Getting Started with the IDG KMC Datasets and Tools
Eryk Kropiwnicki, Eryk Kropiwnicki, Jessica L. Binder, Jessica L. Binder, Jeremy J. Yang, Jeremy J. Yang, Jayme Holmes, Jayme Holmes, Alexander Lachmann, Alexander Lachmann, Daniel J. B. Clarke, Daniel J. B. Clarke, Timothy Sheils, Timothy Sheils, Keith J. Kelleher, Keith J. Kelleher, Vincent T. Metzger, Vincent T. Metzger, Cristian G. Bologa, Tudor I. Oprea, Avi Ma'ayan
bioinformatics
data visualization
disease ontology
drug discovery
drug targets
druggable genome
web applications
Abstract
The Illuminating the Druggable Genome (IDG) consortium is a National Institutes of Health (NIH) Common Fund program designed to enhance our knowledge of under-studied proteins, more specifically, proteins unannotated within the three most commonly drug-targeted protein families: G-protein coupled receptors, ion channels, and protein kinases. Since 2014, the IDG Knowledge Management Center (IDG-KMC) has generated several open-access datasets and resources that jointly serve as a highly translational machine-learning-ready knowledgebase focused on human protein-coding genes and their products. The goal of the IDG-KMC is to develop comprehensive integrated knowledge for the druggable genome to illuminate the uncharacterized or poorly annotated portion of the druggable genome. The tools derived from the IDG-KMC provide either user-friendly visualizations or ways to impute the knowledge about potential targets using machine learning strategies. In the following protocols, we describe how to use each web-based tool to accelerate illumination in under-studied proteins. © 2022 The Authors. Current Protocols published by Wiley Periodicals LLC.
Basic Protocol 1 : Interacting with the Pharos user interface
Basic Protocol 2 : Accessing the data in Harmonizome
Basic Protocol 3 : The ARCHS4 resource
Basic Protocol 4 : Making predictions about gene function with PrismExp
Basic Protocol 5 : Using Geneshot to illuminate knowledge about under-studied targets
Basic Protocol 6 : Exploring under-studied targets with TIN-X
Basic Protocol 7 : Interacting with the DrugCentral user interface
Basic Protocol 8 : Estimating Anti-SARS-CoV-2 activities with DrugCentral REDIAL-2020
Basic Protocol 9 : Drug Set Enrichment Analysis using Drugmonizome
Basic Protocol 10 : The Drugmonizome-ML Appyter
Basic Protocol 11 : The Harmonizome-ML Appyter
Basic Protocol 12 : GWAS target illumination with TIGA
Basic Protocol 13 : Prioritizing kinases for lists of proteins and phosphoproteins with KEA3
Basic Protocol 14 : Converting PubMed searches to drug sets with the DrugShot Appyter
INTRODUCTION
There are approximately 25,000 protein-coding genes (Venter et al., 2001) in the human genome. Abnormal protein expression is associated with many human diseases, which makes proteins critical targets for therapeutic agents. Approximately 15% of protein-coding genes are considered part of the "druggable genome.” This means that these proteins can modulate cellular behavior when targeted by experimental small molecule compounds (Hopkins & Groom, 2002; Johns, Russ, & Fu, 2012; Lipinski, Lombardo, Dominy, & Feeney, 2001; Russ & Lampel, 2005). Moreover, only a few hundred targets represent the existing clinical pharmacopeia, leaving a massive swath of pharmacology that remains unexploited. Therefore, 85% of druggable proteins remain to be explored as potential therapeutic targets. Much of the druggable genome encodes three critical protein families: non-olfactory G-protein-coupled receptors (GPCRs), ion channels, and protein kinases. Critically, we currently lack crucial knowledge about the function of many proteins from these families and their roles in health and disease. A better understanding of these proteins, structurally or functionally, could shed light on new avenues of investigation for basic science and therapeutic discovery (Oprea et al., 2018).
In this article, we provide several protocols to guide users through the use of IDG tools that accomplish specific computational tasks related to illuminating the druggable genome. In Basic Protocol 1, we describe how users can query the Pharos web interface (Sheils et al., 2021) to search for data related to gene targets. Basic Protocol 2 explains how to use Harmonizome (Rouillard et al., 2016), a web application that stores gene-attribute associations from various sources that can be readily visualized and leveraged for machine learning. Basic Protocol 3 describes ARCHS4 (Lachmann et al., 2018), a web application that provides easy access to RNA-sequencing data from human and mouse experiments and also includes gene landing pages for all human genes with gene function predictions based on mRNA co-expression. Basic Protocol 4 describes PrismEXP (Lachmann, Rizzo, Bartal, Jeon, & Clarke, 2021), a machine learning Appyter (Clarke et al., 2021) that improves gene function predictions from gene co-expression correlation data by vertical partitioning the global gene-gene co-expression matrix used by ARCHS4. Basic Protocol 5 teaches the user how to use Geneshot (Lachmann et al., 2019), a web application that facilitates querying of biomedical search terms to retrieve prioritized lists of genes related to the search terms. In Basic Protocol 6, we introduce TIN-X (Cannon et al., 2017), the Target Importance and Novelty eXplorer. We demonstrate how to query and explore interesting disease-target associations based on novelty and importance metrics derived from natural language processing (NLP) of PubMed abstracts. Basic Protocol 7 describes DrugCentral (Avram et al., 2021), a comprehensive database of approved drugs that includes information relating to drug side effects, mode of action, indications, pharmacologic action, and other information. Basic Protocol 8 explains REDIAL-2020 (KC et al., 2021), an ensemble machine learning platform that extends the information available in DrugCentral to predict drugs and small molecules that may have anti-SARS-CoV-2 activity. In Basic Protocol 9 we discuss Drugmonizome (Kropiwnicki et al., 2021), a web application that facilitates drug set enrichment analysis and allows users to submit a drug set of interest to retrieve enriched terms that all, or most, of the members of the input set share. Basic Protocol 10 describes Drugmonizome-ML (Kropiwnicki et al., 2021), an Appyter that extends the information available in Drugmonizome to build on-the-fly machine learning models for predicting novel drug and small molecule attributes. In a similar vein, Basic Protocol 11 discusses Harmonizome-ML, an Appyter that enables users to utilize the datasets from Harmonizome to build machine learning models that predict novel gene-attribute associations. Basic Protocol 12 includes a discussion of TIGA (Yang et al., 2021), Target Illumination GWAS Analytics, a tool that summarizes gene-trait associations derived from genome-wide association studies (GWAS) with rational and intuitive evidence metrics. In Basic Protocol 13, we describe how users can submit an input list of genes or differentially phosphorylated proteins to KEA3 for kinase enrichment analysis (Kuleshov et al., 2021) to infer kinases associated with the input list. Basic Protocol 14 explains how to use DrugShot, an Appyter that allows for the querying of biomedical search terms to retrieve known and predicted lists of drugs and small molecules related to the query term.
Basic Protocol 1: INTERFACING WITH THE PHAROS USER INTERFACE
Pharos is the user interface to the Knowledge Management Center (KMC) for the IDG program, providing facile access to most data types collected by the KMC (Nguyen et al., 2017; Sheils et al., 2020). Given the complexity of the data surrounding any target, efficient and intuitive visualization has been a high priority for users to navigate and summarize search results and rapidly identify patterns. Underlying the interface is a GraphQL API that provides programmatic access to all KMC data, enabling the incorporation of IDG resources with other applications.
Necessary Resources
Hardware
- Desktop or a laptop computer, or a mobile device, with a fast Internet connection
Software
- An up-to-date web browser such as Google Chrome (https://www.google.com/chrome/), Mozilla Firefox (https://www.mozilla.org/en-US/firefox/), Apple Safari (https://www.apple.com/safari/), or Microsoft Edge (https://www.microsoft.com/en-us/edge)
Search targets
1.Navigate to Pharos (https://pharos.nih.gov).
2.To search for a target, click on the search box on the main page or in the top left corner of subsequent pages. Enter STAT3.Note that multiple search types are available in the drop-down menu. (Fig. 1)
3.It is possible to search by pathway or view a list of diseases or ligands associated with a target. Additionally, pressing Enter or Return will allow a text-based search, which will return a list of results featuring ‘STAT3’ anywhere in the text.
4.Press Enter or Return, or click the magnifying glass icon to search for the ‘STAT3’ text string.
5.A list of 81 targets is returned, with ‘STAT3’ being at the top of the list. The rest of the targets will have the phrase ‘STAT3’ somewhere within the target details (Fig. 2).
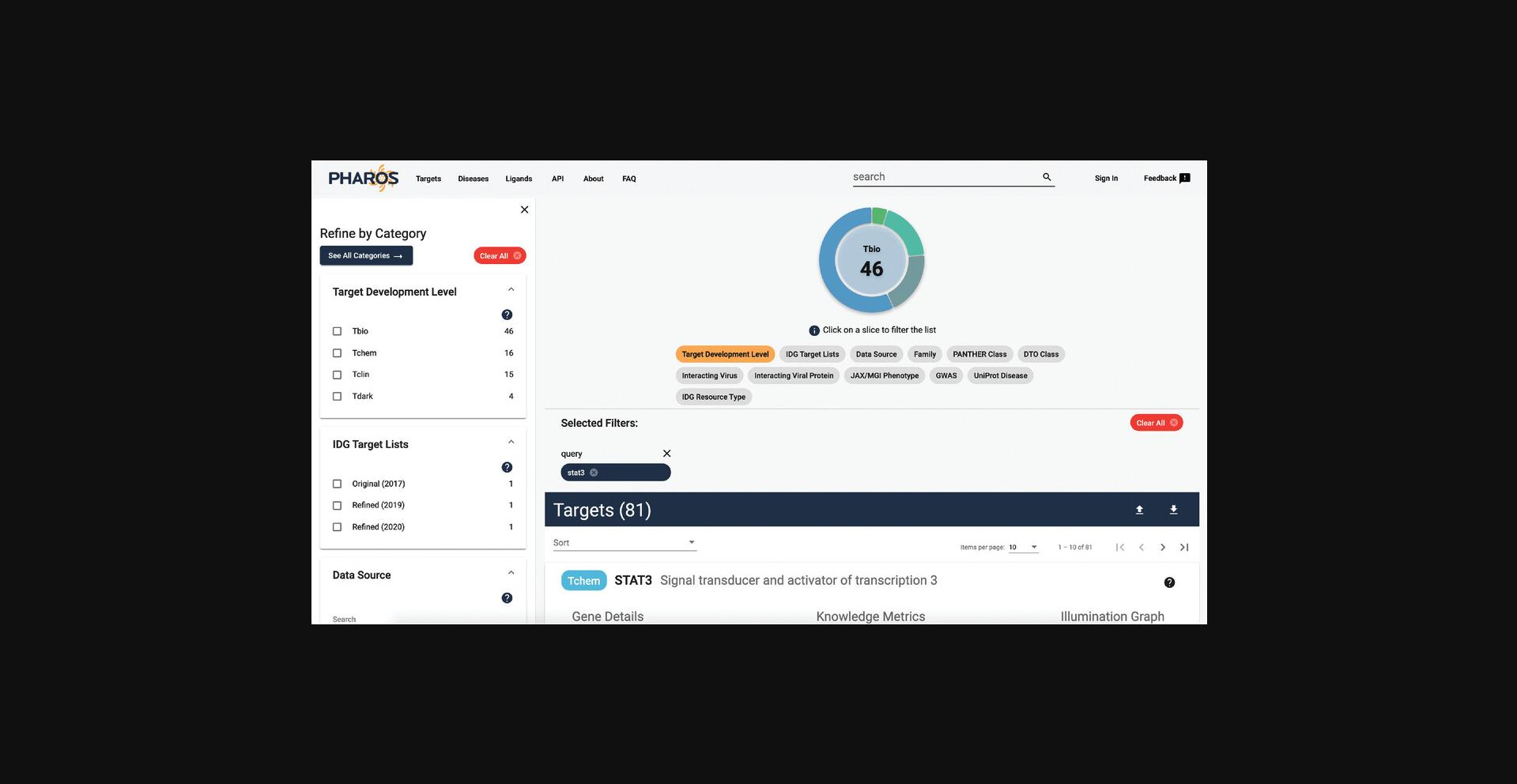
6.Click on the STAT3 card to view the target details.
View target details
7.Follow the steps above, or alternatively, click on the STAT3 (Target) option from the search box auto-complete. This will navigate directly to the STAT3 target details page.
8.The target details page is divided into several sections that highlight an area of knowledge about the target.
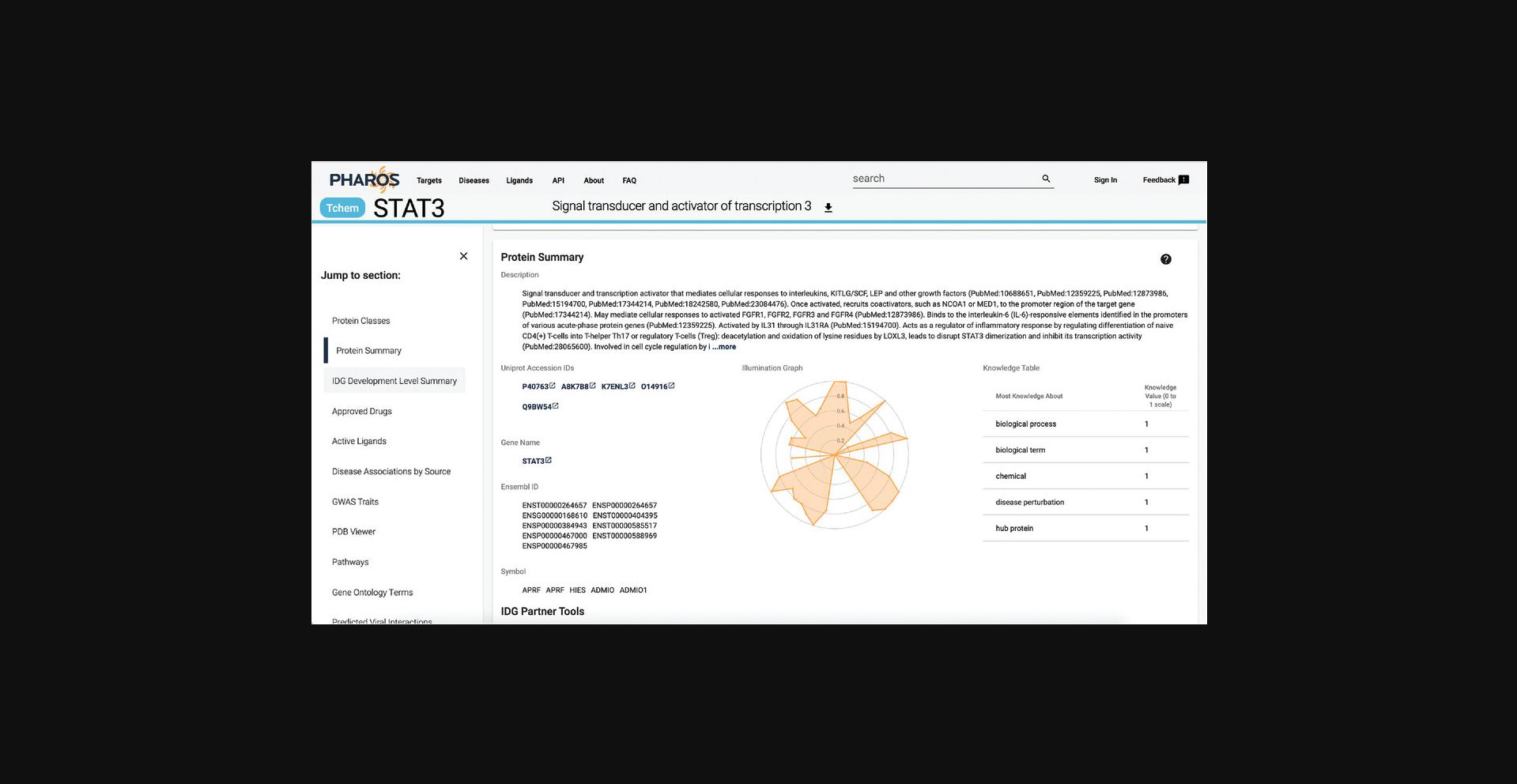
9.Scroll down to the “Protein Summary” section. A brief description of the target, as well as several identifiers, are available. In addition, the central radar plot charts the relative knowledge of a target compared to the rest of TCRD on a 0 to 1 scale. This data is sourced from the Harmonizome, which will be discussed further (Fig. 3).
