity through OTOTO | |
| Fine detail seems obscured, and/or surface appears “chunky” | Excess metal applied during sputter coating | Apply thinner layer of metal |
| Use finer grain or lower atomic number element |
| Problem | Possible cause | Solution |
|---|---|---|
| Little or no label | Antigen not exposed | Use permeabilizing agent (e.g., saponin) |
| Fixation disrupts/alters antigen | Titrate or change fixatives | |
| Primary or secondary concentration too low or not specific | Titrate antibodies; try alternative antibodies | |
| Steric hindrance of large antibodies | Use smaller probes | |
| Loss of structure | Find alternative fixation additives (e.g., alcian blue or ruthenium red) | |
| Cross-reactivity | Change ab species | |
| Quench aldehydes by adding 0.1% glycine | ||
| Excessive label on specimen | Concentration of primary and/or secondary ab too high | Dilute antibody concentration |
| Increase NaCl concentration | ||
| Nonspecific binding to specimen | Change blocking buffer | |
| Excessive label on background substrate | Concentration of primary or secondary ab too high | Titrate ab concentration |
| Add or change blocking agent | ||
| Poor elemental contrast in microscope | Gold size too small | Use larger gold size or enhance with silver or gold |
| Sub-optimal microscope settings | Use larger aperture, greater voltage, bigger spot size, shorter working distance | |
| Coating or OTOTO masked gold | Apply lighter coat of low atomic number metal and/or reduce OTOTO incubation time |
| Problem | Possible cause | Solution |
|---|---|---|
| Specimen moves while imaging | Poor contact with mounting substrate | Press specimen more firmly on surface of mount |
| Poor contact of specimen to mount | Apply conductive paint around specimen to improve contact | |
| Excessive charging of sample | Inadequate bulk conductivity; specimen oxidized | Decrease number of electrons entering sample or increase number of electrons exiting sample (see Table 4) |
| Image appears stretched | Astigmatism from contaminants | Stigmate aperture |
| Unable to stigmate | Misaligned objective aperture | Center beam and aperture |
| Decrease electrons entering | Increase electrons exiting |
|---|---|
| Decrease voltage | Good contact between sample and mount (if necessary, use conductive paint) |
| Increase bias | Screw the mount to the stage for better grounding |
| Reduce spot size | Thicker or different metal coating |
| Insert smaller aperture; if available, change detector (e.g., lower instead of upper) | Increase bulk conductivity (e.g., OTOTO) |
| Increase scanning speed or use frame buffering system | Shorten distance of travel to detector by tilting specimen towards detector |
| Adjust beam current (usually reduced) |
There are a variety of buffer choices used for EM specimen processing. The most commonly used are PB and CAC buffers, which are thought to be less extractive than other buffers. PB buffers are thought to most mimic cytoplasmic environments but have a short shelf life as they easily support bacterial growth and can contaminate specimens when they produce electron-dense precipitates in the presence of calcium ions. CAC buffer works equivalently to PB without the concern for precipitates. However, it contains arsenic, and thus is a hazardous waste, but it has a longer shelf life. Buffers such as HEPES and PIPES are non-toxic, and ions can easily be added although they may not be as effective in buffering capacity as CAC or PB. The buffer must be compatible with the specimen of interest. Tris should not be used during primary fixation with aldehydes as it reacts with GA.
Addition of heavy metals to biological specimens provides both structural stability and elemental contrast that is important for imaging by electron microscopy. OsO4 stabilizes both lipids and proteins. Addition of TA and UA can also increase bulk conductivity of a specimen, enhance membrane stabilization, and improve overall contrast. With successive rounds of OsO4 and TA or TCH as outlined in the OTOTO protocol, enough metal may be infused in the specimen to obviate the need for additional metal coating.
Although this article is limited to room temperature sample preparation for SEM, Figure 29 demonstrates the extraction of cytoplasmic elements in Chlamydia- infected HeLa cells that were high-pressure frozen and freeze fractured following either a brief exposure to PFA (Fig. 29A) or fixation with GA/0.1% MG followed by OTOTO (Fig. 29B). Note the obvious extraction of cytoplasmic elements in the more extensively fixed specimen, which for some studies may be preferred to expose features of interest.
Adequate dehydration of specimens is critical to avoid collapse of structure under vacuum. Biological specimens are composed primarily of water, which needs to be removed from the specimen without introducing shrinkage artifacts (think of plums versus prunes!). Water has strong surface tension due to hydrogen bonds and when evaporated from a biological specimen, the adhesion forces of the water cause crinkling or breakage of membranes or both. Figure 30 shows polarized HeLa cells after insufficient dehydration. The large cracks in Figure 30A could be dehydration artifacts, although these cells were grown on a membrane filter, and mechanical damage during specimen mounting may have contributed to the breakage. The holes in Figure 30B are most likely due to incomplete removal of water or full exchange of the transition agent in the CPD. Surface tension can be reduced with ethanol or acetone. For delicate specimens, a graded series of solvent may be required to prevent damage, and then subsequently replaced with CO2, which can reach its critical point, reducing surface tension to zero under tolerable temperature and pressure for most biological specimens. The gas is slowly released, and the structure should remain largely intact.
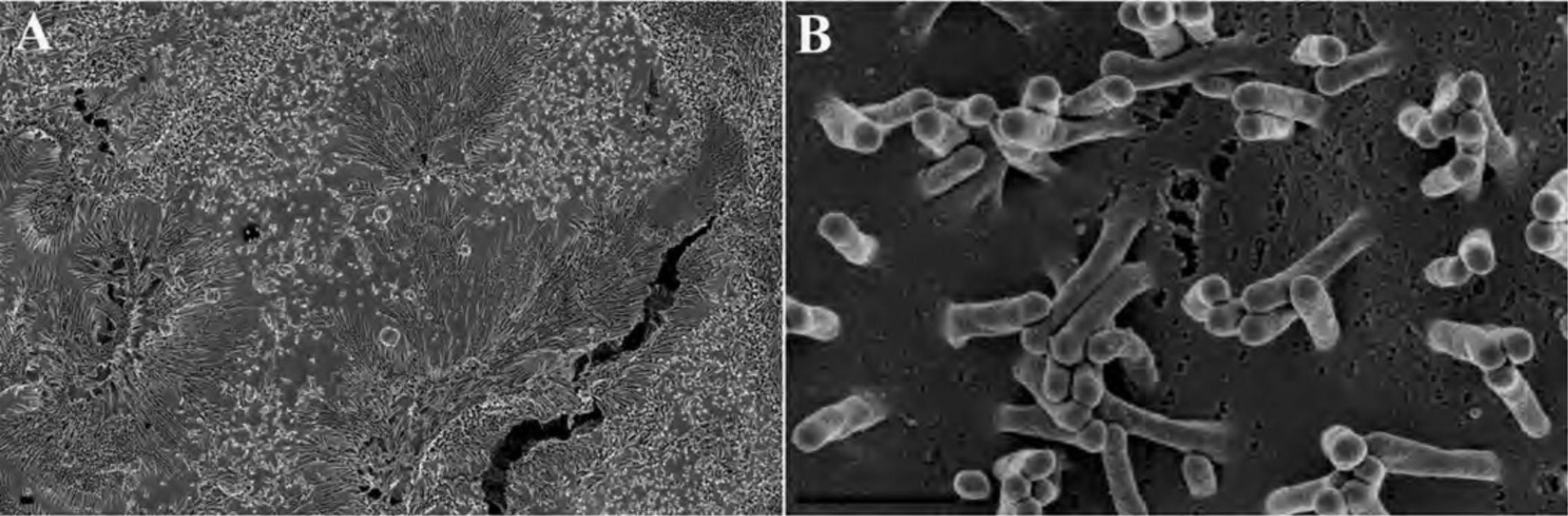
Tissue specimens can be fractured internally after dehydration with dry ethanol. The ethanol acts as a cryo-protectant when the tissue is dropped into liquid nitrogen. If water is present, ice crystals will form within the tissue, causing damage. Pressure from a pre-cooled razor blade causes the tissue to crack, leaving clean edges suitable for imaging. The tissue is then critical point dried as normal.
