Purification of recombinant Low Density Lipoprotein Receptor Related Protein Associated Protein 1 (LRPAP1, RAP) from Escherichia coli
Patricia Yuste-Checa, F Ulrich Hartl, Silvia Gärtner
Abstract
This protocol details how to efficiently purify LDL Receptor Related Protein Associated Protein 1 (LRPAP1 or RAP) from Escherichia coli.
Attachments
Steps
LRPAP1 express4ion
Thaw RbCl-competent Escherichia coli Bl21 cells (DE3) On ice.
Add 1µL of pQTEV-LRPAP1 plasmid without the signal peptide (1-35 amino acids) and incubate 0h 30m 0s On ice.
Heat shock 0h 0m 45s at 42°C.
Incubate On ice 0h 2m 0s, then add 850µL Lysogeny broth (LB) or Super Optimal broth with Catabolite repression (SOC) medium.
Shake for 1h 0m 0s at 37°C.
Centrifuge for 3000x g and remove most of the supernatant.
Resuspend the pellet with the remaining supernatant and plate the bacteria on LB /Ampicillin agar plates and incubate 1h 0m 0s at 37°C.
Prepare preculture: Scrap all colonies with the scraper and inoculate 25-50 mL LB/Ampicillin. Shake at 37°C for 4-6 h.
Measure OD600 of the preculture and inoculate 6L of LB media to an OD600 = 0.05.
Shake flasks at 37°C until approx. OD600 = 0.5-0.8. (2-4 h).
Add isopropyl β-D-1-thiogalactopyranoside (IPTG) at final concentration of 1millimolar (mM).
Shake flasks 1h 0m 0s at 22°C.
Centrifuge bacterial culture at 4000rpm. Discard supernatant.
Resuspend each pellet with Lysis buffer (20 mL/1L bacteria) supplemented with Complete EDTA-free protease inhibitor cocktail (Merck). Flash-freeze in liquid nitrogen for storage at -80°C.
Lysis
Thaw the cell pellets in a water bath at 22°C and add lysis buffer (final volume 200 mL lysis buffer/ 6L bacteria) supplemented with Complete EDTA-free protease inhibitor cocktail (Merck) and Sm DNase 50.
Add 1 lysozyme and incubate gently shaking for 0h 30m 0s at 4°C.
Sonicate lysate On ice, 8 cycles 0h 0m 20s ON, 0h 0m 30s OFF.
Centrifuge lysate at 40000rpm,4°C.
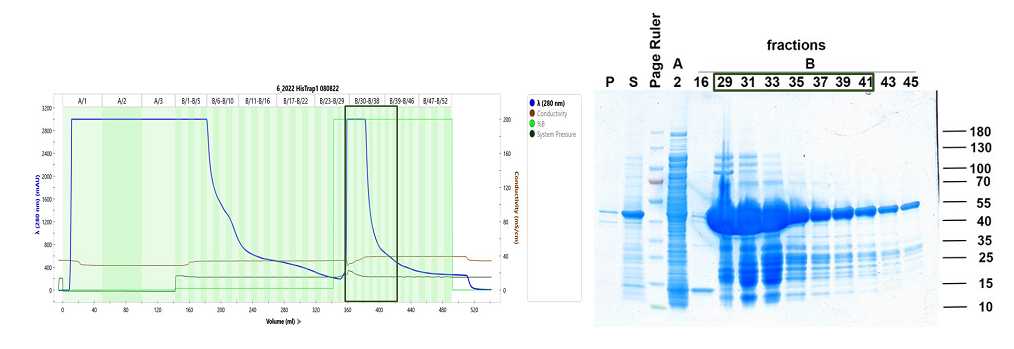
Ni-NTA chromatography
Equilibrate the Ni-NTA column with 10 column volumes (CV, 20 mL) Lysis buffer.
Load lysate supernatant to the Ni-NTA column.
Wash the Ni-NTA column with 10 CV Lysis buffer.
Elute His7-TEV-RAP with 5 CV 100% High salt buffer and collect elution fractions.
Analyze eluted fraction by SDS-PAGE and Coomassie blue staining.
Desalting
In order to reduce the salt concentration, load the eluted protein onto a HiPrep 26/10 desalting column equilibrated with the Low salt buffer.
His-TEV cleavage
Collect eluted fraction containing protein and add glycerol at final concentration of 10%, DTT at final concentration of 1millimolar (mM), EDTA at final concentration of 0.25millimolar (mM) and His-TEV at final concentration of 93U per mg of protein.
Incubate at 4°C 0h 0m 30s.
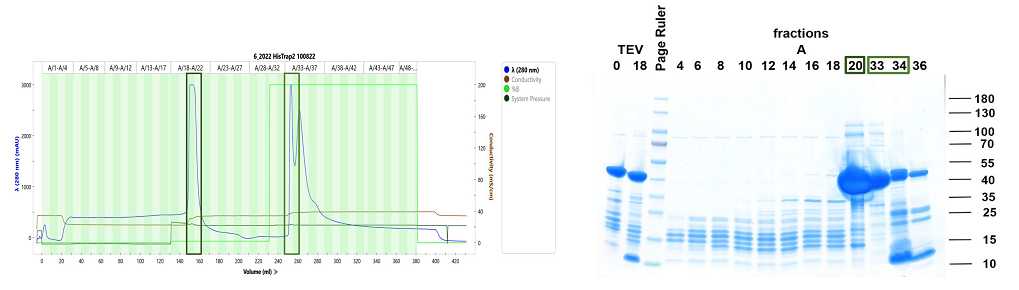
Ni-NTA chromatography (Collect flow through)
Load the cleavage mixture onto a Ni-NTA column previously equilibrated with Lysis buffer and collect the flow through where the cleaved RAP protein should elute.
Wash the column with 5 CV (CV, 20 mL) of Lysis buffer and collect eluted fractions.
Analyze flow though fractions by SDS-PAGE and Coomassie blue staining.
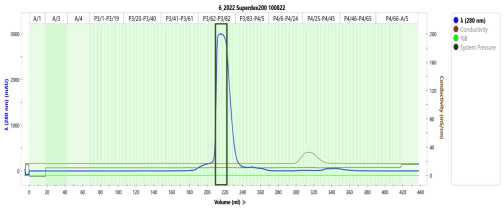
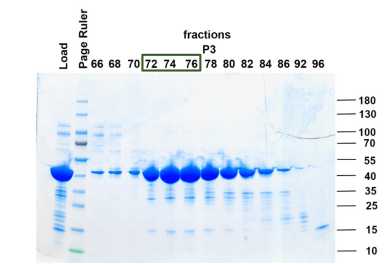
Size exclusion chromatography
Load RAP-containing fractions onto a Superdex-200 column previously equilibrated with SEC buffer.
Pool fractions containing RAP aliquot and flash-freeze in liquid nitrogen for storage at -80°C.