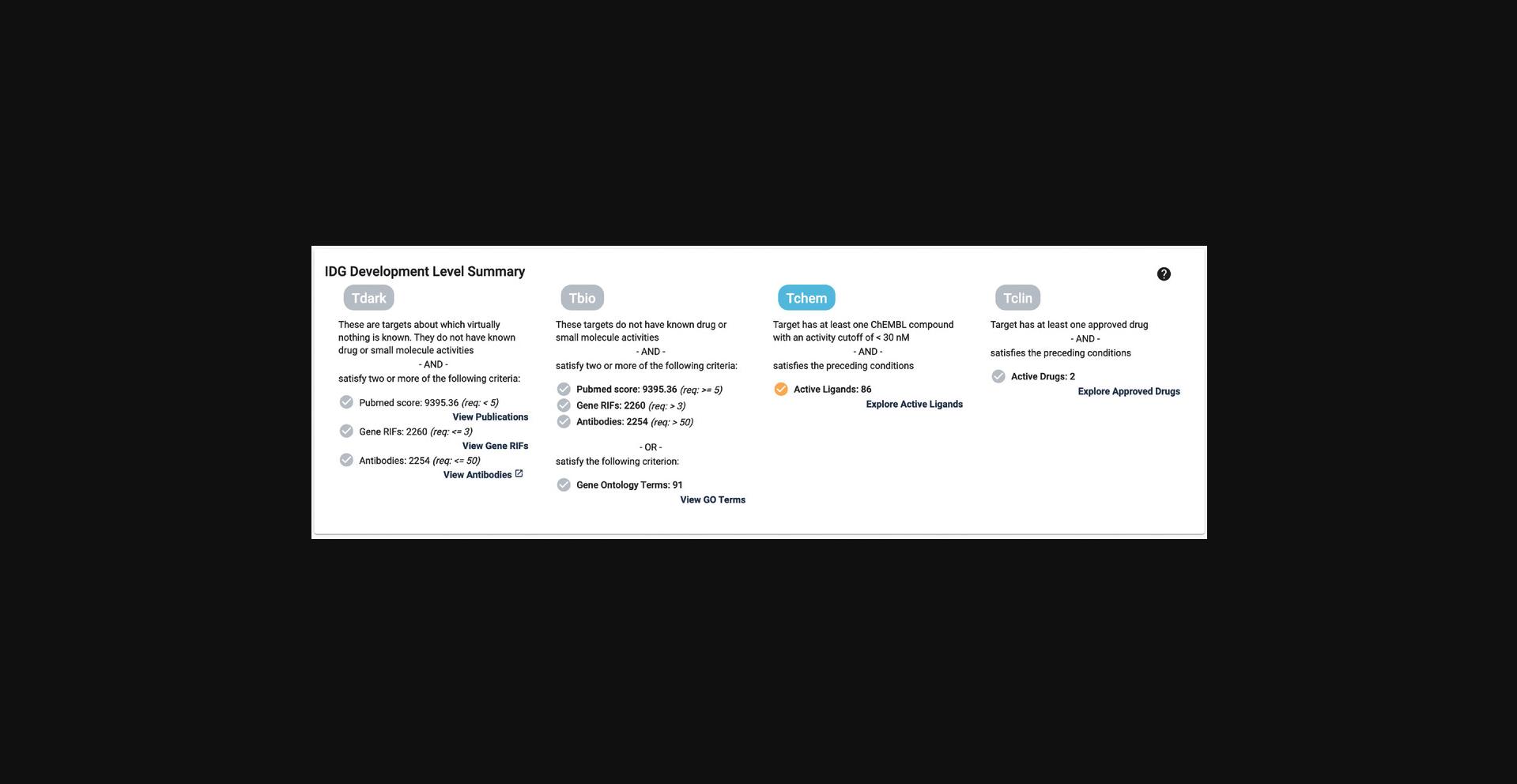
10.Scroll down to the next section, “IDG Development Level Summary.” Displayed here is the current development level . Each level has the criteria listed, as well as links to the data for each property (Fig. 4).
11.On the left side panel, click on “Disease Associations by Source.” This will navigate within the page to a section displaying disease associations from a variety of sources.
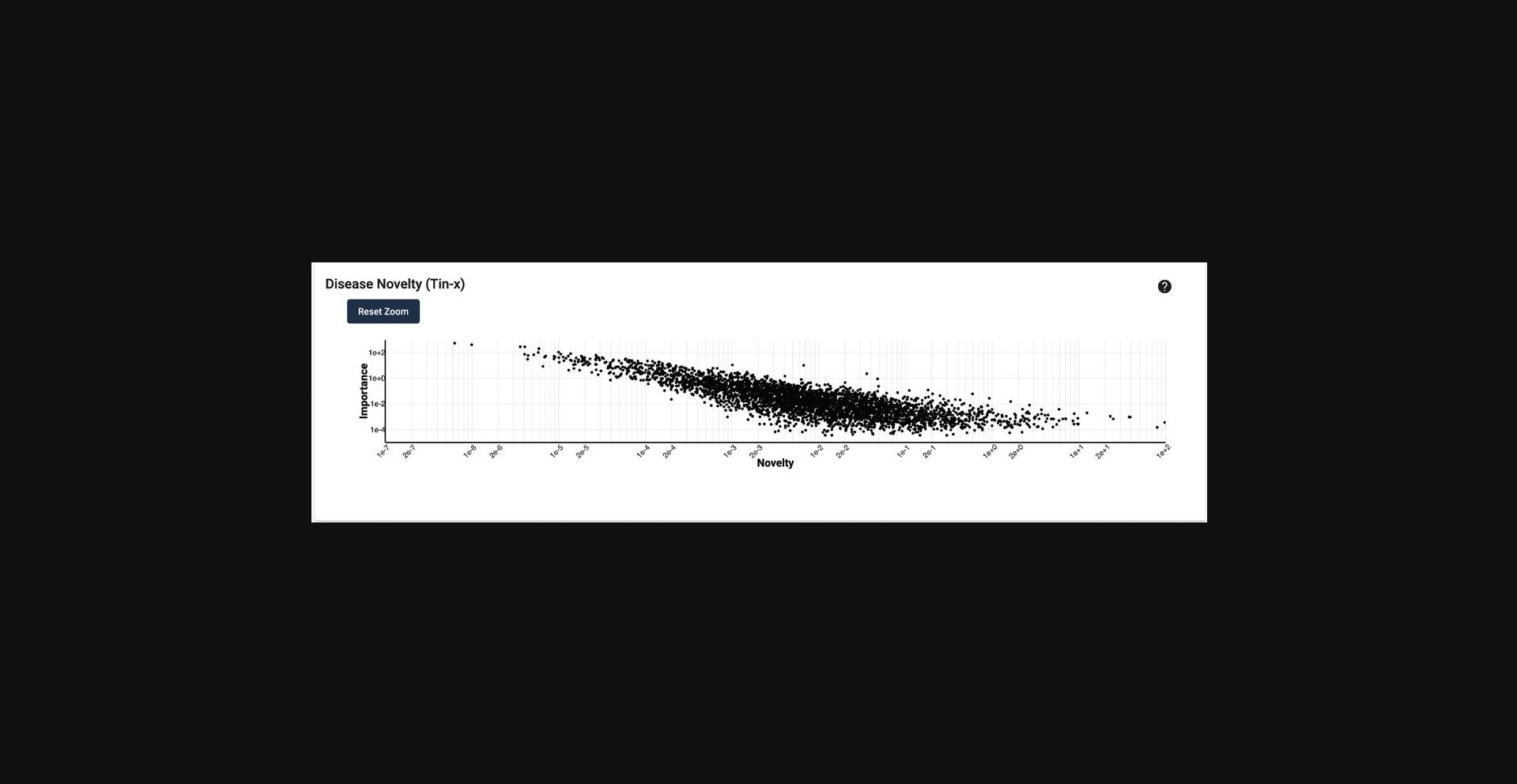
12.Scroll down to the “Disease Novelty (Tin-x)” section, just below Disease Associations. A scatterplot is visible that shows Tin-x data. This data is explained in Basic Protocol 6.Briefly, it is natural language processed PubMed abstracts that chart a target's importance to a disease, as well as the novelty of that target to the disease. A dense chart indicates a large amount of knowledge about a target and its disease associations, whereas a sparser chart would indicate that target is not frequently studied and has fewer disease associations (Fig. 5).
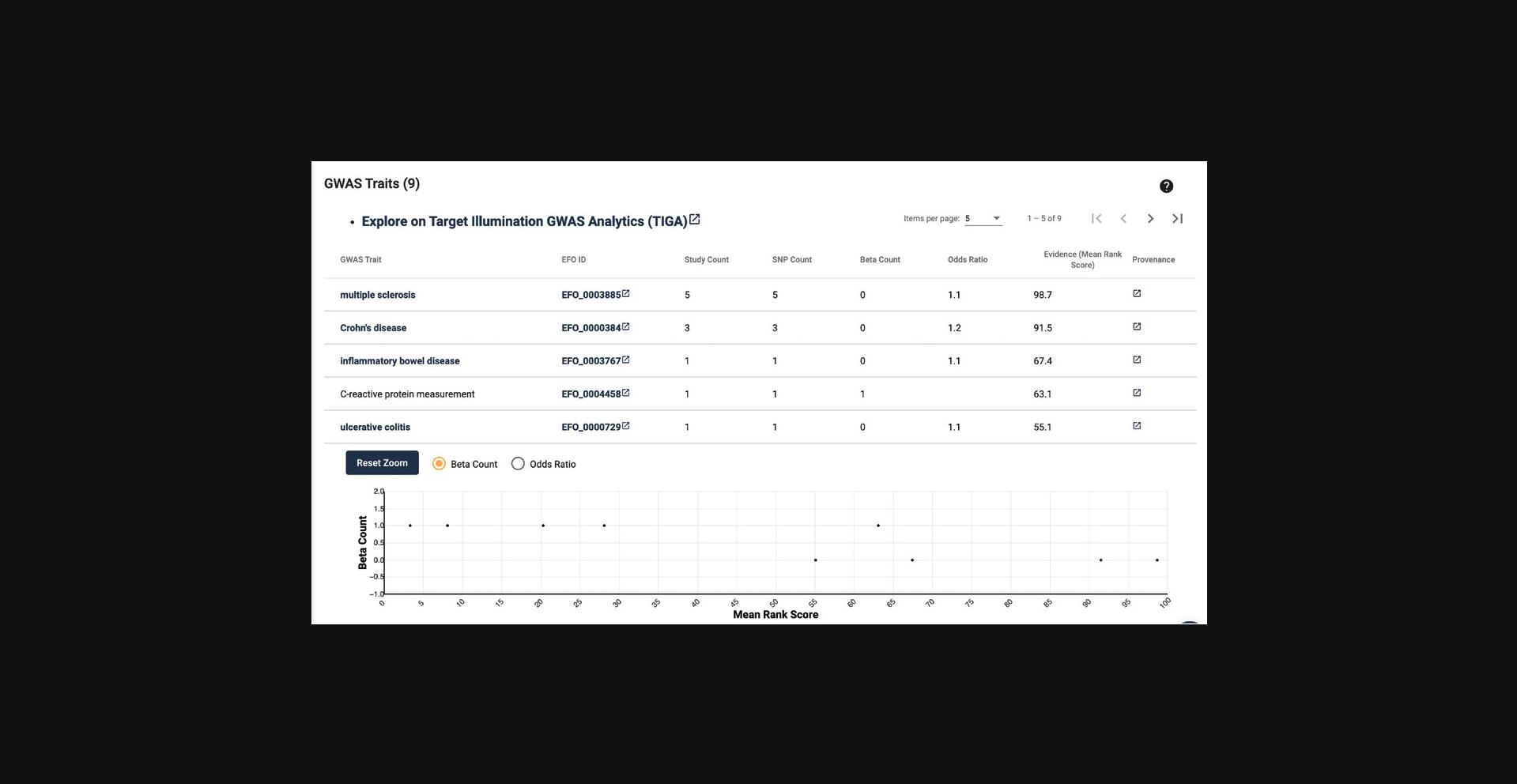
13.Scroll down to the next section “GWAS Traits.” Here a table of GWAS traits is displayed. This list focuses on scoring and ranking protein-coding genes associated with traits from genome-wide association studies. This allows the discovery of traits most associated with a target, but also less emphasized traits (Fig. 6).
Finding a list of under-studied targets that share disease associations with STAT3
14.From the STAT3 target details page, click on “Disease Associations by Source” on the left panel.
15.Click on the “Find Similar Targets” button, directly under the panel header (Fig. 7).
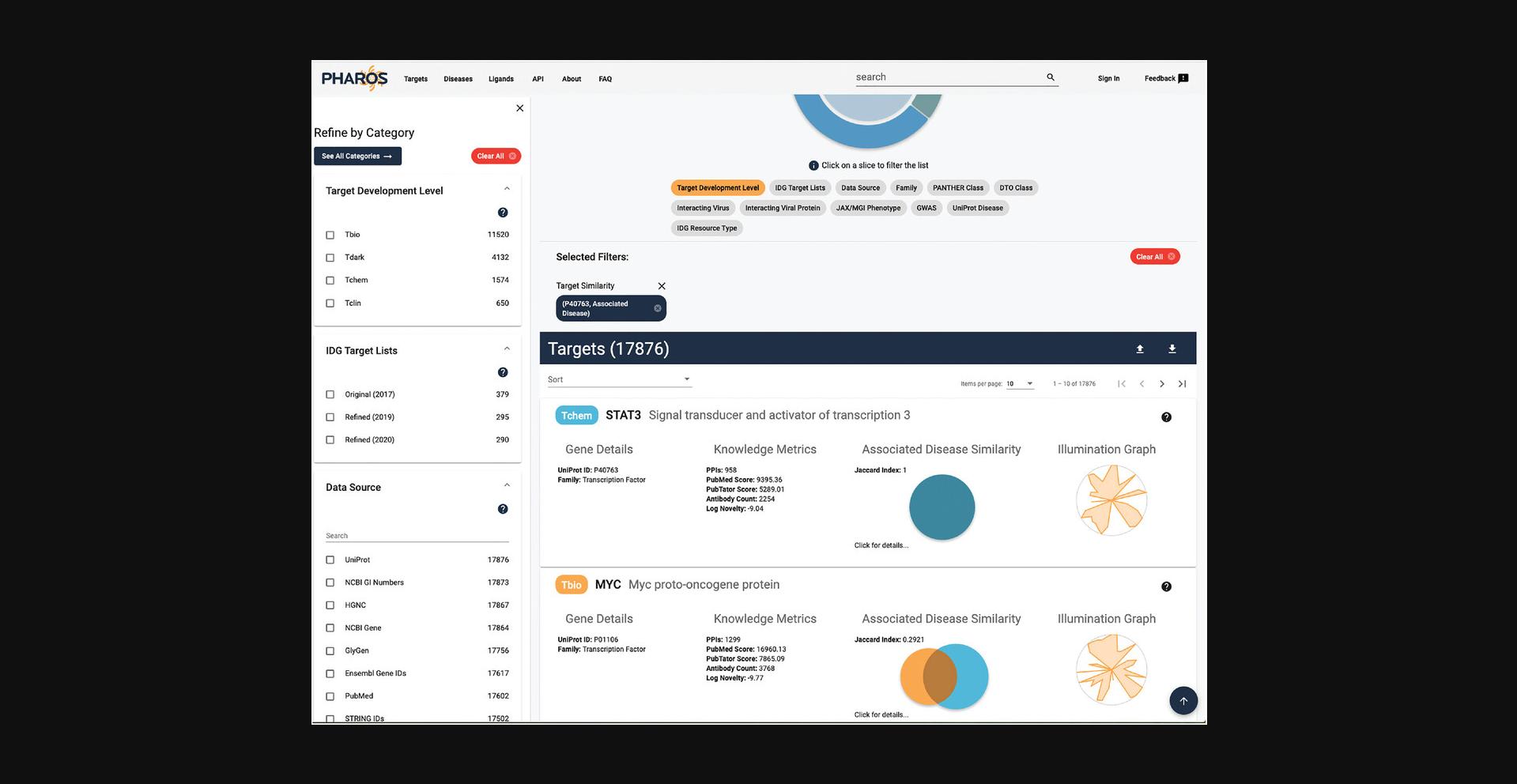
16.The targets list page is now shown, with a target similarity filter applied, showing 17,876 targets (Fig. 8).
17.To refine this list for targets of interest to the IDG program (mentioned in Basic Protocol 1), click on the “Refined (2020)” checkbox in the IDG Target Lists filter panel on the left side of the page. The list of targets shown is reduced to 290.
18.To find only dark targets in this list, click the “Tdark” value in the Target Development Level filter panel, returning 48 targets (Fig. 9).