Some specimens may not fit in a CPD or may be unable to withstand the turbulence for which there are chemical alternatives such as HMDS. Although best application is for rigid specimens, chemical alternatives can be useful for some delicate specimens.
After drying, specimens are mounted on a SEM mount or stub that is microscope specific. The specimen can be placed on a conductive adhesive and/or attached using conductive paint and allowed to dry. Tissues or cell layers can be mechanically fractured to expose internal structures using an adhesive tape gently applied to the surface or with a scalpel or syringe tip, depending on the tissue. The sample should be coated after fracturing so a conductive layer is applied to newly exposed surfaces.
Metal selection will be somewhat determined by available equipment, although there are several points to consider. Conductivity is of major importance. Metals such as silver, gold, and copper all have good conductivity. During metal deposition, the metal coats the surface of the specimen and initially serves to nucleate a film as more metal is deposited. Gold is susceptible to thermal fluctuation, expanding and contracting, which can cause cracks and impede conductivity. Gold also has a strong cohesive force with itself, and as it migrates can form puddles of gold interfering with surface topography. Frequently, gold targets are mixed 60:40 with palladium (Au/Pd) to reduce the cohesiveness and prevent clustering. With improved resolution of modern microscopes, the Au/Pd mixture can interfere with high-resolution imaging. Alternatives that provide consistent and more refined layers include iridium and chromium. Iridium provides better elemental contrast and chromium, with its lower atomic number, does not interfere with gold visualization, although is more easily oxidized and not as stable over time. Charge variation on the specimen surface can prevent even distribution of the metal, and wettability can be improved by depositing a light layer of carbon or chromium prior to addition of the heavier metal.
Topography of a specimen can be enhanced by coating at a fixed angle with a heavy metal and then adding a continuous film of a lighter element. For instance, in Figure 31, proteins were fixed on a silicon chip, critical point dried, and then rotary shadowed with iridium (Fig. 31A) or shadowed with Pt, followed by rotary shadowing with carbon to improve conductivity (Fig. 31B). Note the difference in apparent dimensionality created by application of Pt from a fixed angle to create a shadow effect. It should be noted that at higher magnification, the grain size of the Pt becomes obvious.
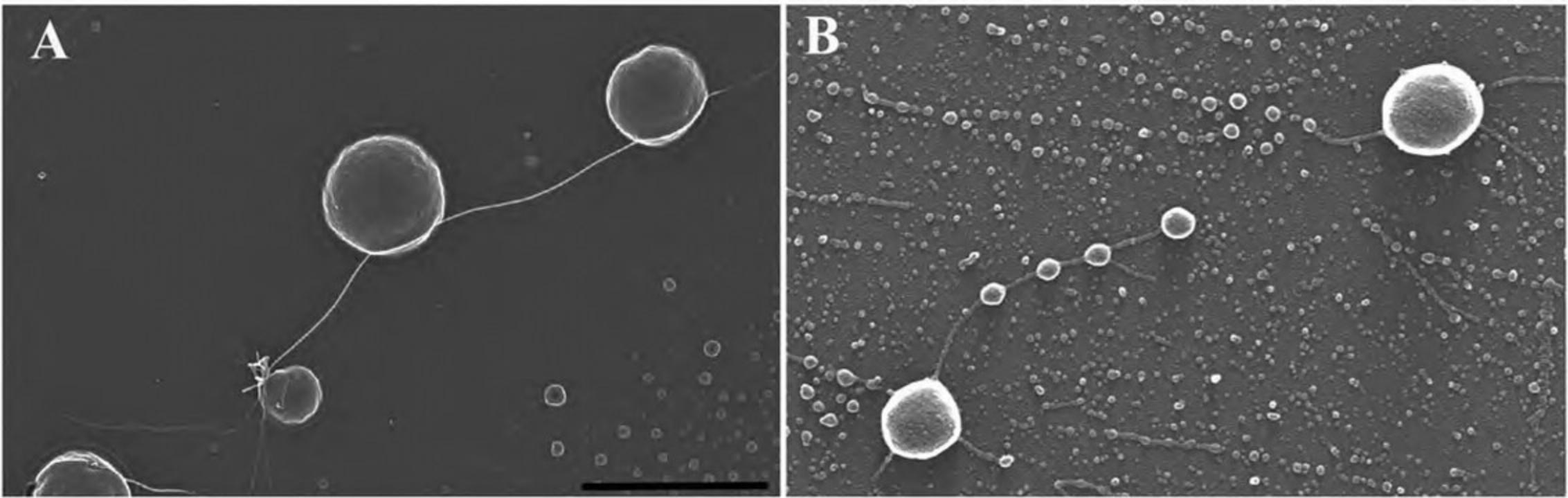
In some instances, such as gold or quantum dot labeling, impregnating specimens with heavy metals may provide enough conductivity to a specimen making coating unnecessary, as described in Alternate Protocol 7.
Immunology
The ability to localize antigens is an incredibly powerful tool. Electron-dense probes, such as colloidal gold, Nanogold, Aurion Ultrasmall gold, or quantum dots, allow high spatial resolution of macromolecular structures to provide superior insights into cellular and subcellular composition and functional relationships. Although there are many reagents to choose from and permutations to improve labeling, it is helpful to understand some basic strategies including the labeling, chemical and mechanical post-processing, necessary controls, and interpretation of results.
All protocols involve specific labeling with a primary antibody or probe, either directly conjugated to or subsequently labeled with an electron-dense probe. Optimal concentrations should be experimentally determined through titration of both primary and secondary antibodies.
Primary antibodies can be polyclonal or monoclonal. Polyclonal antibodies may recognize multiple epitopes on the antigen of interest. Monoclonal antibodies typically recognize a single epitope. Many polyclonal and monoclonal antibodies to a variety of cellular antigens are commercially available but must be tested for activity under the appropriate conditions.
Gold colloids are usually complexed with species-specific secondary antibodies. In most cases, it is time and cost effective to purchase commercially available probes. Gold probes can vary in size from <1 nm to >80 nm. For directly viewing gold in an SEM, 20 to 40 nm sized particles are generally preferred for ease of visualization at mid-range magnifications. Steric hindrance may impede efficiency of binding, and, in this case, smaller gold probes may be beneficial. If using Nanogold or Aurion Ultrasmall gold, the signal can be amplified through either gold or silver enhancement techniques.
