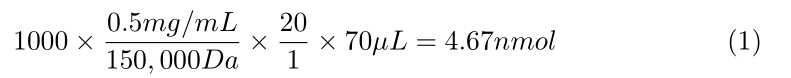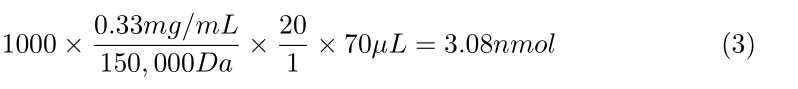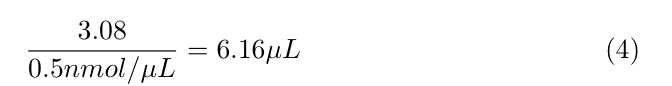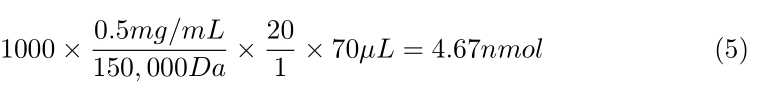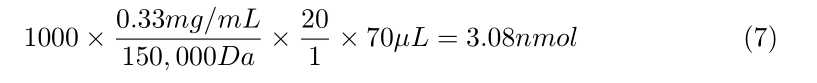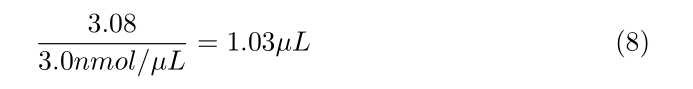MSD Gold Streptavidin Antibody Preparation and Plate Run Protocol
Maria Sckaff, Claire D Clelland, Kamaljot Gill
Buffer Exchange
Biotinylate the Antibody
Sulfo-tag the Antibody
MSD
ELISA
protein quantification
streptavidin
antibody
protein
Abstract
This protocol describes how to conjugate antibodies and run the Meso Scale Discovery (MSD) Sandwich enzyme-linked immunosorbent assay (ELISA) on MSD GOLD 96-well Small Spot Streptavidin SECTOR Plates. This protocol is adapted from MSD GOLD™ Streptavidin Plate and Avidin Plates Quick Guide 1 and MSD GOLD™ SULFO-TAG NHS-Ester Conjugation Quick Guide 2 and optimized for C9orf72 dipeptide repeat detection from human iPSC derived neurons.
Attachments
Steps
Buffer Exchange the Antibodies
Chill PBS (or MSD Conjugation Buffer) and ultrapure water On ice.
Equilibrate Zeba Spin Desalting Columns, MSD Storage Buffer, and Sulfo-NHS-LC-Biotin at Room temperature.
Use one Zeba column per 70µL of antibody.
In order to both biotinylate and sulfo-tag the antibody, you need at least 140µL of antibody at an optimal concentration of 1.0mg/mL.
It still possible to move forward with a less concentrated sample.
Dilute the antibodies with ice-cold PBS if necessary.
Remove the Zeba columns' bottom closure and loosen the cap.
Place the column in a collection tube to remove the storage buffer.
Spin at 1500x g. Empty collection tube.
Wash 1 : Add 300µL of PBS (or MSD Conjugation Buffer) to the column. Spin at 1500x g. Empty collection tube.
Wash 2 : Add 300µL of PBS (or MSD Conjugation Buffer) to the column. Spin at 1500x g. Empty collection tube.
Wash 3 : Add 300µL of PBS (or MSD Conjugation Buffer) to the column. Spin at 1500x g. Empty collection tube.
Change collection tube to a clean Eppendorf tube for sample recovery. Label one Eppendorf tube/sample for each sample to be biotinylated or sulfo-tagged.
Pipette 70µL of the antibody to the spin column.
Spin at 1500x g,0h 0m 0s for 3-4 minutes.
Save the eluent On ice.
Biotinylate the Antibody
Calculate how much Sulfo-NHS-LC-Biotin you need per antibody, using the following formula:
- 1,000 × ([Concentration of antibody in mg/mL]/ 150,000 Da) × 20 ×
70µLof antibody = nmol of Biotin needed - This nmol of Biotin needed divided by 0.5 nmol/μL Biotin reagent = μL of Sulfo-NHS-LC Biotin needed
- See attached examples at the end of this document
Add 180µL of ultrapure H2O to the 1mg vial to Sulfo-NHS-LC-Biotin.
Dilute the Sulfo-NHS-LC-Biotin by adding 10µL of the stock to 190µL of cold water. Once formed, this is highly unstable and should be used immediately.
Add the calculated volume of diluted reconstituted Sulfo-NHS-LC-Biotin to each antibody.
Let the antibody and biotin incubate at Room temperature for 2h 0m 0s in the dark.