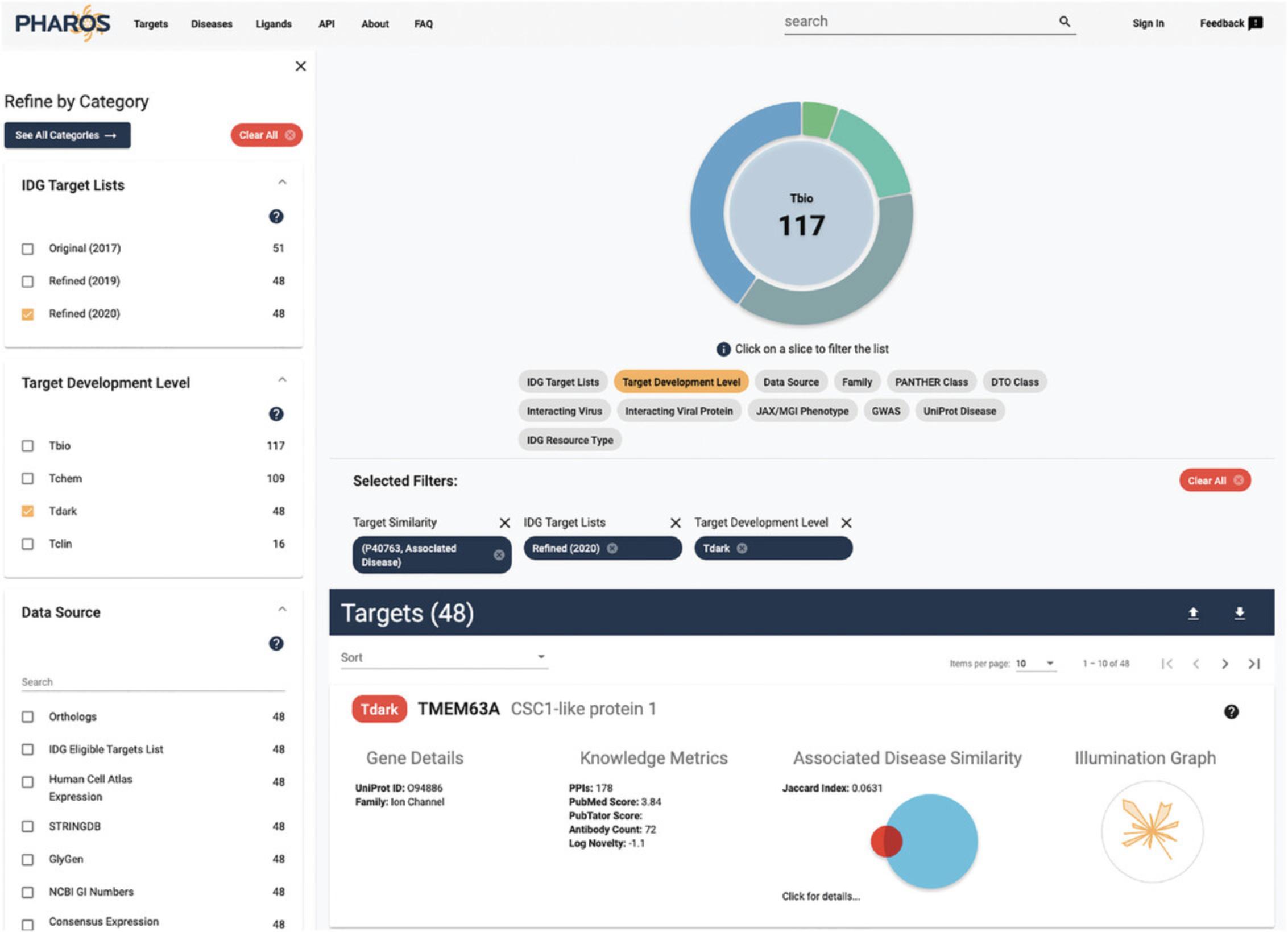
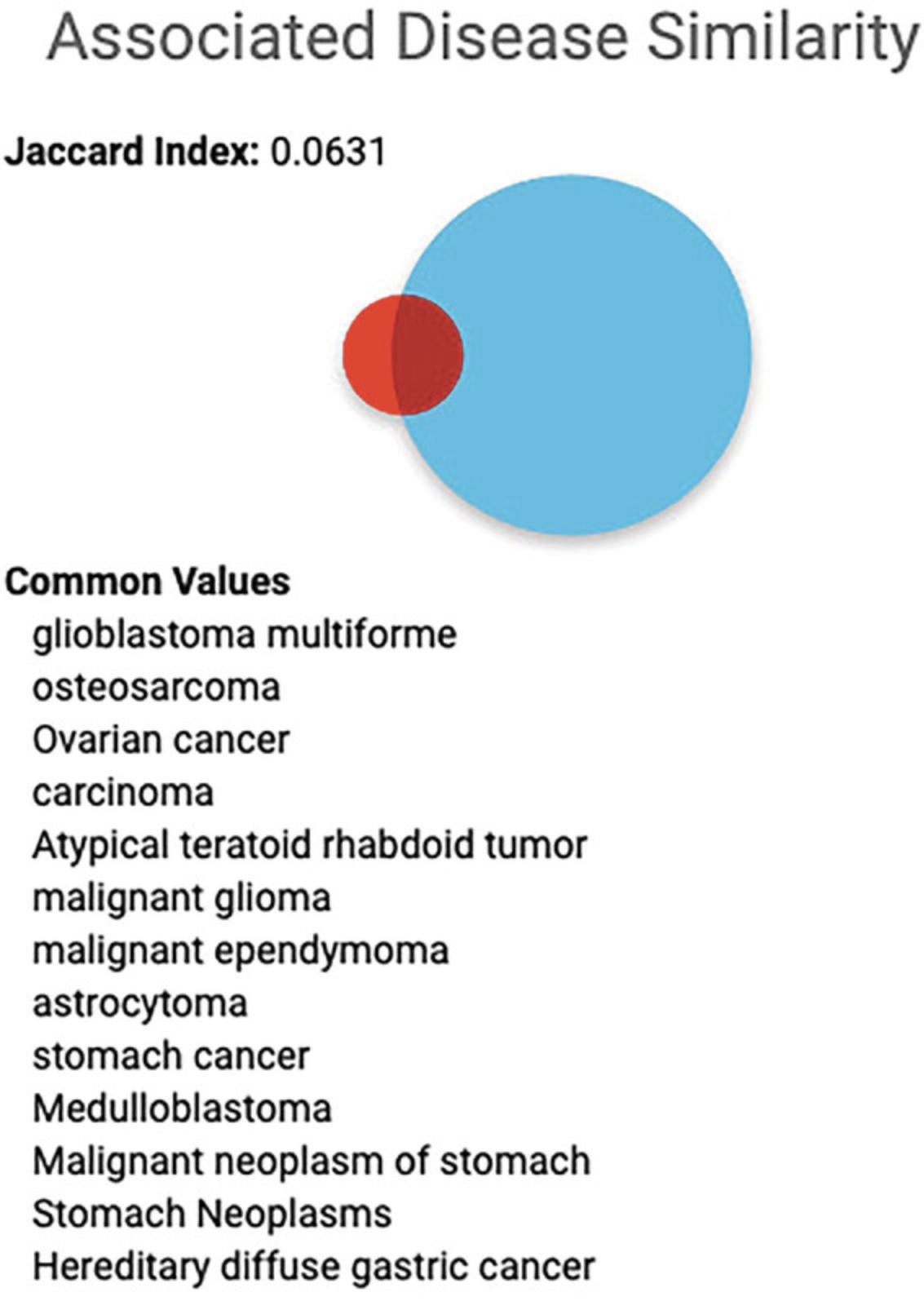
19.Click on the “click for details…” text on the TMEM63A target card to view a list of associated diseases that this target shares with STAT3 (Fig. 10).
Download target list
20.Click on the downward-facing arrow on the right side of the Targets header (Fig. 11).

21.A window will pop open displaying a list of fields that can be selected (Fig. 12).
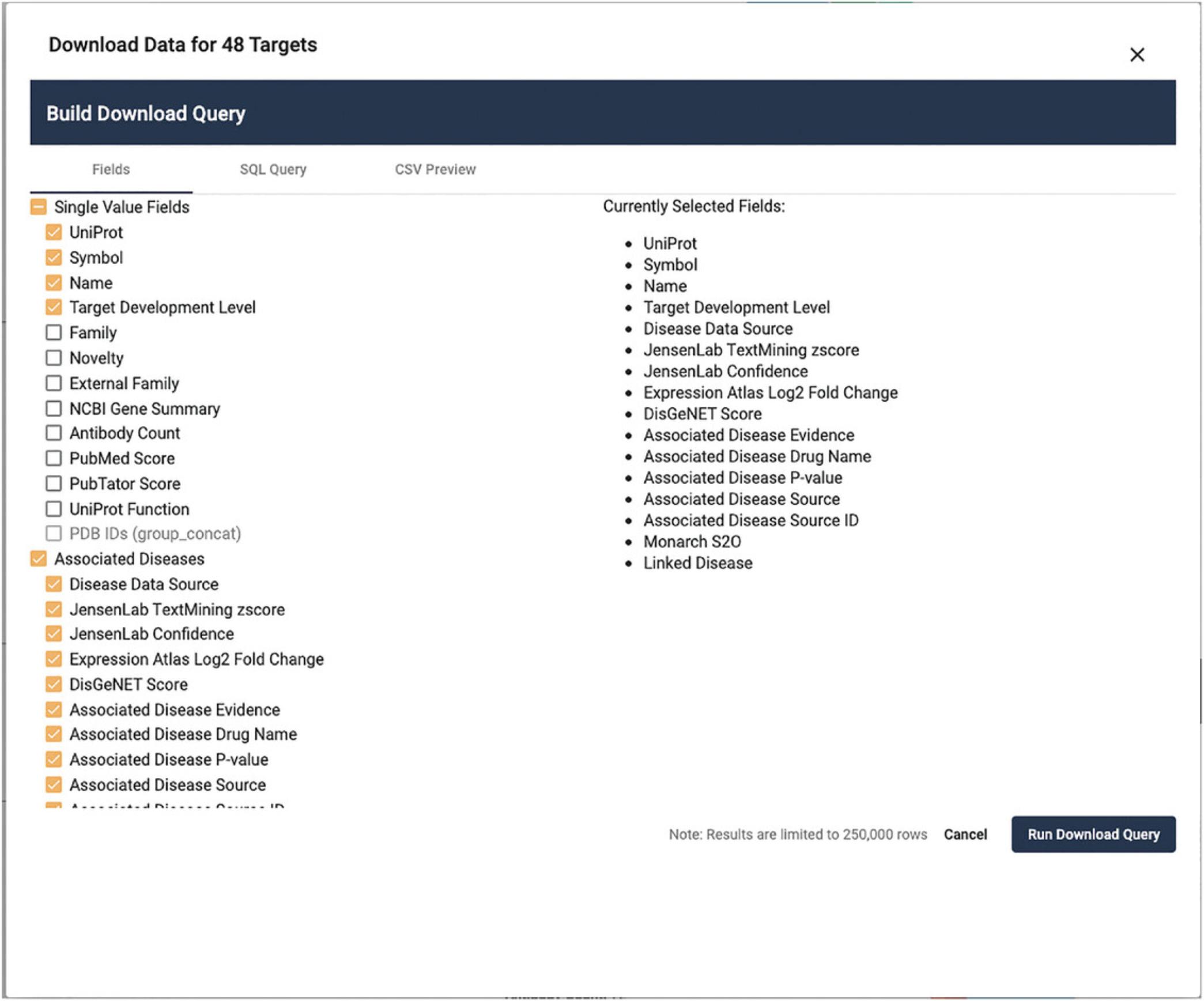
22.Click on the Associated Diseases checkbox. Note that many fields are deactivated, to reduce the overall file size.
23.Click on Name and Target Development Level under the Single Value Fields heading.
24.Click the Run Download Query Button. A file download dialog will open. Depending on the complexity of the target list and the fields selected, this may take some time.
25.After the file is downloaded, this list of targets can be used as a starting point for many of the protocols listed below.
GraphQL queries
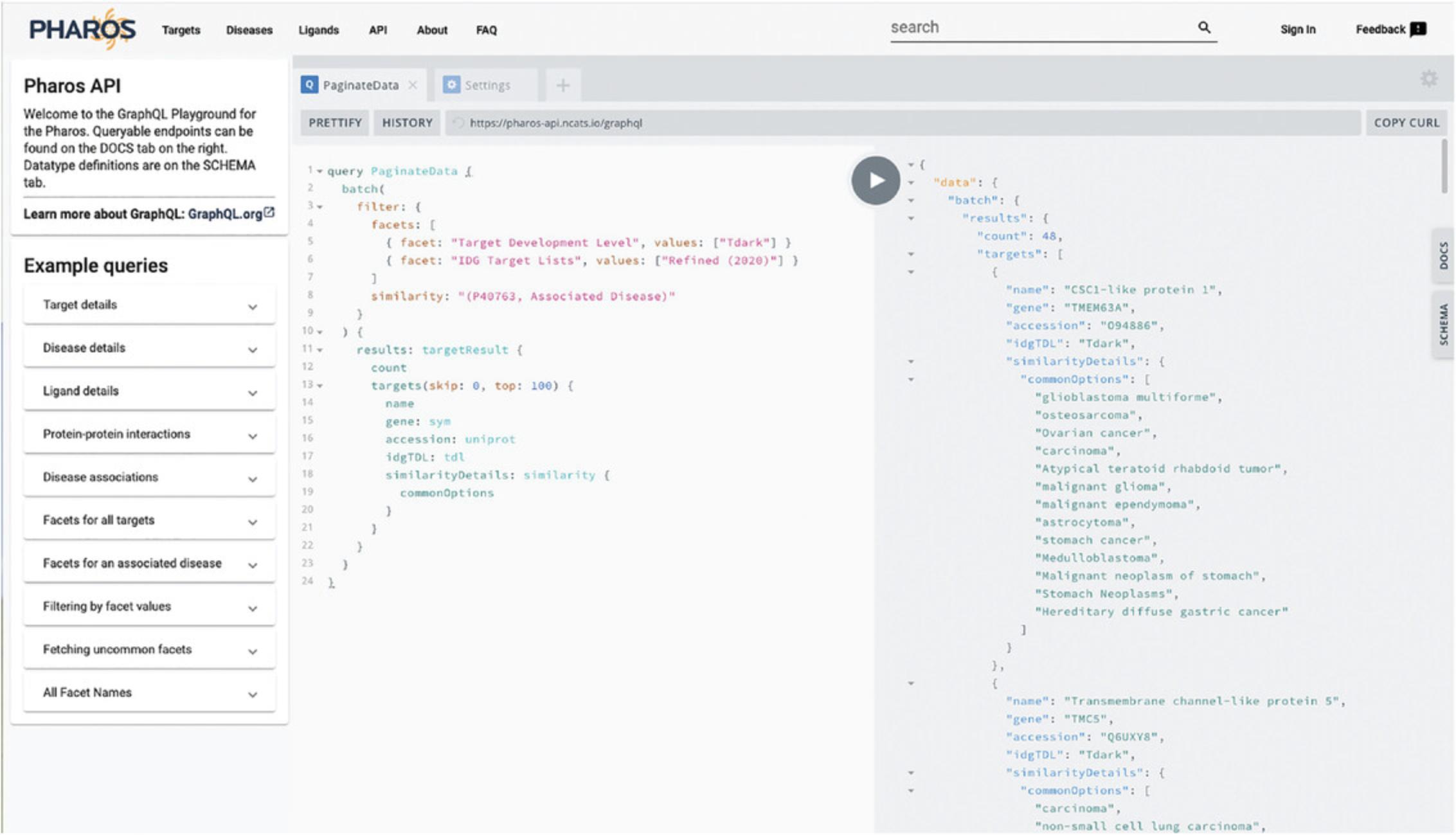
26.Click on API on the main Pharos header.
27.A code “sandbox” is now visible, allowing testing of GraphQL queries to fetch complex data from Pharos. A distinct feature of GraphQL is the ability of the consumer to determine the exact fields returned from the query, as opposed to a SQL query, where the data returned is determined by the database developer.
28.Click the “Edit & Run” button for one of the Sample Queries on the left panel, and then the “Play” button in the top center. This will execute the query on the server and display the JSON results in the right panel.
29.Click on the “Docs” tab on the right side of the page. A menu will open up that displays the queries available, the inputs required, and the responses and properties returned. Click on the “Docs” tab again to close the menu.
30.Replace the text in the left column with this query:
- query PaginateData {
- batch(
- filter: {
- facets: [
- { facet: "Target Development Level", values: ["Tdark"] }
- { facet: "IDG Target Lists", values: ["Refined (2020)"] }
- ]
- similarity: "(P40763, Associated Disease)"
- }
- ) {
- results: targetResult {
- count
- targets(skip: 0, top: 100) {
- name
- gene: sym
- accession: uniprot
- idgTDL: tdl
- similarityDetails: similarity {
- commonOptions
- }
- }
- }
- }
- }
31.Press the play button. This query fetches all dark targets of interest to the IDG that share associated diseases with STAT3. Returned are the target name, gene symbol, Uniprot id, IDG TDL, and shared associated diseases (Fig. 13).
Entire relational database download page
32.Navigate to the TCRD website (http://juniper.health.unm.edu/tcrd/).
33.Click on the “Downloads” tab on the navigation bar at the top of the page to be redirected to a table of downloadable, e.g., MySQL dump of the full TCRD (latest.sql.gz).
Basic Protocol 2: ACCESSING THE DATA IN HARMONIZOME
The Harmonizome resource contains processed datasets detailing functional associations between genes/proteins and their attributes extracted from 66 online resources. The information from the original datasets was distilled into attribute tables that define significant associations between genes and their attributes, where attributes could be other genes, proteins, pathways, cell lines, tissues, experimental perturbations, diseases, phenotypes, drugs, or other entities depending on the dataset. The Harmonizome web application can be accessed from https://maayanlab.cloud/Harmonizome/ (Rouillard et al., 2016).
Necessary Resources
Hardware
- Desktop or a laptop computer, or a mobile device, with a fast Internet connection
Software
- An up-to-date web browser such as Google Chrome (https://www.google.com/chrome/), Mozilla Firefox (https://www.mozilla.org/en-US/firefox/), Apple Safari (https://www.apple.com/safari/), or Microsoft Edge (https://www.microsoft.com/en-us/edge)
- Text editor or development environment of choice, such as Visual Studio (https://visualstudio.microsoft.com/vs/); most updated version of Python
- (https://www.python.org/downloads/); Python module for Harmonizome (https://maayanlab.cloud/Harmonizome/static/harmonizomeapi.py)
Metadata search
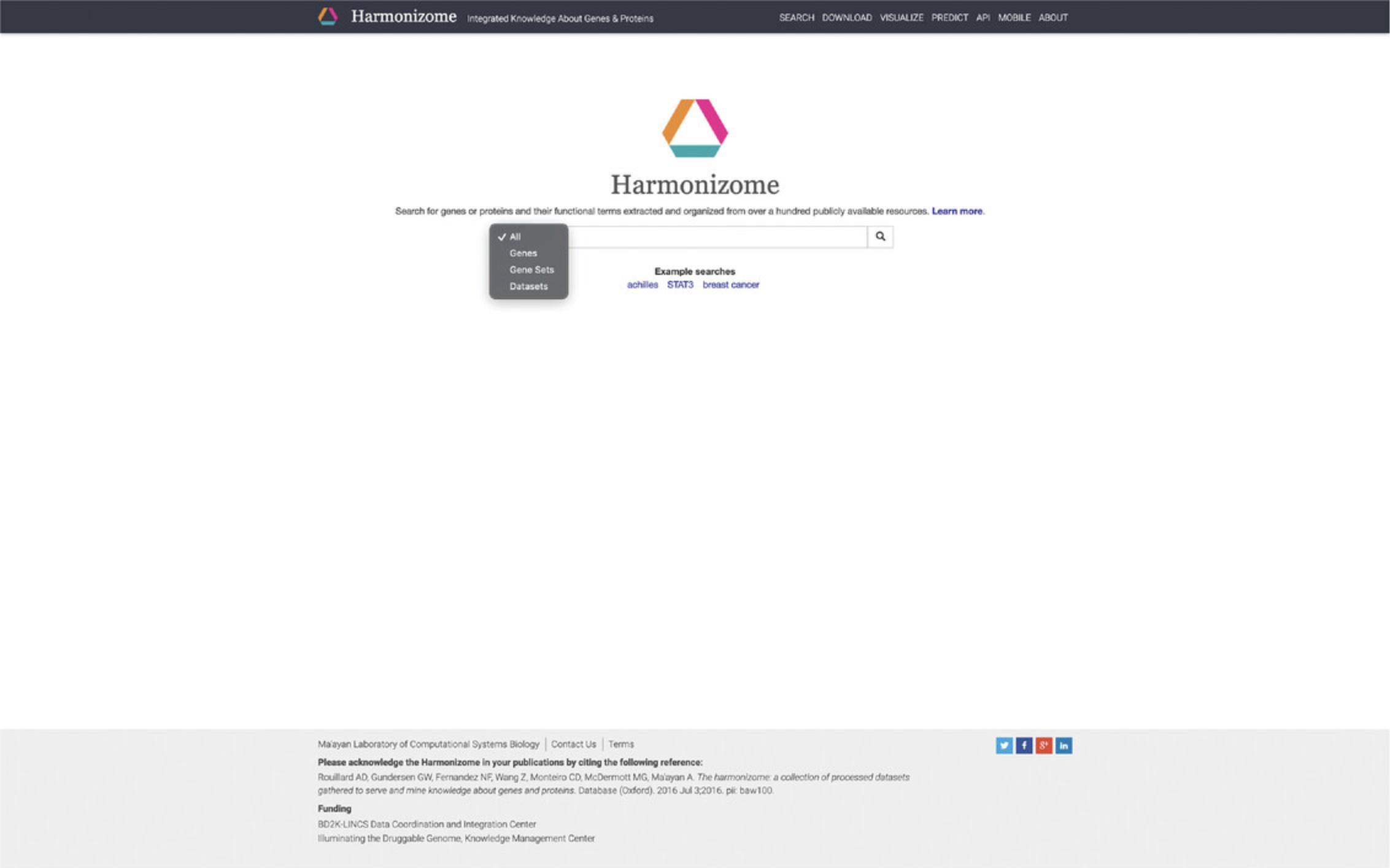
1.Navigate to the Harmonizome website (https://maayanlab.cloud/Harmonizome/).