Plan ahead! Proper controls are absolutely essential for proper analysis of the results. The controls should include, where appropriate, uninfected cells, non-expressing cells, isotype-matched irrelevant primary antibody control, known positive antibody control, secondary only (substitute blocking buffer for primary), and if applicable, silver or gold enhancement only controls. Some degree of nonspecific (background) binding is almost always inevitable. Tips for reducing background labeling are shown in Table 2. Figure 32 shows three different labeling outcomes. The focal plane in Figure 32A suggests that the bacteria are well-labeled, although at closer inspection, excessive background gold is evident, clouding interpretation. Figure 32B shows labeling that appears specific above a lesser amount of background levels, and Figure 32C shows a highly specific interaction with little or no background labeling.
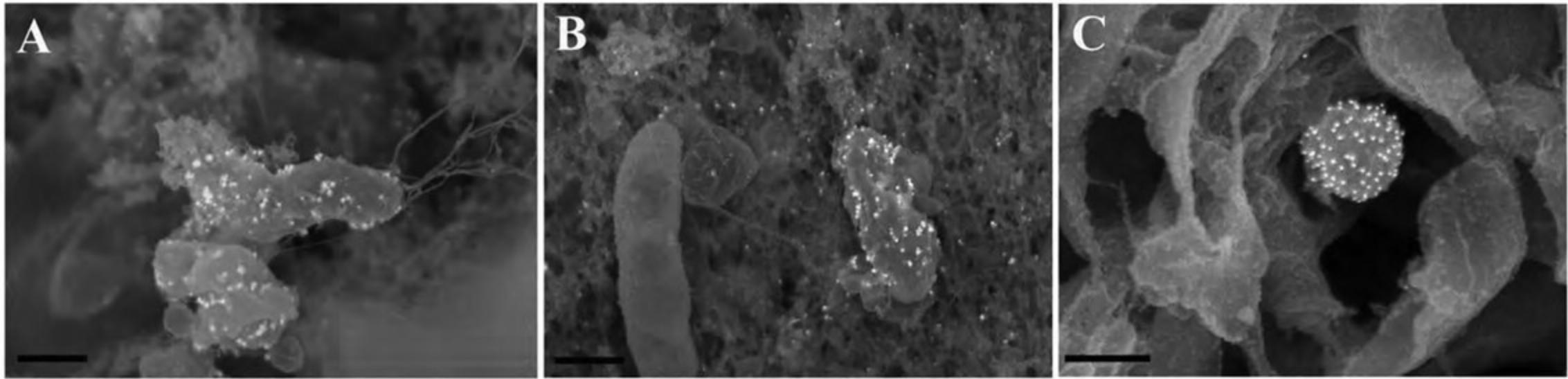
Immune-labeling for SEM, in general, labels surface-exposed antigens, and the immunology is usually performed following mild fixation, usually 2% to 4% PFA in suitable buffer to immobilize a cell (this is not always necessary for viruses, bacteria, or macromolecular complexes unless required for biosafety or for stabilization). Figure 33 demonstrates specific labeling of material that appears to have sloughed off during preparation, suggested by what appears to be specific labeled material on the mounting substrate. Stabilization of the structure, by addition of alcian blue/lysine to the primary fixative, resulted in successful retention of structures, as shown previously in Figure 4.
Blocking steps often help reduce nonspecific binding and can include nonfat milk, fish gelatin, globulin-free bovine serum albumin, or fetal bovine serum. Addition of salts such as sodium chloride or potassium chloride, or mild detergents such as Tween 20, can help reduce background excess. The blocking buffer or a reduced protein version should be used as a vehicle for the primary and secondary antibodies, and the washing steps in between. The number and duration of washes can help reduce excess binding. After labeling, specimens should be thoroughly rinsed with buffer and distilled water to remove excess gold, and then the specimen processed according to preferred protocol.
Choice of gold size and metal coatings should be carefully considered. Chromium or the OTOTO methods do not obscure most gold particles. Enhancement of the gold prior to coating can add elemental contrast for viewing. Microscope-specific parameters will be discussed in the following section.
Although cryo-SEM techniques are not discussed in the article, it is worth pointing out that this technique also allows viewing labeled specimens by freeze fracture after high-pressure freezing and could be useful for complementary techniques, as shown by cryo-SEM and room temperature TEM in Figure 34 panels A and B, respectively.
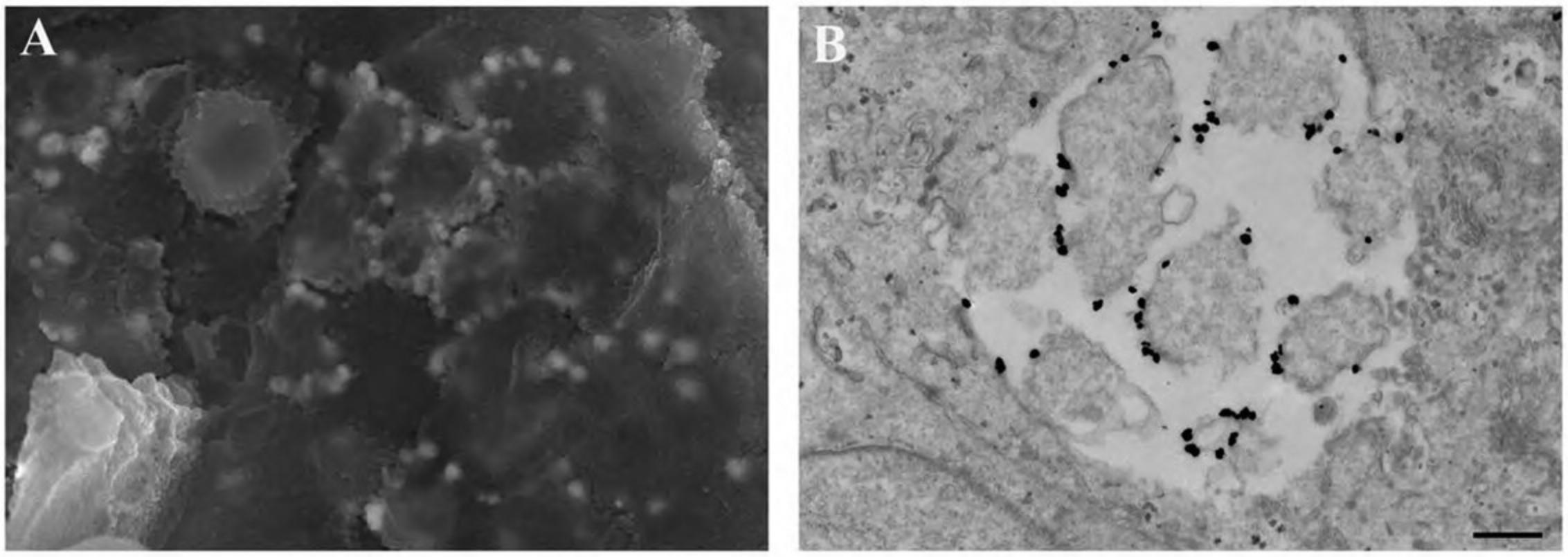
Microscope settings
The following section offers practical guidelines for imaging biological specimens by SEM and Tables 3 and 4 offer suggestions for ameliorating specific conditions. A more complete discussion behind the theory and limitations of microscope resolution and operations can be found in a variety of sources (Goldstein et al., 2002).