Sulfo-tag the Antibody
Calculate how much SULFO-TAG NHS-Ester you need per antibody, using the following formula:
- 1,000 × ([Concentration of antibody in mg/mL]/ 150,000 Da) × 20 ×
70µLof antibody = nmol of Sulfo-Tag reagent needed - This nmol of Sulfo-Tag needed divided by 3.0 nmol/µL = µL of sulfo-tag ester solution needed
- See attached examples at the end of this document
Add 50µL of ice-cold ultrapure H2O to the 150nmol SULFO-TAG NHS-Ester Vial to make a 3nmol/μL solution. Once formed, this is highly unstable and should be disposed.
Vortex the solution lightly.
Add the calculated volume of diluted reconstituted SULFO-TAG NHS-Ester reagent to each antibody.
Let the antibody and SULFO-TAG NHS-Ester incubate at Room temperature for 2h 0m 0s in the dark.
Clean-up the Biotinylated Antibody
Equilibrate Zeba Spin Desalting Columns, MSD Storage Buffer at Room temperature.
Place the column in a collection tube to remove the storage buffer.
Remove the columns' bottom closure and loosen the cap
Spin at 1500x g. Empty collection tube.
Wash 1 : Add 300µL of MSD Conjugate Storage Buffer to the column. Spin at 1500x g. Empty collection tube.
Wash 2 : Add 300µL of MSD Conjugate Storage Buffer to the column. Spin at 1500x g. Empty collection tube.
Wash 3 : Add 300µL of MSD Conjugate Storage Buffer to the column. Spin at 1500x g. Empty collection tube.
Change collection tube for sample recovery.
Pipette 70µL of the unpurified biotinylated antibody to the spin column.
Spin at 1500x g,0h 0m 0s for 3-4 minutes.
Save the eluent 4On ice.
Cleanup the Sulfo-Tagged Antibody
Equilibrate Zeba Spin Desalting Columns, MSD Storage Buffer at 4Room temperature.
Remove the columns' bottom closure and loosen the cap.
Place the column in a collection tube to remove the storage buffer.
Spin at 1500x g. Empty collection tube.
Wash 1 : Add 300µL of MSD Conjugate Storage Buffer to the column. Spin at 1500x g. Empty collection tube.
Wash 2 : Add 300µL of MSD Conjugate Storage Buffer to the column. Spin at 1500x g. Empty collection tube.
Wash 3 : Add 300µL of MSD Conjugate Storage Buffer to the column. Spin at 1500x g. Empty collection tube.
Change collection tube for sample recovery.
Pipette 70µLof the unpurified SULFO-TAG NHS-Ester tagged antibody to the spin column.
Spin at 1500x g,0h 0m 0s for 3-4 minutes.
Save the eluent 4On ice.
Day 1 of MSD: Coating the Plate with Capture Antibody
Dilute biotinylated capture antibodies in 1x DPBS to your desired concentration.
Add 30µL of the diluted antibody to the corner of each well of the MSD 96 Streptavidin Small. Spot Plate according to the plate format.
Tap the plate on its edges to ensure the antibody is spread evenly across the well.
Seal the plate with parafilm to avoid loss of antibody due to evaporation.
Store in the plate at 4°C to incubate 2h 0m 0s without shaking.
Day 2 of MSD
Tap out the plate to dispose of the capture antibody.
Add 150µL/well of Blocking Solution (3% Blocker A (or BSA) + PBS) per well.
| A | B |
|---|---|
| 3% Blocker A in 1x PBS (Store at 4°C) | |
| For 100 mL | |
| Blocker A | 3 g |
| 1X PBS | 100 mL |
| (Stir or shake overnight at 4°C to make sure it is dissolved completely.) |
Seal the plate and incubate at 4Room temperature with shaking at 750rpm.
Prepare the lysate samples according to plate layout, by diluting the protein samples in the lysate buffer to the desired lysate concentrations.
Tap out the plate.
Wash 1x with 150µL/well of PBS - T (0.05% Tween).
Discard the wash solution, without letting the plate dry.
Add 25µL/well of the lysate to the target wells.
Seal the plate and incubate at 4Room temperature with shaking at 750rpm for 1-2 hours.
While the lysates incubate, prepare the detection antibodies (your sulfo-tagged antibodies) in 1% MSD Blocker A in 1x DPBS.
Discard the lysate solution.
Wash with PBS - T (0.05% Tween).
Wash with 150µL/well of PBS - T (0.05% Tween). (1/3)
Wash with 150µL/well of PBS - T (0.05% Tween). (2/3)
Wash with 150µL/well of PBS - T (0.05% Tween). (3/3)
Discard the wash solution, but do not let the plate dry.
Add 25µL/well of sulfo-tag antibodies (in 1% Blocker A in DPBS) to the plate layout.
Seal the plate and incubate at 4Room temperature with shaking at 750rpm.
Tap out the plate.
Wash with PBS - T (0.05% Tween).
Wash with 150µL/well of PBS - T (0.05% Tween). (1/3)
Wash with 150µL/well of PBS - T (0.05% Tween). (2/3)
Wash with 150µL/well of PBS - T (0.05% Tween). (3/3)
Tap out the plate.
Add 150µL/well of Read Buffer A using reverse pipetting to avoid making bubbles.
Read the plate immediately.