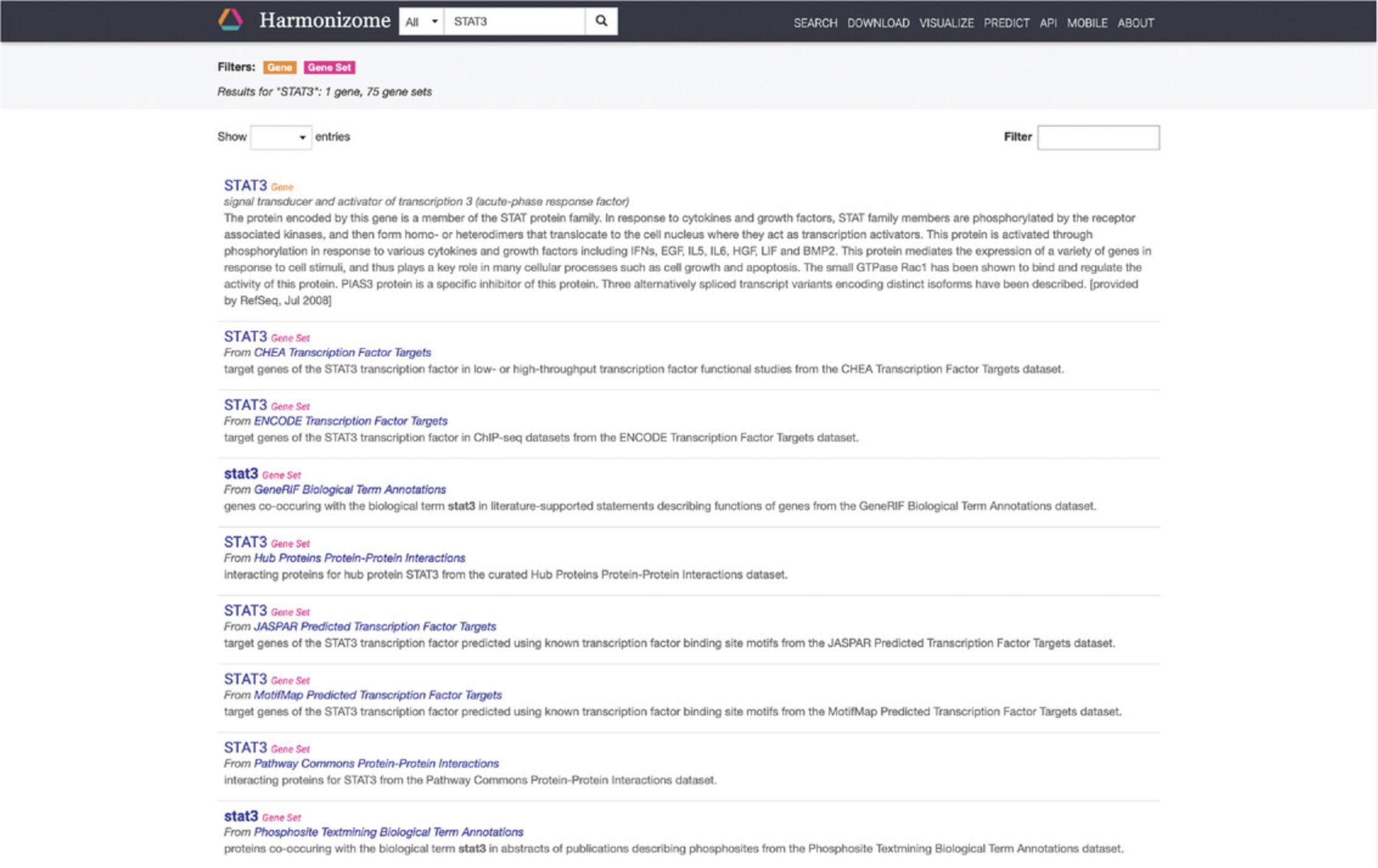
2.The front page features a search bar where keywords of interest can be input. Click the filter button on the left of the search bar to narrow searches to “genes,” “gene sets,” or “datasets” (Fig. 14). Type STAT3 into the search bar and click the submit button. The results page includes a single-gene landing page for STAT3 and 75 gene sets with STAT3 as an attribute (Fig. 15).
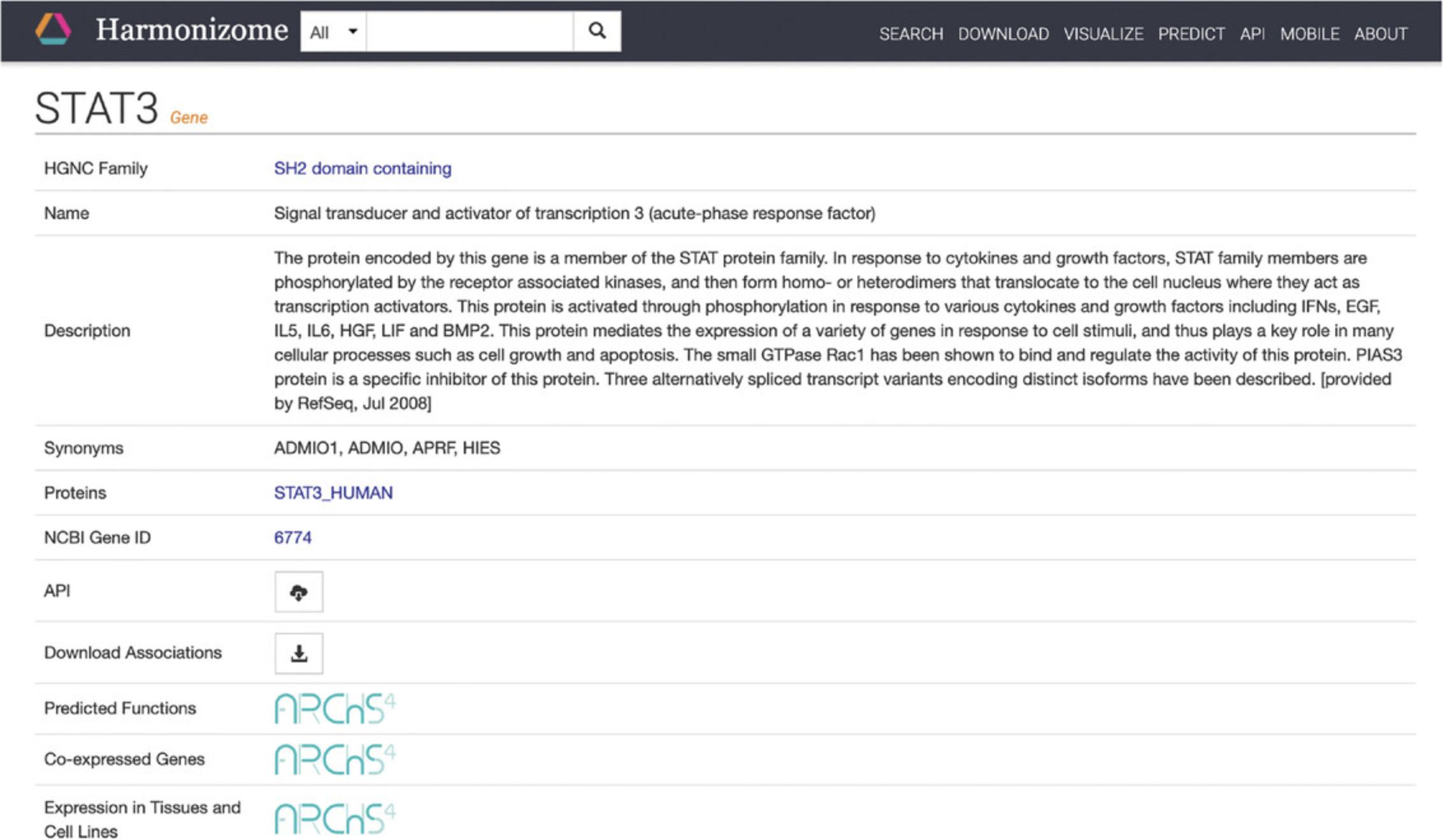
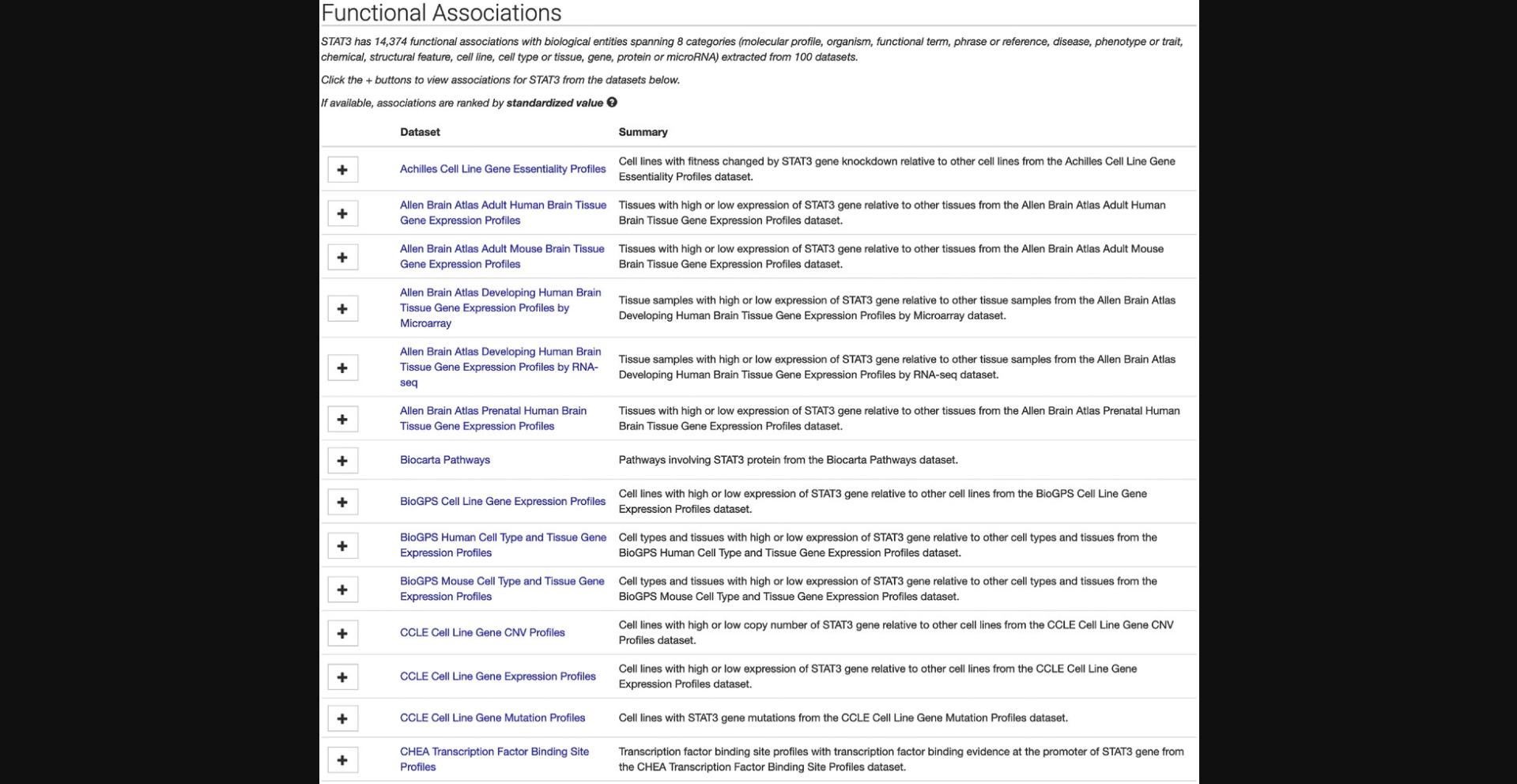
3.Click on the STAT3 “gene” result to be redirected to a single-gene landing page (Fig. 16). The page includes identifying metadata for the gene, download links for accessing functional associations between STAT3 and other attributes, and links to other gene-related information from ARCHS4 (Lachmann et al., 2018). Additionally, a list of functional associations for STAT3 from the various processed datasets included in Harmonizome is available (Fig. 17). Click the “+” button to view associations for STAT3 for any of the datasets.
4.Click on any of the STAT3 “gene set” results. The gene set results page includes metadata for the STAT3 gene set; in this case the gene set includes all target genes of STAT3.All of the genes included in the gene set are found in the “Genes” section (Fig. 18). Click on any of the gene symbols to be redirected to a single-gene landing page.
Download page
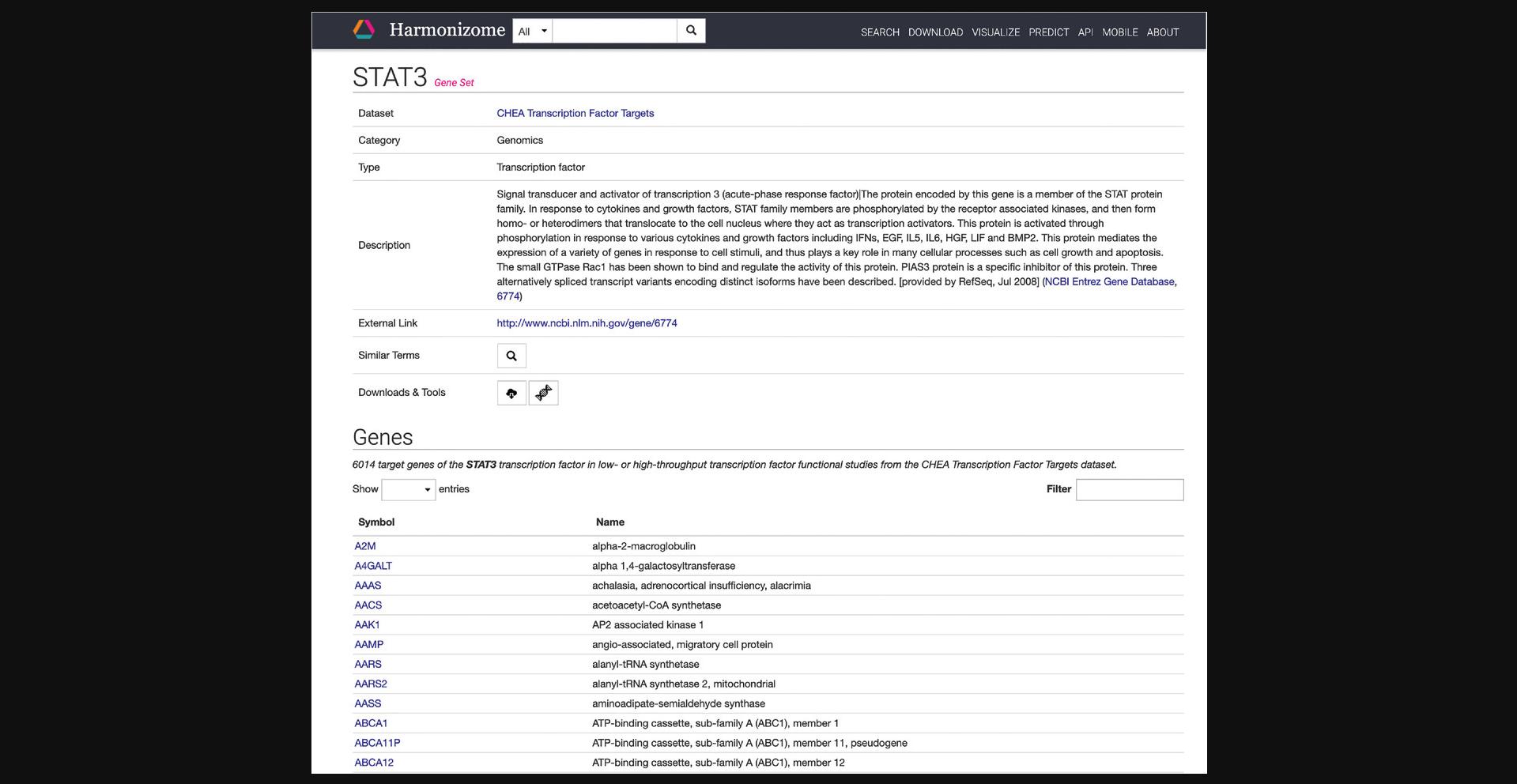
5.Click on the “Download” section on the navigation bar at the top of the page to be redirected to a table of all the datasets included in Harmonizome (Fig. 19).