In theory, higher voltages, smaller apertures, smaller spot sizes, and shorter working distances are tantamount to better resolution. In practice, however, optimal settings vary widely depending on the application. Of course, a well-aligned microscope is essential in producing high-quality images, and alignments, focusing, and proper stigmation should be performed at magnifications higher than used for imaging.
Voltage
Abbe's Law defines resolution as directly proportional to the wavelength used for imaging; the shorter the wavelength, the closer two objects can be and remain distinct. Electrons travel in much shorter wavelengths, by a factor of x <103, compared to photons, giving electron microscopes much higher resolving power than light microscopes. Electrons have an increasingly shorter wavelength as the voltage increases, corresponding to better resolution. However, they also travel with more energy and the beam penetrates more deeply, especially into biological specimens, which degrades resolution.
There are two types of scattering events that occur as the electron beam strikes a surface, elastic or inelastic, and depending on the event, cause either backscattered or secondary electrons that can be preferentially detected. Elastic scattering events occur when the electron beam strikes the specimen and deflects with little or no loss of energy, and the electron “bounces” off the sample as a backscattered electron (BSE). BSE generation is greater for higher atomic numbered elements and the frequency of interactions in a biological specimen labeled with 20- to 40-nm colloidal gold is much greater with the gold atoms than on adjacent carbon atoms. Thus, more BSEs are generated; providing elemental contrast to allow identification of gold particles on biological specimens. BSEs are selectively collected by backscatter detectors. Inelastic events occur when electrons striking a sample interact with other electrons, and with great loss of energy cause emission of secondary electrons and characteristic X-rays (which can be used for elemental analysis). Secondary electron images are produced based on specimen topography and detector geometry, and thus are collected to image surface contour. Biological specimens are susceptible to deep penetration by the electron beam, and the resulting interaction volume can result in loss of signal or generation of secondary electrons from a broad depth that degrades resolution. Adequate coating, metal impregnation, and appropriate voltage requirements based on the specimen should be considered to optimize imaging conditions.
A lower voltage beam is more susceptible to the effects of chromatic aberration and results in lower signal but does not penetrate the specimen as deeply. The “correct” voltage is sample dependent (Fig. 35). For lightly coated specimens, imaging with a field emission SEM at 2 kV (Fig. 35A) provides adequate signal and good definition of surface features. For cell monolayers or most tissues with adequate coating, 5 kV generally provides better signal without excessive beam penetration (Fig. 35B). Higher voltage at 10 kV in this case may result in loss of resolution due to deep penetration of the electron beam. Note the ghost-like appearance in Figure 34C.
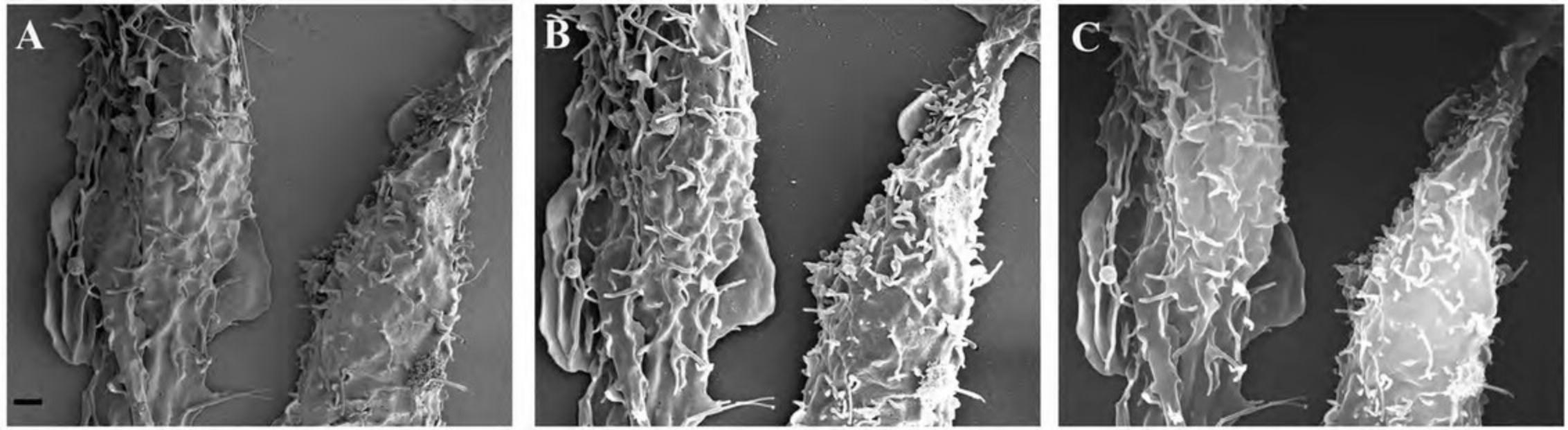
Conversely, for specimens that lack depth, increasing voltage may improve both signal and resolution, as shown in Figure 36. In this case, protein fibrils processed by OTOTO that were uncoated showed charging artifacts (Fig. 36A). When the same preparation was coated with 20 Å of chromium, imaging at higher voltage allowed better visualization of surface topography (Fig. 36B-E). Specimens shadowed with 15 Å of Pt and rotary coated with 20 Å of carbon obscured fine detail and metal grains became more evident (Fig. 36F).