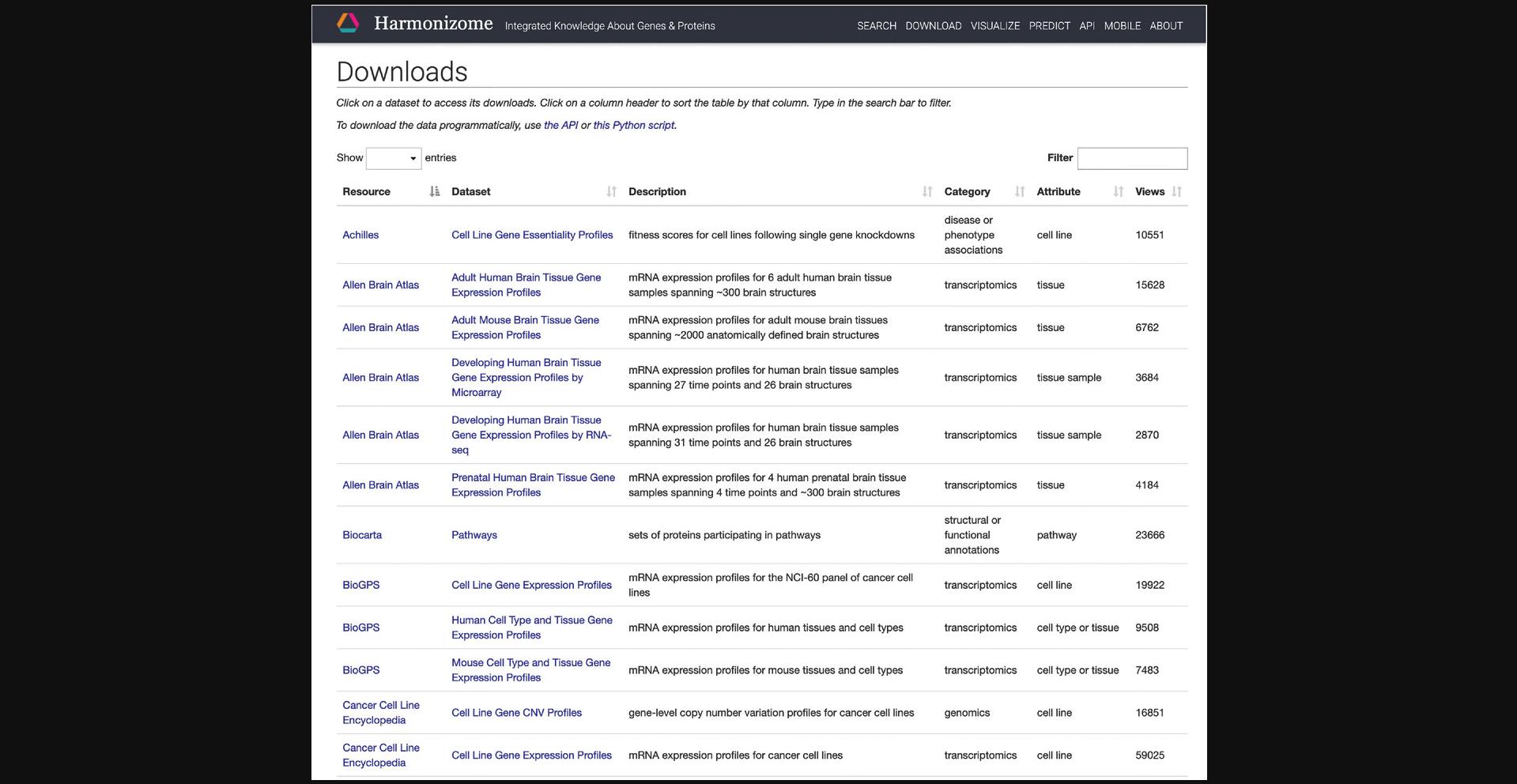
6.Click on “Achilles” in the resource column to be redirected to a page with identifying metadata for the resource and a list of all datasets derived from the resource (Fig. 20).
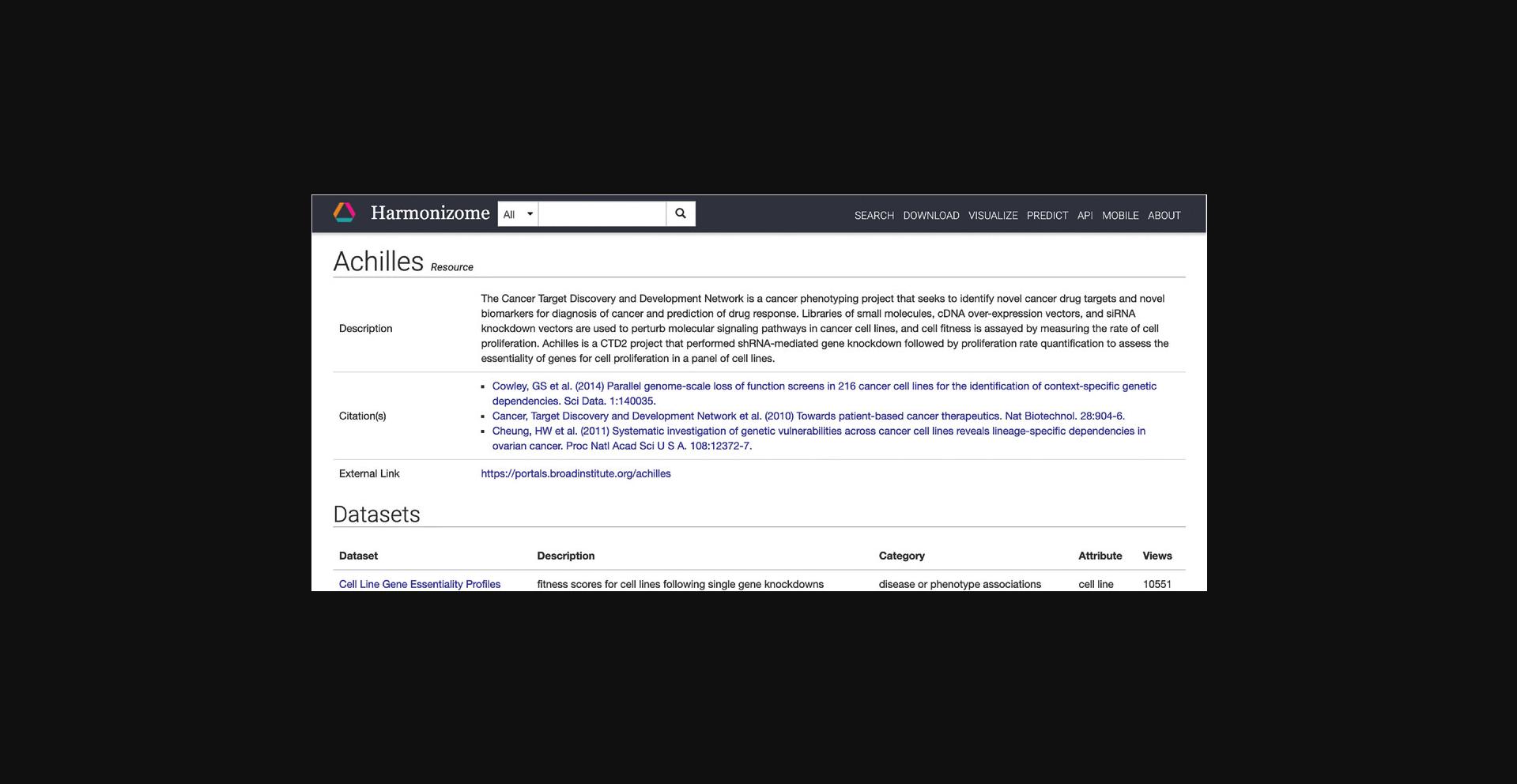
7.Click on “Cell Line Gene Essentiality Profiles” in the dataset column to be redirected to a page with identifying metadata for the dataset and links to downloadables contained within this dataset (Fig. 21). Further down the page are links to visualizations of the dataset contents and a table of gene sets (Fig. 22). Click on any of the gene set names to be redirected to a gene set specific page.
Visualize
8.Click on the “Visualize” section on the navigation bar at the top of the page, and a drop-down menu will appear (Fig. 23).

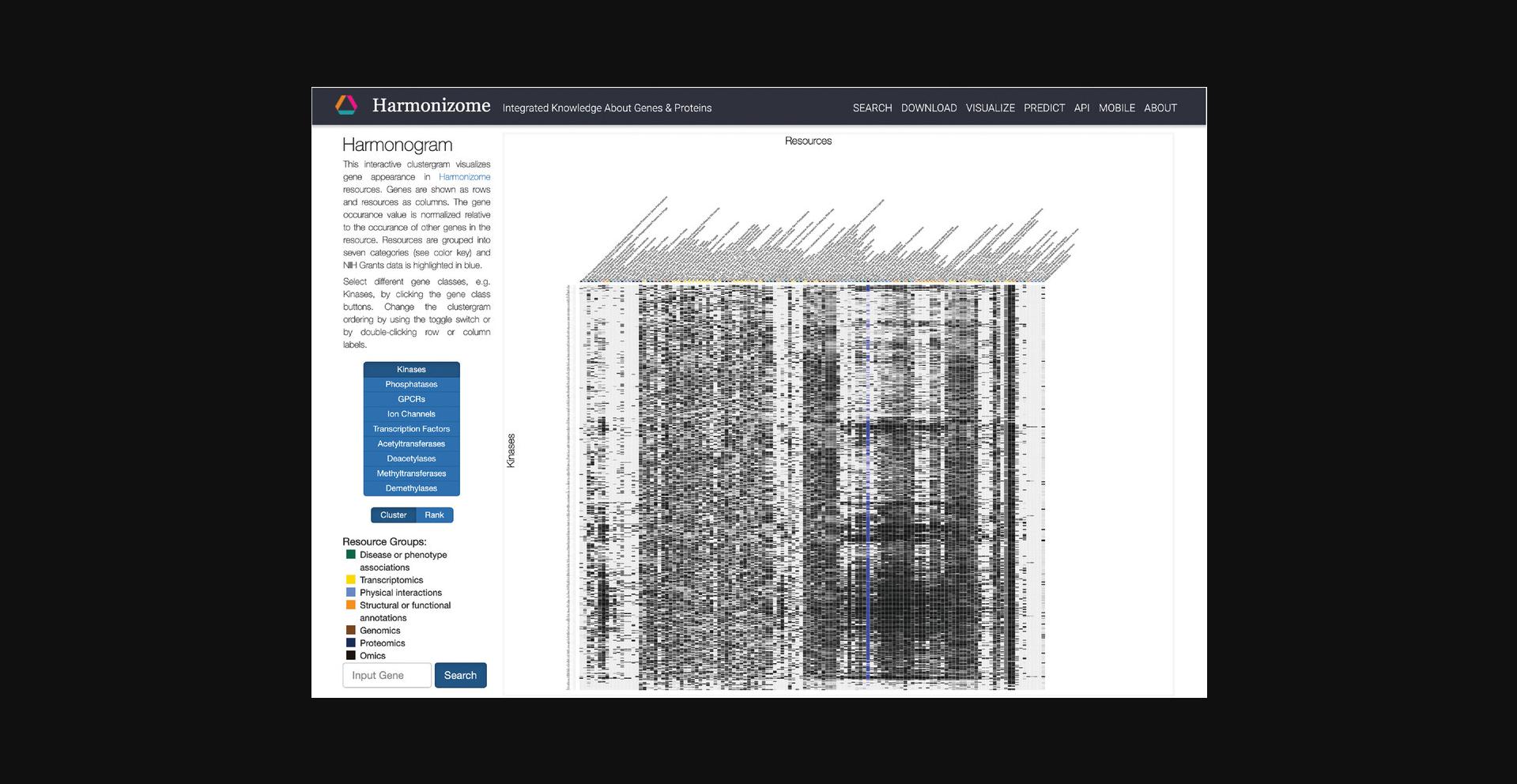
9.Click on “Global Heat Map” within the drop-down menu to be redirected to an interactive clustergram that visualizes the appearance of each gene in Harmonizome. Select different gene classes with the buttons on the left. Switch the ordering of the clustergram between “cluster” and “rank” by clicking the corresponding button (Fig. 24).
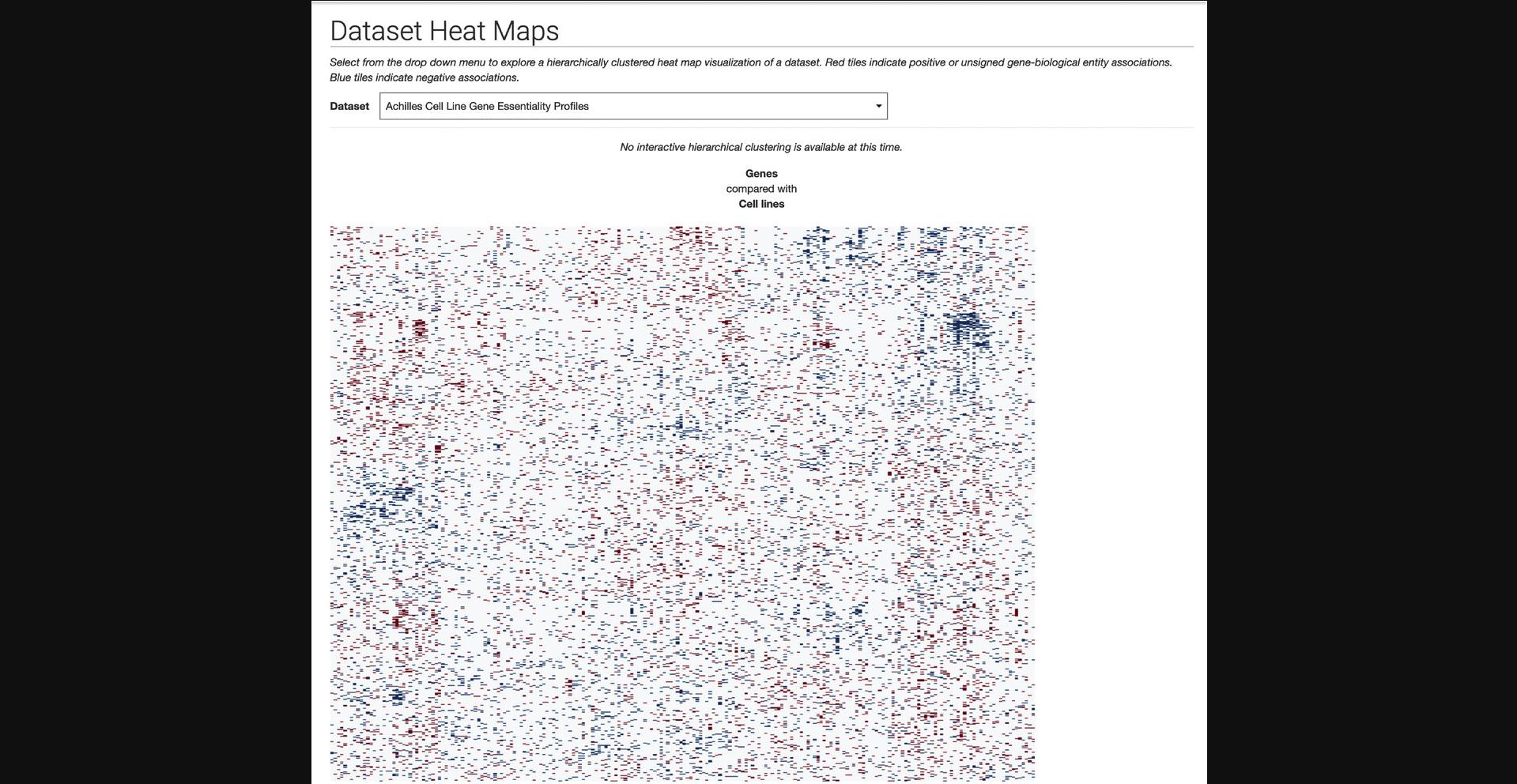
10.Click on “Dataset Heat Maps,” “Gene Similarity Heat Maps,” or “Attribute Similarity Heat Maps” within the drop-down menu to be redirected to a page with a drop-down menu of Harmonizome datasets. Open the drop-down menu and select any dataset to generate a hierarchically clustered heat map visualization of the dataset (Fig. 25).
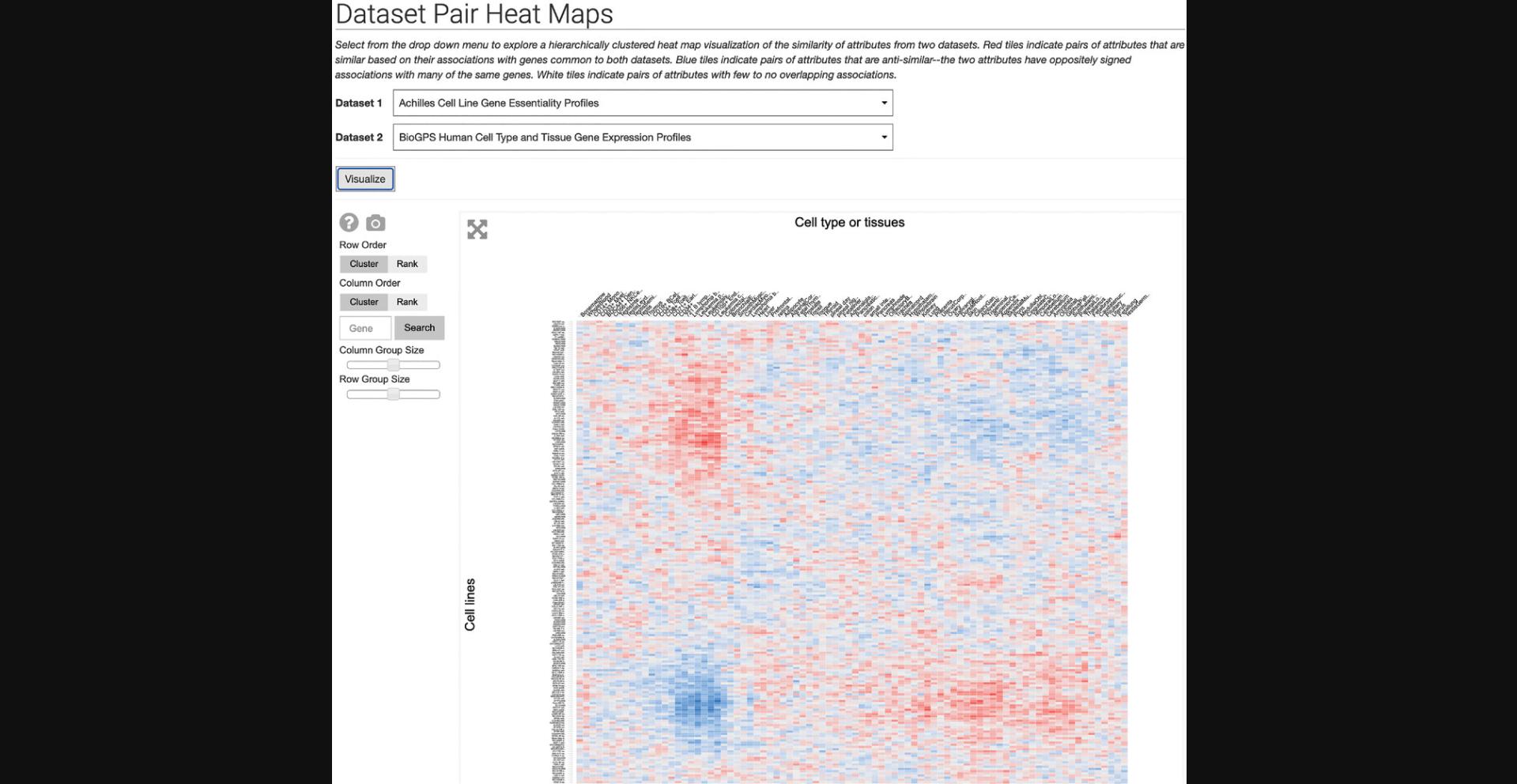
11.Click on “Dataset Pair Heat Maps” within the drop-down menu to be redirected to a page with a drop-down menu of Harmonizome datasets. Open the drop-down menu and select a dataset. A second drop-down menu will appear for selecting a second dataset to compare. Click visualize to generate a hierarchically clustered heat map visualization of the two datasets (Fig. 26).
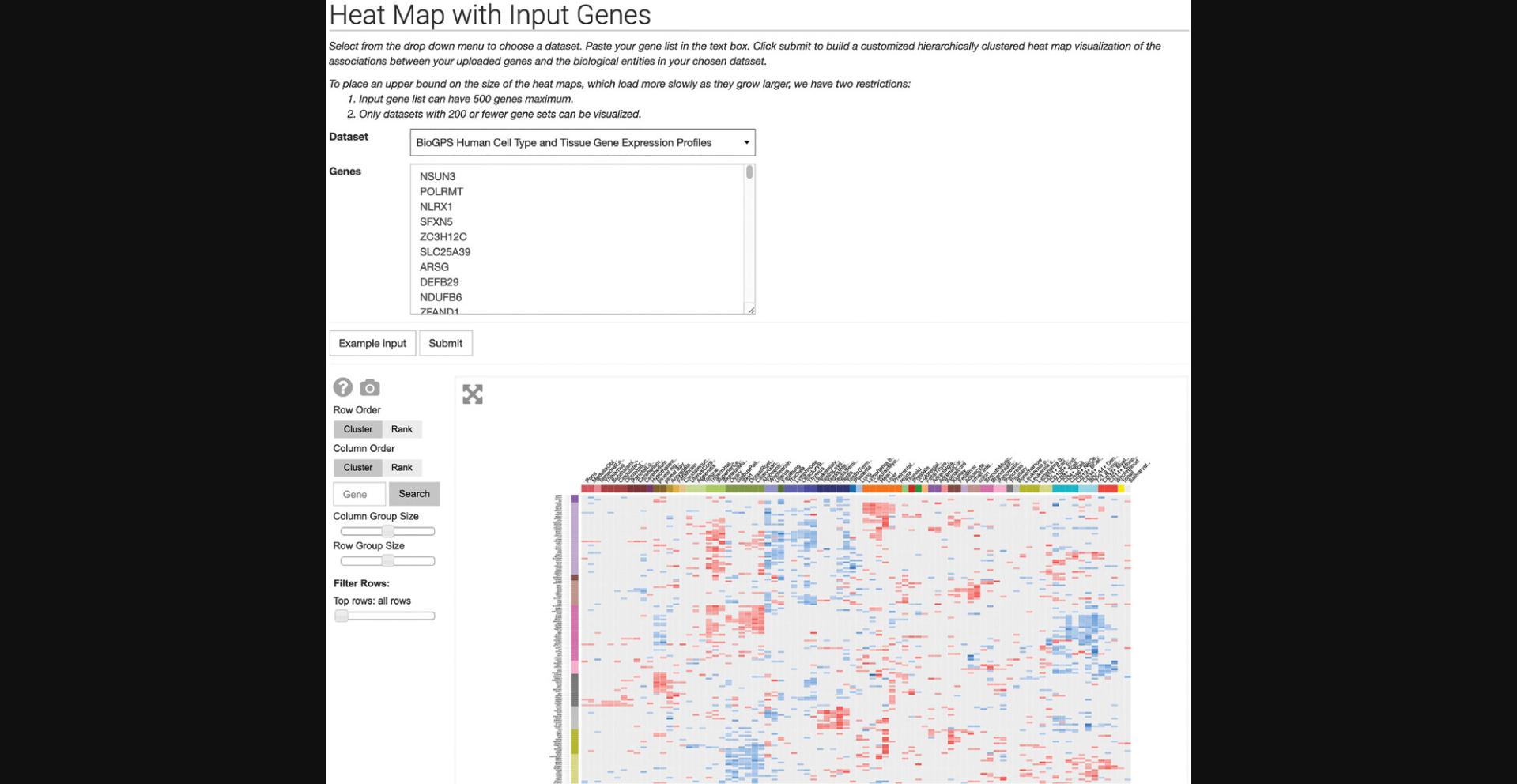
12.Click on “Heat Map with Input Genes” within the drop-down menu to be redirected to a page with a drop-down menu of Harmonizome datasets and a gene list text box. Click the “Example input” button to populate the fields with an example dataset and gene set. Click “Submit” to generate a hierarchically clustered heat map visualization of the associations between the uploaded genes and biological entities in the dataset (Fig. 27).
Predict
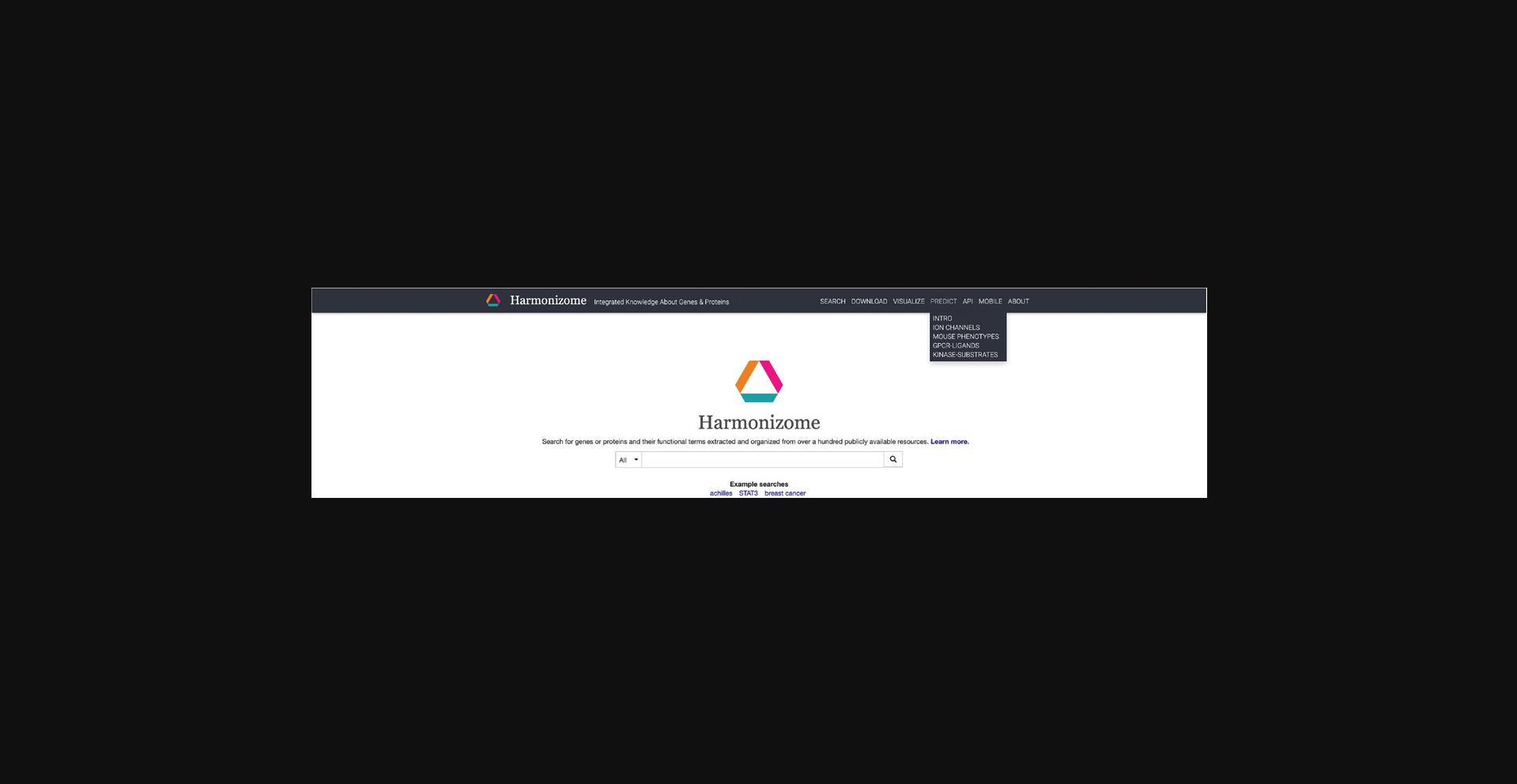
13.Click on the “Predict” section on the navigation bar at the top of the page and a drop-down menu will appear (Fig. 28). Click “Intro” within the drop-down menu.
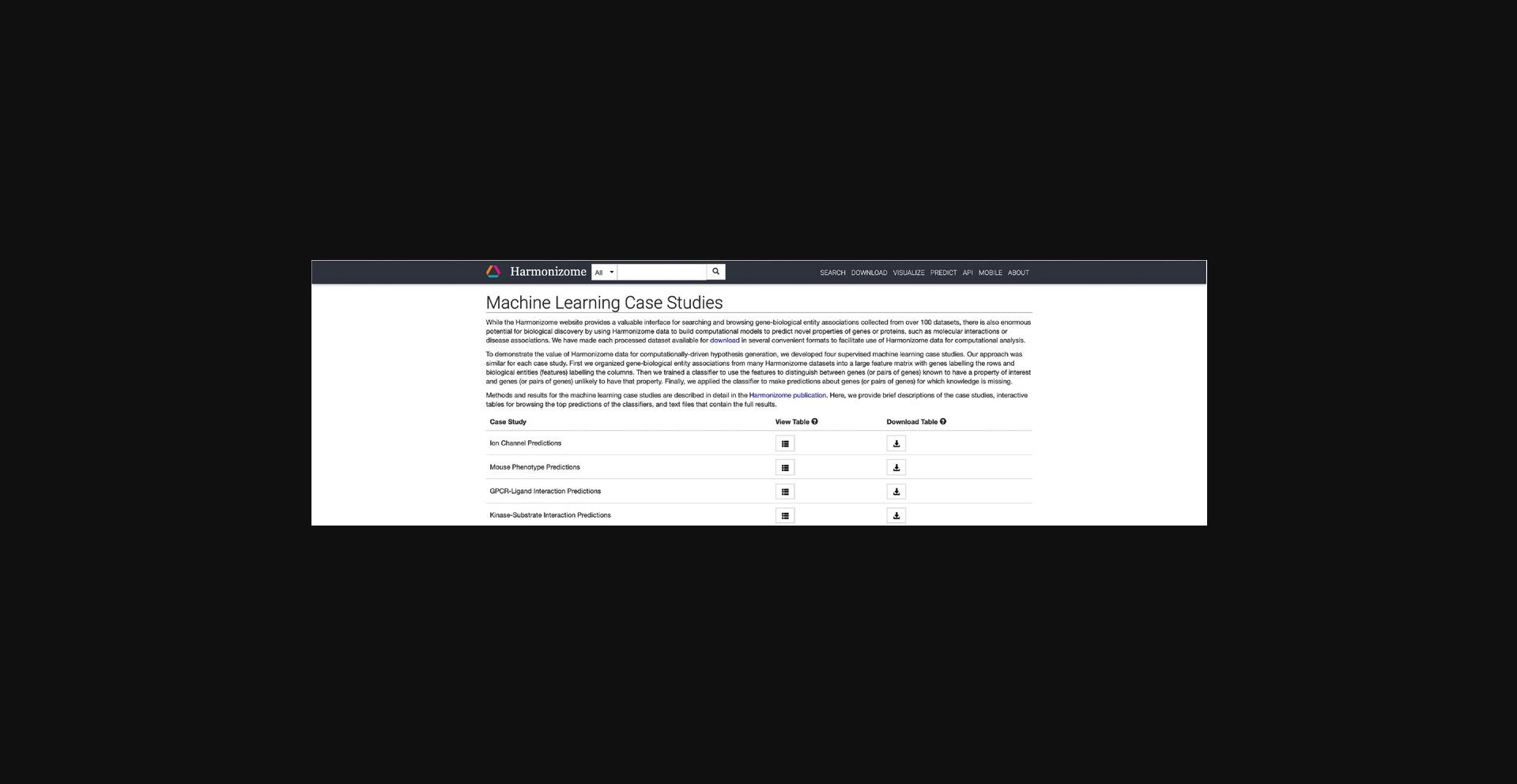
14.The intro page contains information about how machine learning studies were devised using the Harmonizome datasets. A table with four separate case studies: “Ion Channel Predictions,” “Mouse Phenotype Predictions,” “GPCR-Ligand Interaction Predictions,” and “Kinase-Substrate Interaction Predictions” contains links to view and download tables of predicted associations (Fig. 29).
Using the Harmonizome API
15.These are the entity types supported by the Harmonizome API:
- DATASET, GENE, GENE_SET, ATTRIBUTE, GENE_FAMILY, NAMING_AUTHORITY, PROTEIN, RESOURCE
Open a new or existing Python code file. Import the required Harmonizome API Python module at the top of the file:
- from harmonizomeapi import Harmonizome, Entity
The Harmonizome object includes several methods to read, parse, and download data from the Harmonizome API. The Harmonizome object includes .get(), .next() and .download() methods. For example, to display the datasets available in Harmonizome, run the following code block:
- entity_list = Harmonizome.get(Entity.DATASET)
- more = Harmonizome.next(entity_list)
In order to minimize database queries and request times, the Harmonizome API uses a technique called "cursoring" to paginate large result sets. Therefore, the first line in the above code block returns the first 100 datasets, whereas the second line continues from where the previous entity list left off and retrieves the subsequent 14 datasets that are available in Harmonizome. The Harmonizome.get()and Harmonizome.next() methods can be used for all entity types supported by the Harmonizome API.
16.To download datasets available in Harmonizome to a local directory, use the Harmonizome.download() generator function. Alternatively Harmonizome.download_df() can be used to download files and load them in directly as sparse (with an added sparse=True argument) or dense Pandas DataFrames (assumed). The function takes a list of datasets and downloadables as arguments. Leaving the datasets argument empty will download all datasets by default. Leaving the what argument empty will download all downloadables for each dataset by default. In the example code below, the gene_attribute_matrix.txt.gz downloadable from the “CTD Gene-Chemical Interactions” dataset is downloaded, decompressed, and saved to a local directory named after the dataset if it has not already been processed:
dl, = Harmonizome.download(datasets=[ˈCTD Gene-Chemical Interactionsˈ],
- what=[ˈgene_attribute_matrix.txt.gzˈ])
More information regarding the Harmonizome API is available at https://maayanlab.cloud/Harmonizome/documentation.
Basic Protocol 3: THE ARCHS4 RESOURCE
ARCHS4 (Lachmann et al., 2018) is a web resource that provides access to published RNA-seq gene- and transcript-level data from human and mouse experiments. FASTQ files from RNA-seq experiments deposited in the Gene Expression Omnibus (GEO) were aligned using a cloud-based infrastructure. The ARCHS4 web interface facilitates the exploration of the processed data through querying tools, interactive visualizations, and single-gene landing pages that provide average expression of a specific gene across cell lines and tissues, top co-expressed genes, and predicted biological functions and protein–protein interactions for each gene based on prior knowledge combined with co-expression.
Necessary Resources
Hardware
- Desktop or a laptop computer, or a mobile device, with a fast Internet connection
Software
- An up-to-date web browser such as Google Chrome (https://www.google.com/chrome/), Mozilla Firefox (https://www.mozilla.org/en-US/firefox/), Apple Safari (https://www.apple.com/safari/), or Microsoft Edge (https://www.microsoft.com/en-us/edge)
- Most updated version of R (https://www.r-project.org/); R Studio (https://www.rstudio.com/); rhdf5 library (https://www.bioconductor.org/packages/release/bioc/html/rhdf5.html)
Metadata search
1.Navigate to the ARCHS4 web application (https://maayanlab.cloud/archs4/).
2.Click the “Get Started” button on the homepage to proceed to the data search and visualization page (Fig. 30).