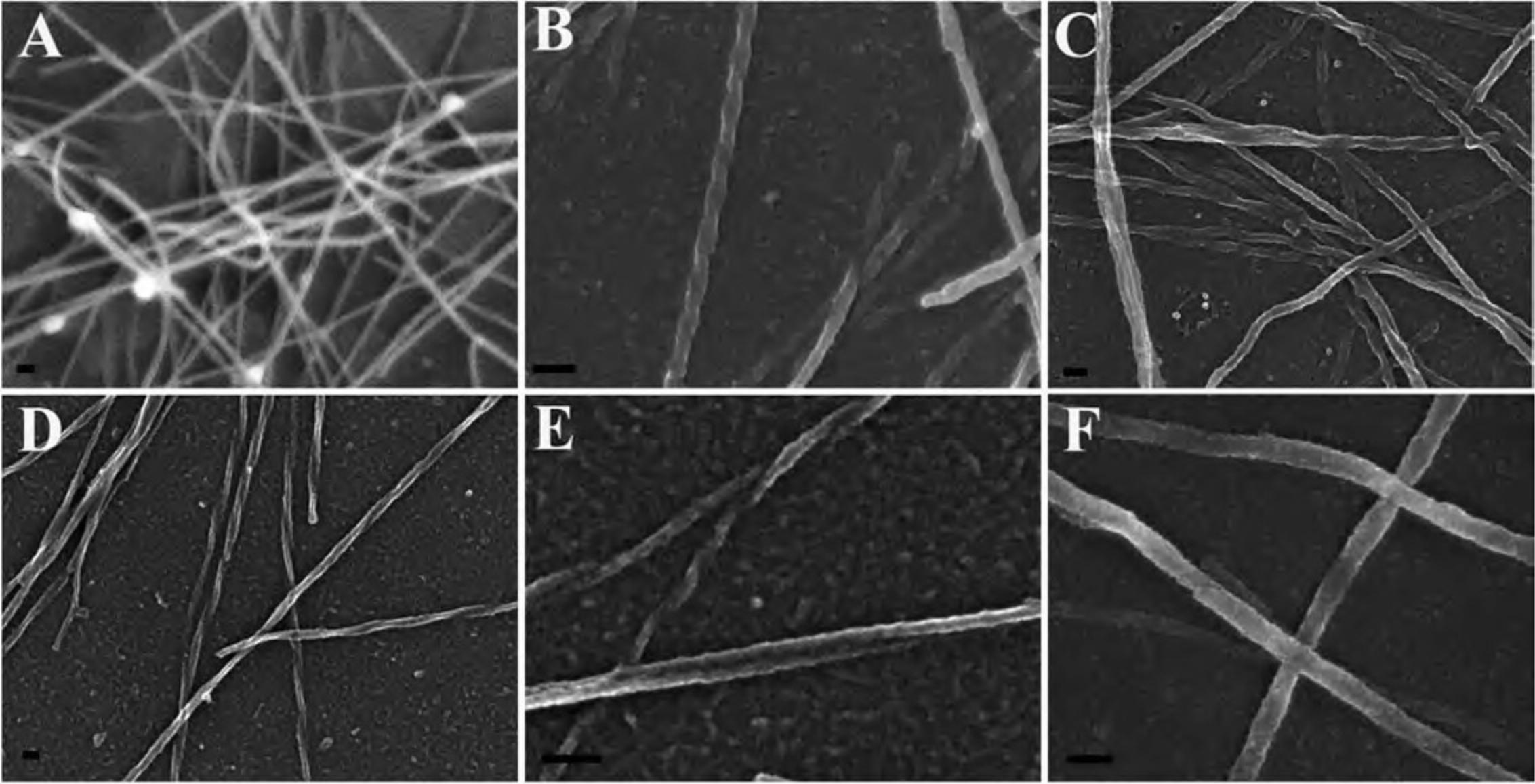
Depth of field
One of the great features of SEMs is the tremendous depth of field capacity. Relatively large objects can be imaged entirely in focus if the working distance is set accordingly. Again, compromise may be required. Placing an object closer to the objective lens will increase the resolution but it also limits the focal plane. The “correct” working distance is established by the desired imaging effect or limitations of the stage. For high-resolution imaging of relatively flat surfaces, 0.1- to 5-mm distances are generally quite good. For larger specimens, the working distance may be increased to 45 mm, and for specimens in between, 8- to 15-mm distances are usually preferred. The distance used will also depend on detector geometry and how much signal is sacrificed when using long working distances. Figure 37 shows an HIV-infected lymphocyte at 5- vs 17-mm distance from the objective lens. Note that although the “top” of the cell is well focused in Figure 37A, the image becomes blurred quickly in the background compared to the cell in Figure 37B, where the cell and background substrate are both in focus.
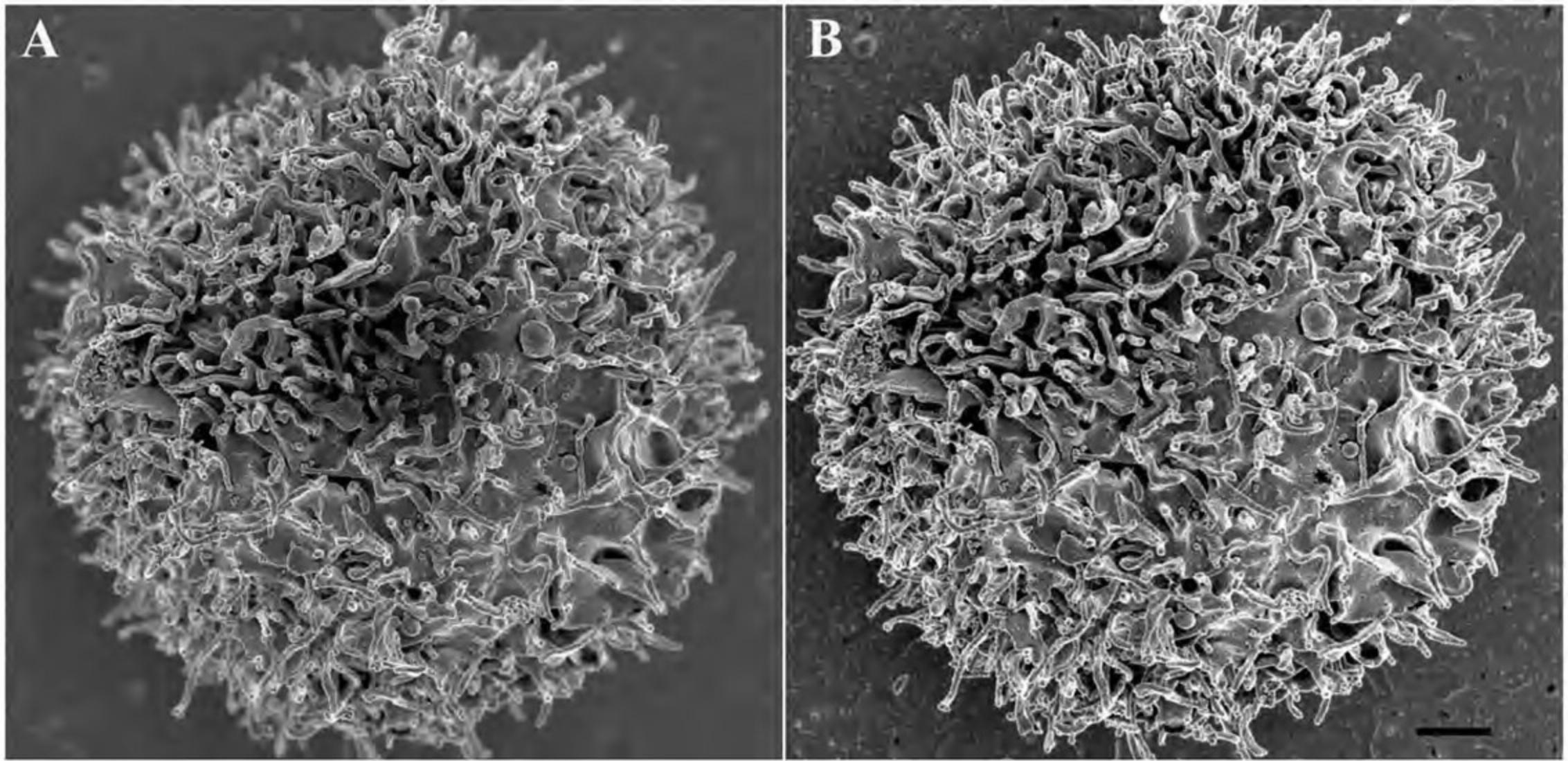
Apertures are inserted to reduce spherical aberration. In theory, smaller apertures are most effective, but the compromise is reduction in signal. Objective apertures typically range from 30 to 100 µm in diameter. Generally, 50-µm apertures are suitable for most applications. Since elemental contrast is dependent on number of beam interactions, a larger aperture may provide better signal and improved contrast. After the electron beam and apertures have been centered, it is important to correct for astigmatism. This is most easily accomplished by going through focus and using the stigmator control, correcting the stretch in the image, first in one direction, and then in the other. Several iterations of this must sometimes be performed to refine the images. Figure 38 demonstrates the difference between astigmatism in the x direction (Fig. 38A), y direction (Fig. 38B), corrected but out of focus (Fig. 38C), and well stigmated and focused (Fig. 38D).