3.The data search and visualization page by default shows an interactive 3D t-SNE scatter plot of all the human gene expression samples found in ARCHS4 (Fig. 31). The metadata search field on the left enables querying of specific terms that will be highlighted in the 3D scatter plot. Searching for the term “Pancreatic Islet” and then clicking on the search button results in the highlighting of the relevant samples. The samples that are related to the search term cluster in the scatter plot because the samples contain similar expression profiles (Fig. 32).
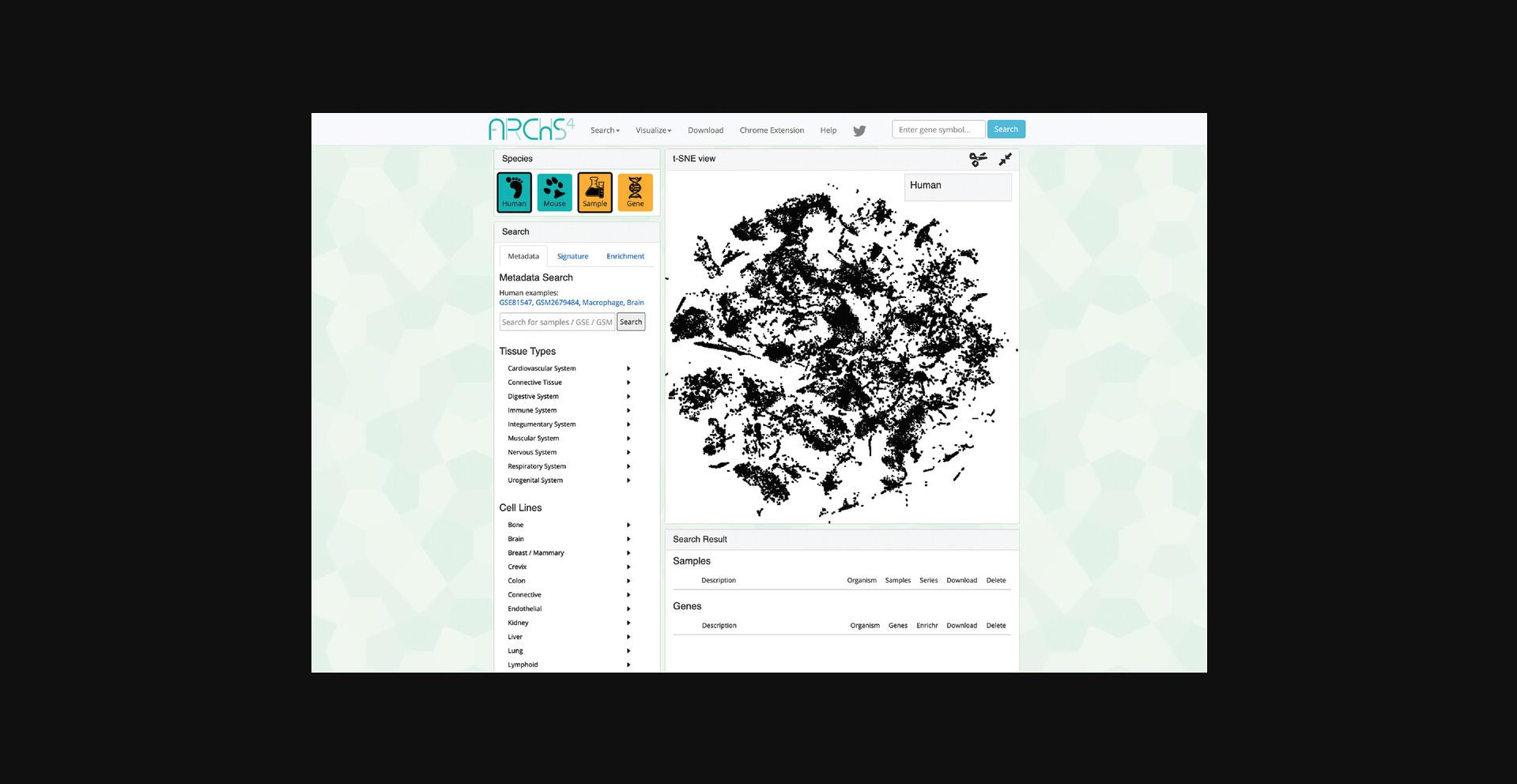
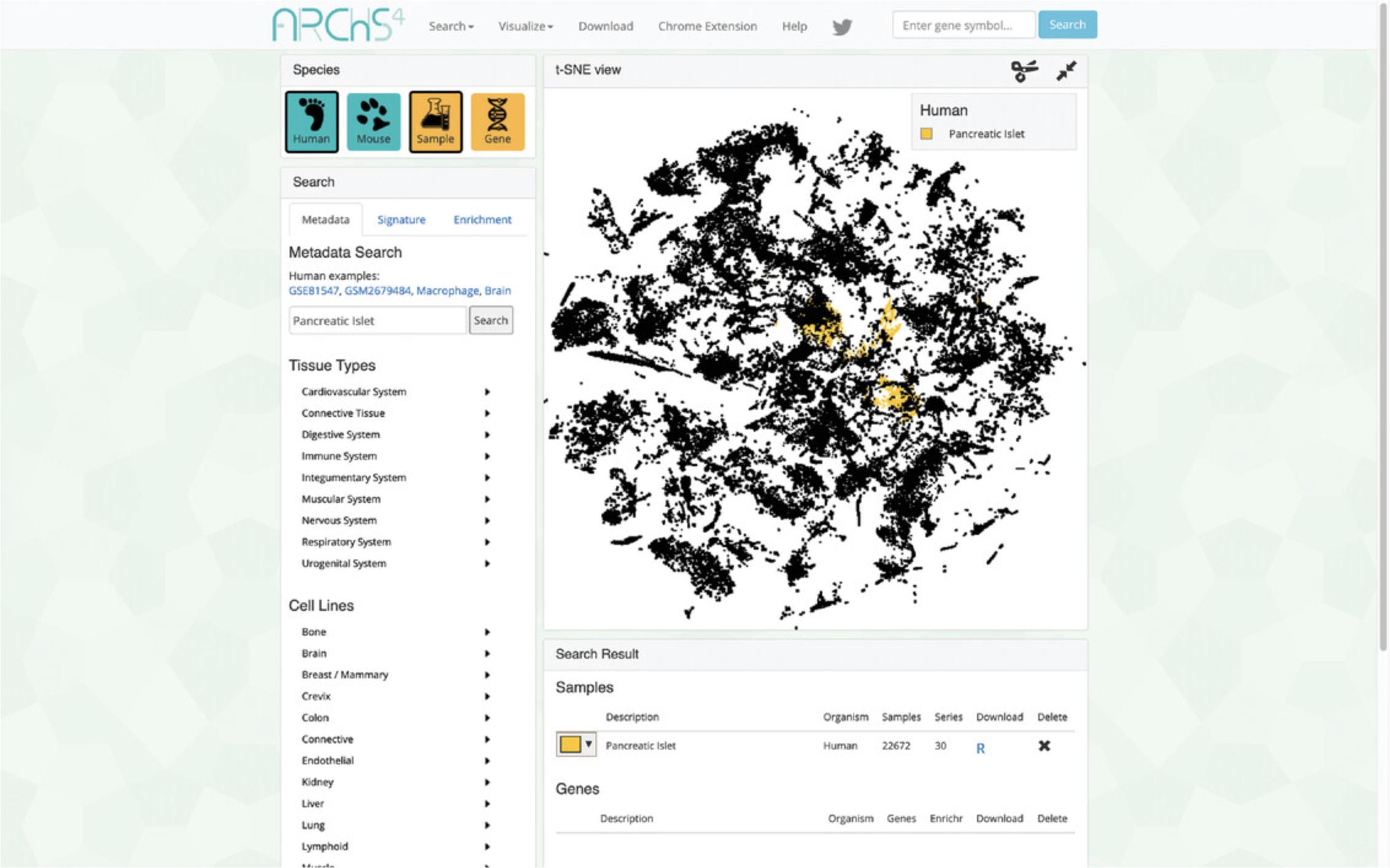
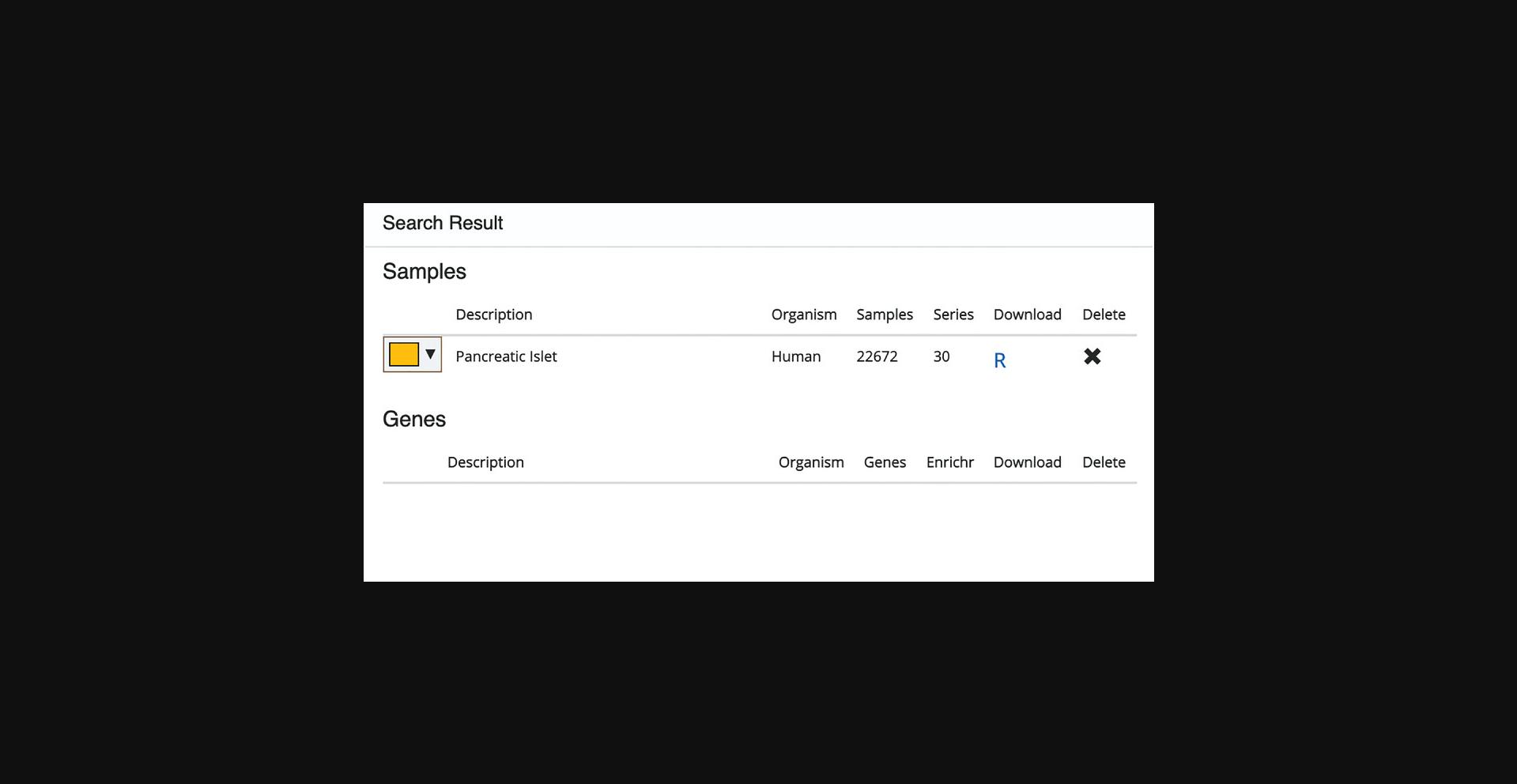
4.Any submitted search term will be found in its corresponding section within the “Search Result” table below the interactive t-SNE scatter plot visualization. The table contains metadata regarding the organism, number of samples, and number of series, as well as a button to download an R script that can be used to retrieve the identified sample files. An X button is also available to delete the query (Fig. 33).
Signature search
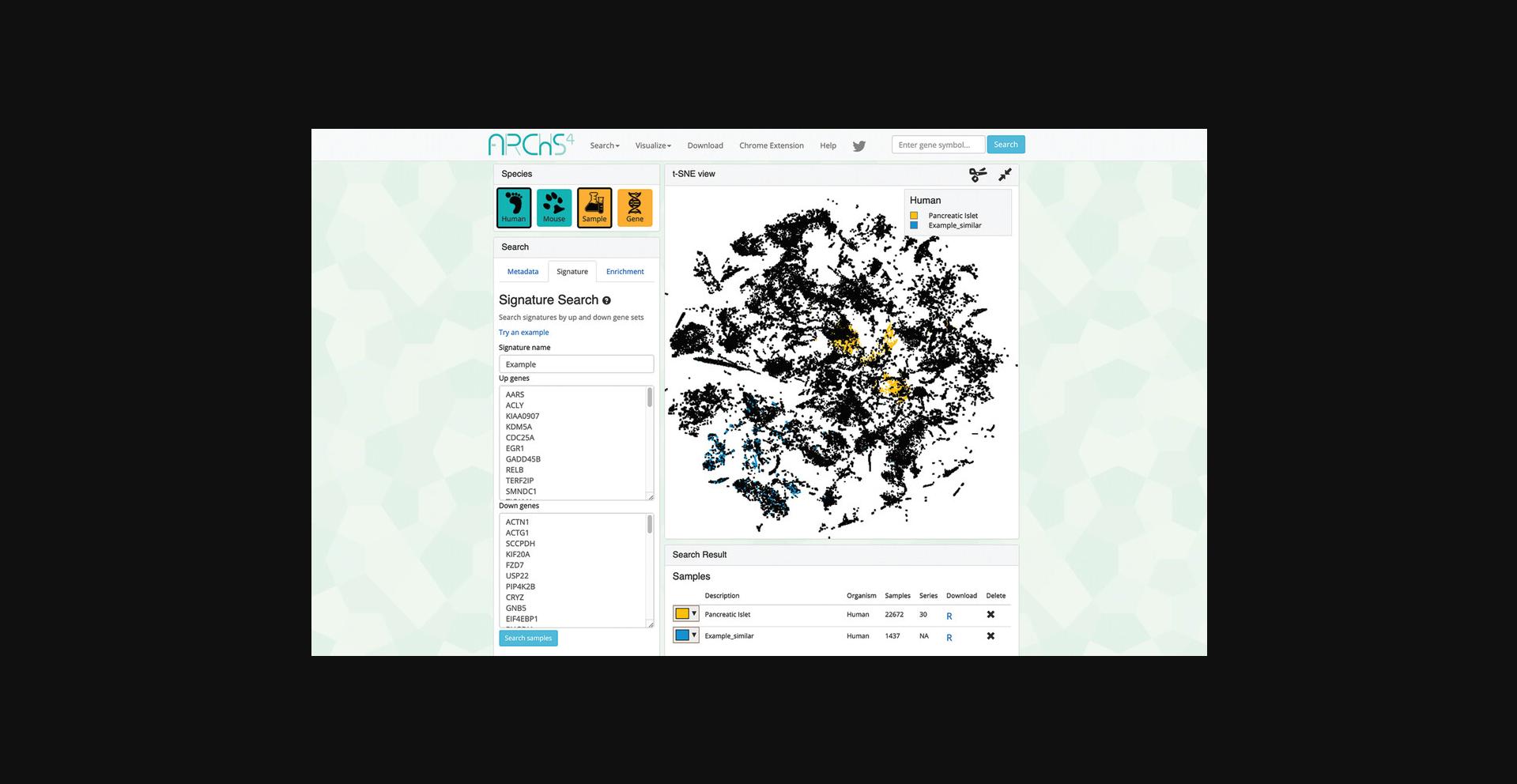
5.Switching to the signature search functionality can be done by clicking on the corresponding tab within the “Search” field on the left (Fig. 34). The signature search uses a set of highly and lowly expressed genes from each sample to identify matching samples to the given input.