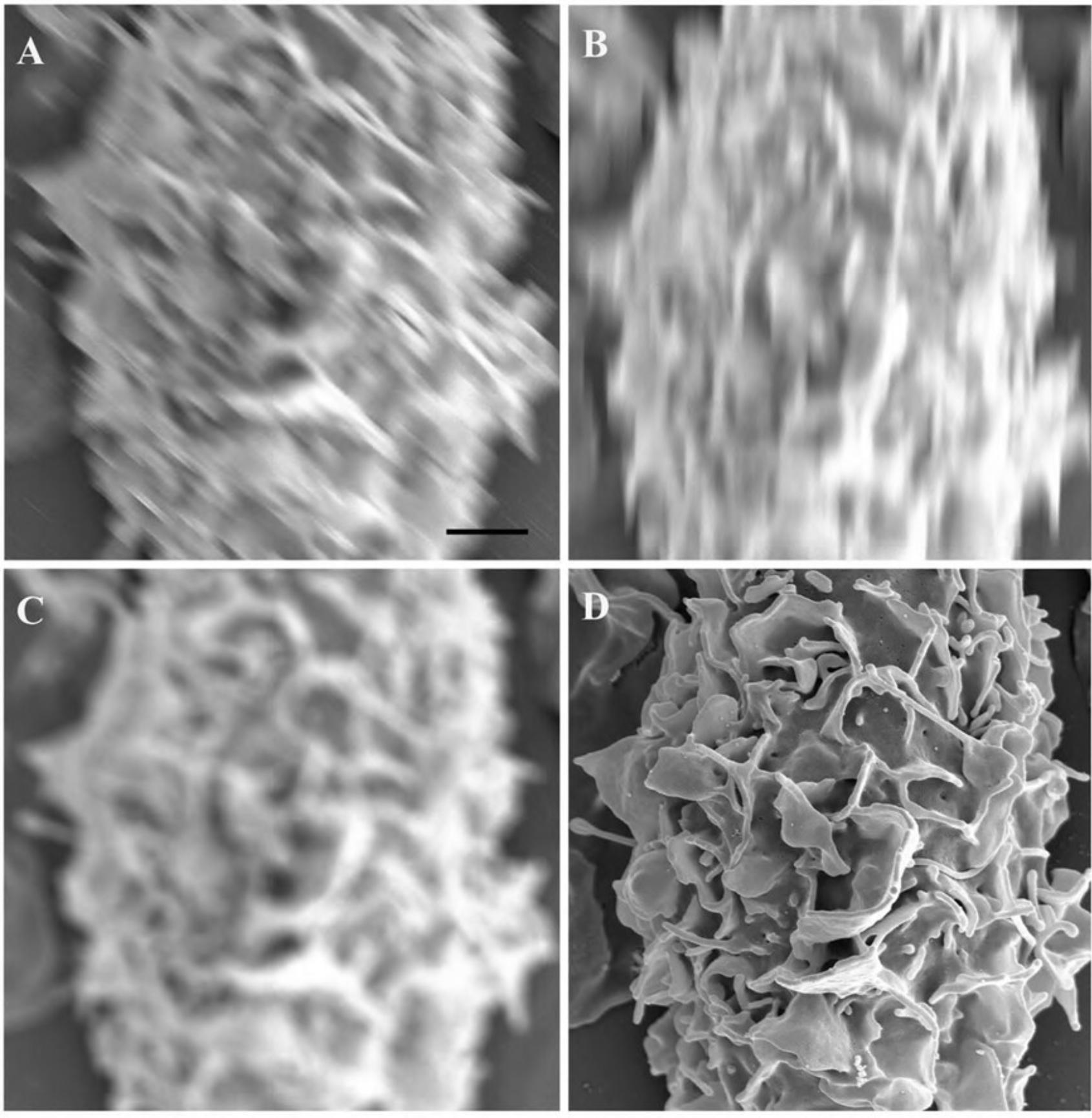
Charge artifact
Entropy is induced when the electron beam strikes a specimen, where regions of both positive and negative charge are produced. If an area becomes more positive, it displays as a dark region since it attracts more electrons. More commonly, negative regions show up very bright as they repel electrons, displayed as streaking, flattening and, in extreme cases, smearing of electrons and damage to specimen, preventing quality visualization and imaging (Fig. 39). Neutralizing a specimen requires the number of electrons entering a sample equaling the number leaving a sample. Charging occurs when the number of electrons leaving a sample are greater or lower than the number of electrons entering. Table 4 provides methods to reduce charging by either decreasing the number entering or increasing the number exiting a specimen.
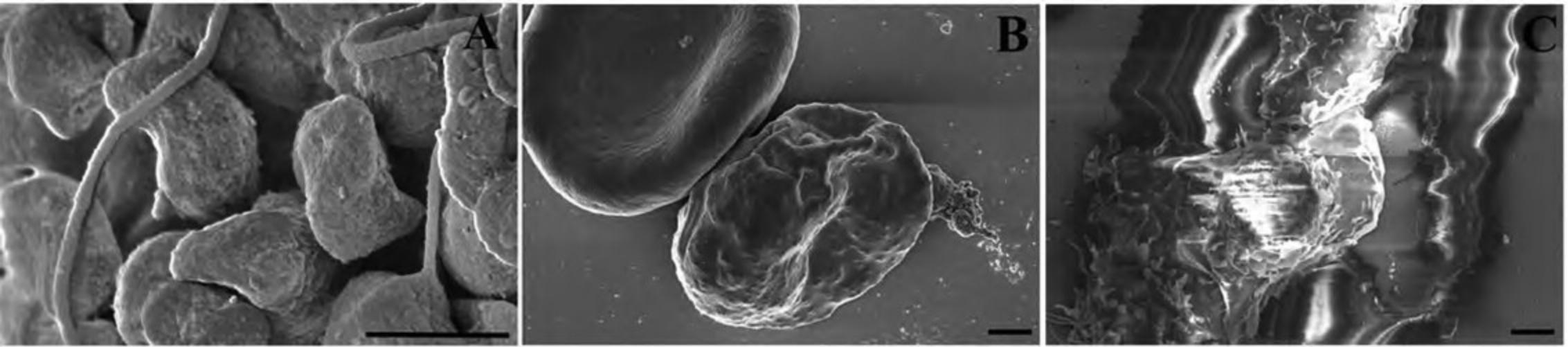
Understanding Results
General SEM preparation and imaging
Successful imaging by SEM begins with quality specimens whose structures have been preserved in a state representative of how they exist in nature. Specimens should be properly attached and fixed on a mounting substrate and coated or prepared in a manner such as OTOTO to reduce artifacts such as charging or movement. Dehydration artifacts should be minimized through proper dehydration techniques.
Selection of appropriate SEM voltage requires a balance between good signal-to-noise ratio, depth of penetration consideration, and resolution requirements. Working distance should be adjusted depending on the range of depth in the z -axis to achieve desired effects. For instance, in Figure 40, the feature of interest is the centered HeLa cell infected with both streptococcal bacteria and Varicella Zoster Virus (VZV). The working distance of the specimen is adjusted so that most of the cell is in focus, and the surrounding “background” cells are slightly out of focus. This draws the viewers’ eyes to the region of interest and appears to add depth to the specimen.
Selection of voltage can greatly influence the ability to resolve small structural details, and the gain in resolution at high voltage can be undone by deep beam penetration within the specimen. Flat specimens, such as purified proteins or complexes well adhered to mounting substrate, can often be imaged at very high voltages since they have little depth to their structures.
Immune-labeling, imaging, and interpretation
Although immune-labeling provides a powerful tool for the localization of cellular proteins, correct interpretation of results is dependent on the quality and quantity of the label, and proper controls. The amount of gold particles present should convincingly exceed background levels, which should be visible in the image. Controls should include the use of an irrelevant primary antibody, specimens that lack protein or structure of interest, and when appropriate, silver- or gold-only enhancement. Although it is tempting to use the amount of gold label for quantification studies, this cannot be done without normalization for variables such as binding efficiency and antigen accessibility; however, qualitative observations can often be made.
Imaging with appropriate voltage, magnification, and other microscope parameters in BSE or mixed BSE/SE imaging modes are all important considerations for visualizing the gold particles. Note in Figure 41A, SE imaging does not provide the elemental contrast needed to visualize the gold particles labeling a glycoprotein associated with the biofilm produced by staphylococcal bacteria. By increasing the number of BSEs collected compared to SEs, the gold particles are readily identifiable as shown in Figure 41B.
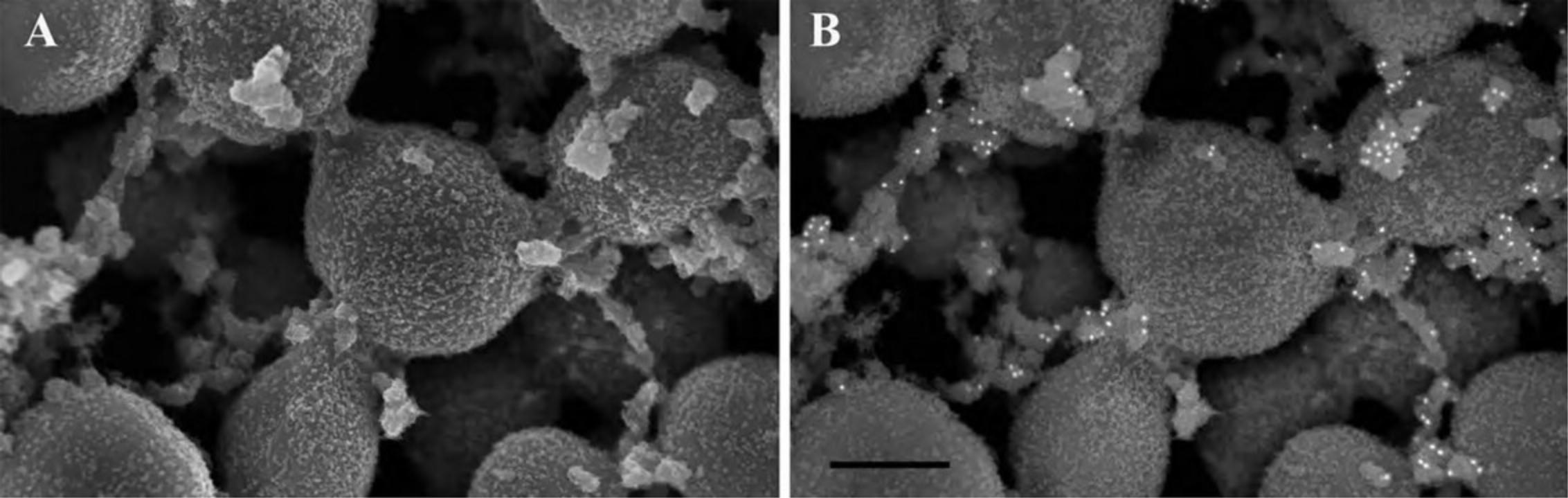
Time Considerations
Conventional specimen preparation
SEM specimen preparation is relatively quick. Specimens are washed, fixed, and dehydrated within 2 hr. Bulk materials may require extended incubation time in the various fixative components, or additional steps to improve conductivity of the specimen, although overall chemical processing still takes <1 day. Microwave technologies have greatly reduced the time required for many, as discussed in Support Protocol 3.
Improper dehydration is a primary culprit in deterioration of cell membranes, and special care should be taken with fragile specimens that may require a graded series of ethanol or acetone, and possibly longer exchanges in the CPD or chemical dehydrant. It is recommended that specimens be allowed to dry overnight in a desiccator prior to placement under vacuum.
Sputter coating, depending on the system, can take minutes or hours. In general, since a continuous film is built on nucleating events as the metal strikes the surface of the specimen, it is better to coat more quickly to avoid migration and “puddling” of the metal through more rapid deposition of nucleating centers.
Immune-labeling
Successful immune-labeling generally requires blocking, antibody incubations, and multiple washing steps prior to fixation, and subsequent steps for general SEM processing. In the case of external antigens, labeling with specific antibodies can be performed in 2 to 3 hr, and the fixing and drying as described above may take an additional 2 to 3 hr. Titration of antibody concentration may be required to achieve optimal results and can require several attempts for satisfactory yield. It can be advantageous to perform preliminary experiments to determine optimal buffer and blocking conditions, and titration of aldehydes and antibodies, using fluorescent secondary antibodies.
Labeling internal antigens requires additional permeabilization steps, and if gold or silver enhancement is necessary, several hours will be added to the procedure.
Acknowledgments
The authors would like to thank Dr. Ted Hackstadt for critical review of the manuscript and Dr. Raymond Rosado for technical assistance with the microwave oven protocol, and Austin Athman, Sophia Antonioli-Schmit, and Benjamin Crews for help with figures. We would like to thank the following individuals for providing samples: Dr. Harlan Caldwell, Dr. Eliza Carneiro Alves-Ferreira, Dr. Jean Celli, Dr. Jeffrey Cohen, Dr. Frank DeLeo, Dr. Inta Gribonika, Dr. Ted Hackstadt, Dr. B. Joseph Hinnebusch, Dr. Vicky Jensen, Dr. Louis Miller, Dr. Seth Pincus, Dr. Suzette Priola, Dr. Tom G. Schwan, Dr. Olivia Steele-Mortimer, Dr. Tom Wellems, and numerous other NIAID intramural investigators. Support and funding for this work is from the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Author Contributions
Elizabeth R. Fischer : Conceptualization; methodology; project administration; resources; supervision; validation; writing original draft. Bryan T. Hansen : Methodology; writing review and editing. Vinod Nair : Investigation; methodology; writing review and editing. Forrest H. Hoyt: Methodology; writing review and editing. Cindi L. Schwartz : Investigation; methodology; writing review and editing. David W. Dorward : Investigation; methodology; writing review and editing.
Conflict of Interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Literature Cited
- Boyd, A., & Franc, F. (1981). Freeze-drying shrinkage of glutaraldehyde fixed liver. Journal of Microscopy , 122, 75–86. https://doi.org/10.1111/j.1365-2818.1981.tb01244.x
- Boyd, A., & Maconnachie, E. (1979). Freon 113 freeze-drying for SEM. Scanning , 2, 164–166. https://doi.org/10.1002/sca.4950020306
- Braet, F., deZanger, R., & Wisse, E. (1997). Drying cells for SEM, AFM and TEM by hexamethyldisilazane: A study on hepatic endothelial cells. Journal of Microscopy , 186, 84–87. https://doi.org/10.1046/j.1365-2818.1997.1940755.x
- Brown, W. J., & Farquhar, M. G. (1989). Immunoper-oxidase methods for the localization of antigens in cultured cells and tissues by electron microscopy. Methods in Cell Biology , 31, 553–569. https://doi.org/10.1016/S0091-679X(08)61626-X
- Burghardt, R. C., & Droleskey, R. (2006). Transmission electron microscopy. Current Protocols in Microbiology , Chapter 2. https://doi.org/10.1002/9780471729259.mc02b01s03
- Burnett, L. C., Lunn, G., & Coico, R. (2009). Biosafety: guidelines for working with pathogenic and infectious microorganisms. Current Protocols in Microbiology , 13, 1A.1.1–1A.1.14. https://doi.org/10.1002/9780471729259.mc01a01s13
- Corwin, D. (1979). An improved method for cleaning and preparing ticks for examination with the electron microscope. Journal of Medical Entomology , 16, 352–353. https://doi.org/10.1093/jmedent/16.4.352
- Current Protocols. (2006). Commonly Used Reagents. Current Protocols in Microbiology , 00, A.2A.1–A.2A.15. https://doi.org/10.1002/9780471729259.mca02as00
- DeLeo, F. R., & Otto, M. (2008). Bacterial pathogenesis: Methods and protocols. In D. W. Dorward (Ed.), Methods in Molecular Biology (Vol. 431, pp. 173–187). Humana Press.
- Dixon, B. R., Petney, T. N., & Andrews, R. H. (2000). A simplified method of cleaning Ixodid ticks for microscopy. Journal of Microscopy , 197, 317–319. https://doi.org/10.1046/j.1365-2818.2000.00663.x
- Fassel, T. A., Mozdiak, P. E., Sanger, J. R., & Ed-minston, C. E. (1997). Paraformaldehyde effect on ruthenium red and lysine preservation and staining of the staphylococcal glycocalyx. Microscopy Research and Technique , 36, 422–427. https://doi.org/10.1002/(SICI)1097-0029(19970301)36:5<422::AID-JEMT12>3.0.CO;2-U
- Giberson, R. T., & Demaree, R. S. Jr. (2002). Microwave techniques and protocols. Humana Press.
- Goldstein, J. I., Newbury, D. E., Echlin, P., Joy, D. C., Fiori, C., & Lifshin, E. (2002). Scanning electron microscopy and x-ray microanalysis. Plenum Press.
- Hayat, M. A. (1993). Stains and chemical methods. Plenum Press.
- Hitchcock, P. J., Brown, T. M., Corwin, D., Hayes, S. F., Olszewski, A., & Todd, W. J. (1985). Morphology of three strains of contagious equine metritis organism. Infection and Immunity , 48(1), 94–108. https://doi.org/10.1128/iai.48.1.94-108.1985
- Karnovsky, M. J. (1965). A formaldehyde-glutaraldehyde fixative of high osmolality for use in electron microscopy. Journal of Cell Biology , 27, 137A.
- Lawton, J. R. (1989). An investigation of the fixation and staining of lipids by a combination of malachite green or other triphenylmethane dyes with glutaraldehyde. Journal of Microscopy , 154, 83–92. https://doi.org/10.1111/j.1365-2818.1989.tb00570.x
- Lee, J. T., & Chow, K. L. (2012). SEM sample preparation for cells on 3D scaffolds by freeze-drying and HMDS. Scanning , 34(1), 12–25. https://doi.org/10.1002/sca.20271
- Luft, J. H. (1971). Ruthenium red and violet. The Anatomical Record , 171, 347–377. https://doi.org/10.1002/ar.1091710302
- Lunn, G., & Lawler, G. (2011). Safe use of hazardous chemicals. Current Protocols in Microbiology , 23, 1A.3.1–1A.3.36. https://doi.org/10.1002/9780471729259.mc01a03s23
- Meisenhelder, J., & Semba, K. (2006). Safe use of radioisotopes. Current Protocols in Microbiology , Chapter 1 , 1A.4. https://doi.org/10.1002/9780471729259.mc01a04s01
- Nation, J. L. (1983). A new method using hexamethyldisilazane for preparation of soft insect tissues for scanning electron-microscopy. Stain Technology , 58, 347–351. https://doi.org/10.3109/10520298309066811
- Palade, G. E. (1951). A study of fixation for electron microscopy. Journal of Experimental Medicine , 95, 285–297. https://doi.org/10.1084/jem.95.3.285
- Sabatini, D. D., Bensch, K., & Barrnett, R. J. (1963). Cytochemistry and electron microscopy: The preservation of cellular ultrastructure and enzymatic activity by aldehyde fixation. Journal of Cell Biology , 17, 19–58. https://doi.org/10.1083/jcb.17.1.19
- Specia, R. D., & Neutra, M. R. (1981). The surface topography of the colonic crypt in rabbit and monkey. The American Journal of Anatomy , 160, 461–472. https://doi.org/10.1002/aja.1001600409
Key References
- Glauert, A. M. (1974). Fixation, dehydration, and embedding of biological specimens. Elsevier.
- Hayat, M. A. (1989). Colloidal gold: Principles, methods, and applications (Vols. 1–3). Academic Press.
- Hayat, M. A. (2000). Principles and techniques of electron microscopy: Biological applications. Cambridge University Press.
The following books are excellent resources for general EM preparative techniques covering general principle for conventional and immunological preparation.
Internet Resources
- http://www.tedpella.com/
- http://www.emsdiasum.com/microscopy/
- http://www.2spi.com/
- http://www.ebsciences.com/
The above four websites are some common sources for electron microscopy supplies, reagents and useful tips for immune-labeling and microwave oven processing.
Advanced Microscopy Facility, University of Victoria. A University site containing particularly useful microwave oven theory and protocols.
The above three websites are additional resources for commercially available antibodies and small gold probes.
Adobe Photoshop.