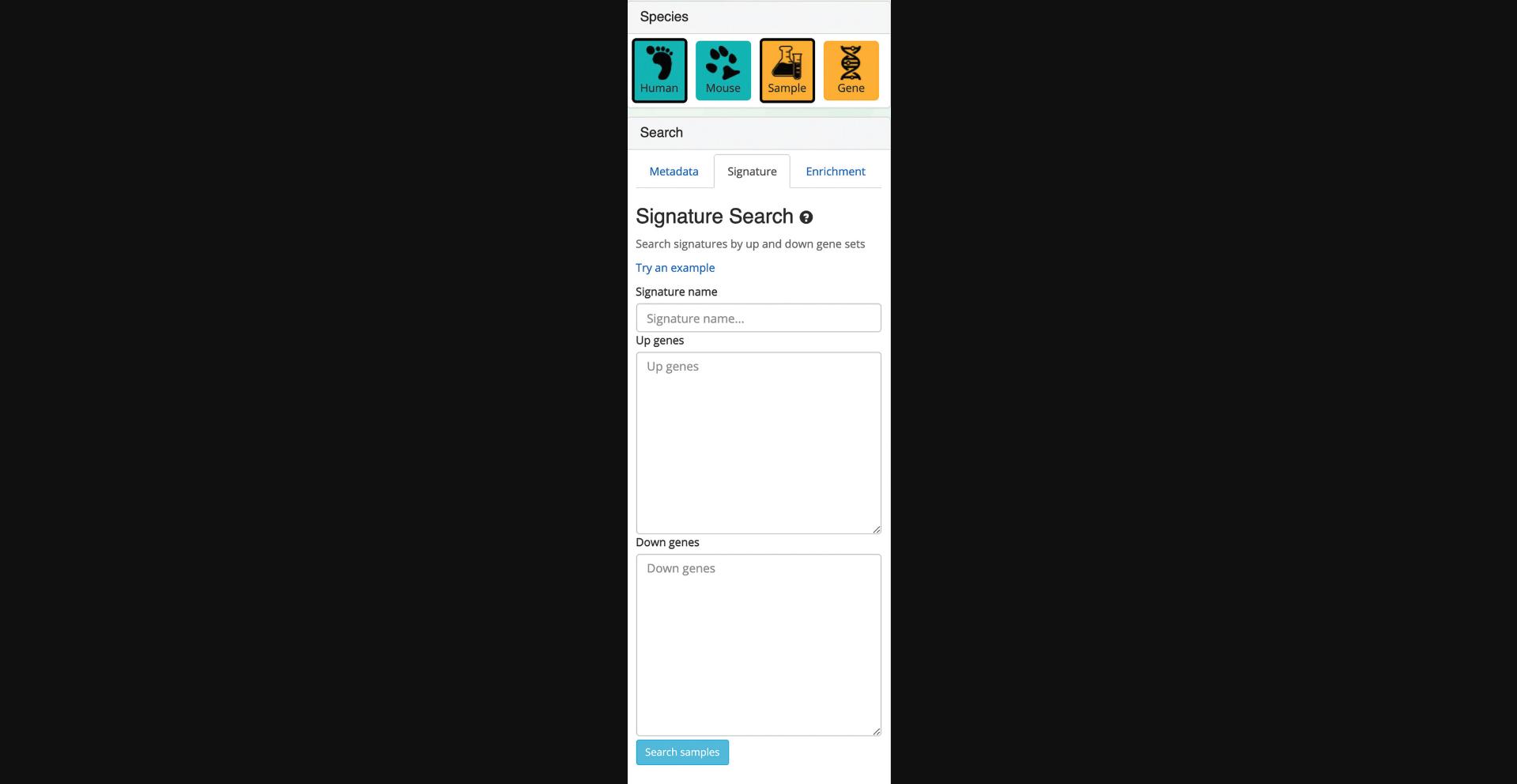
6.Query the example up and down gene sets by clicking “Try an example.” The corresponding samples are highlighted within the scatter plot and are added to the “Search Result” table (Fig. 35). Note that the previous query of “Pancreatic Islet” is still visualized within the scatter plot and listed in the “Search Result” table.
Enrichment analysis
7.Switch to the enrichment search by clicking on the corresponding tab within the “Search” field on the left (Fig. 36). The enrichment search highlights samples that are enriched in gene sets from eight gene set libraries. Select the gene set library, gene set of interest within the selected library, and a signature direction.
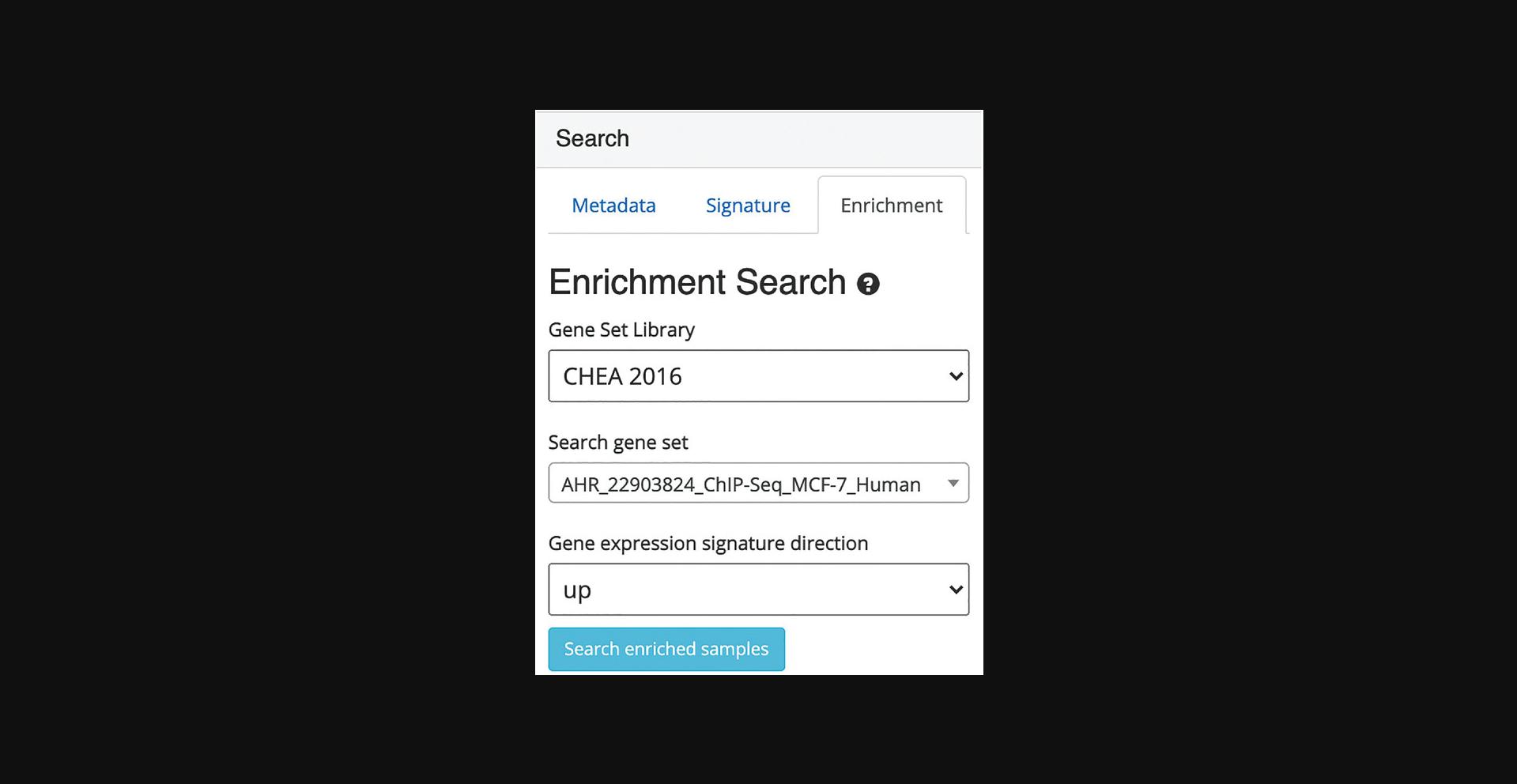
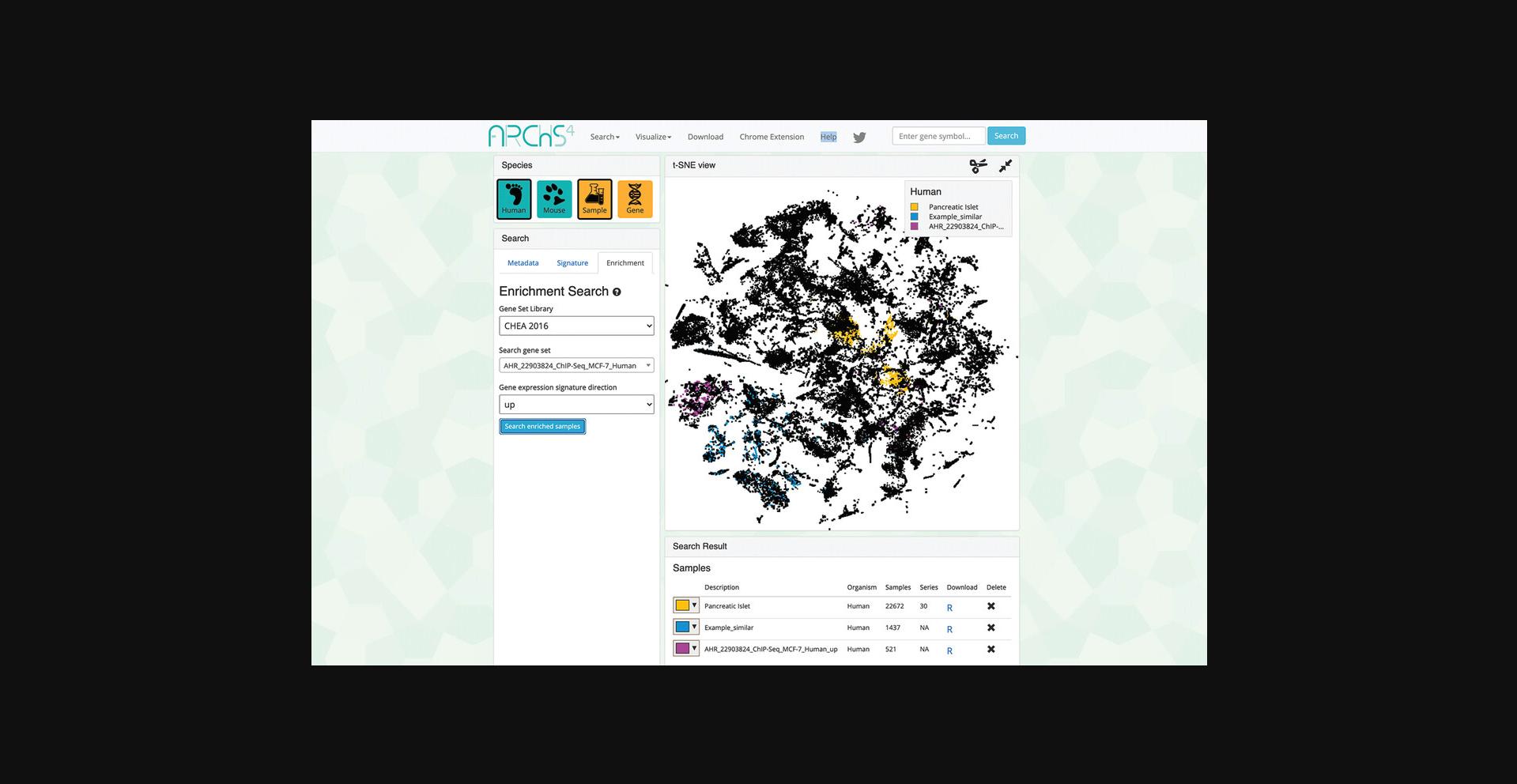
8.Query the example by clicking “Search enriched samples.” The corresponding samples are highlighted within the scatter plot and added to the “Search Result” table along with the previous queries (Fig. 37).
Gene-centric visualization
9.Switch to gene-centric searches by clicking on the orange button under the “Species” field in the upper left. Use this field to also switch between human and mouse samples by clicking the corresponding teal button (Fig. 38).

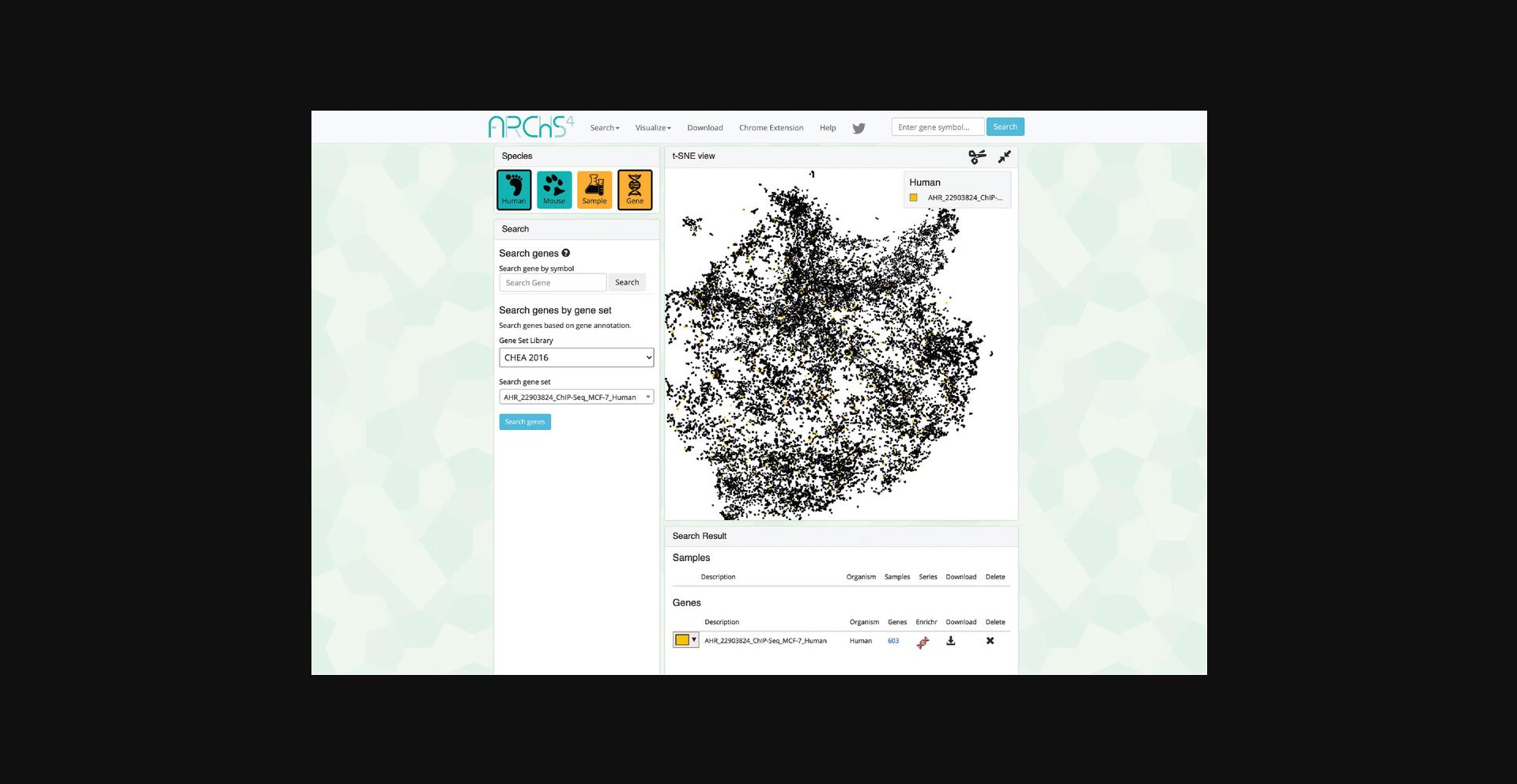
10.The page will now contain an interactive t-SNE scatter plot where each point represents a gene instead of a sample (Fig. 39).
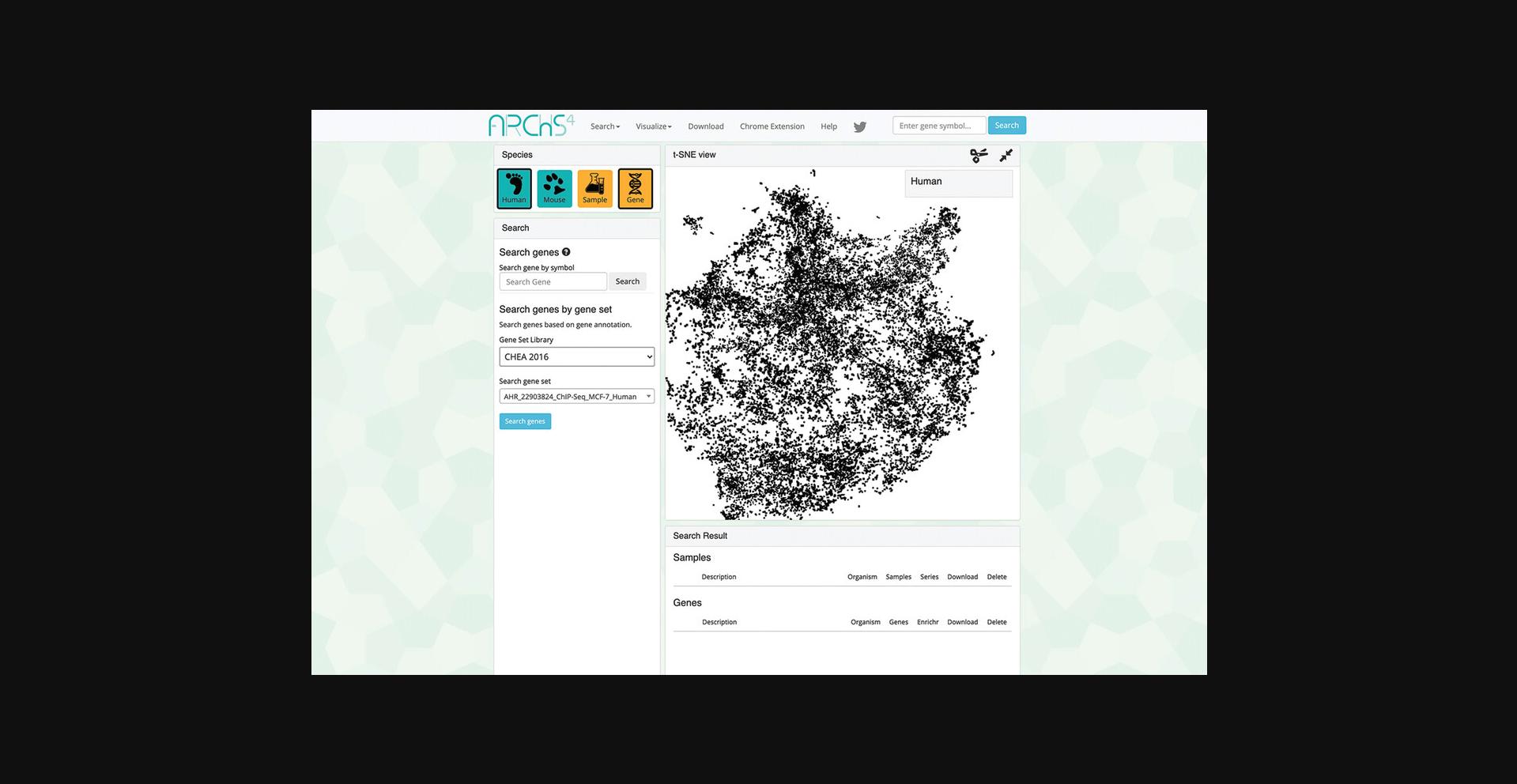
11.Choose a gene set library and a gene set within the “Search” field on the left (Fig. 40). Query the default options by clicking “Search genes.”
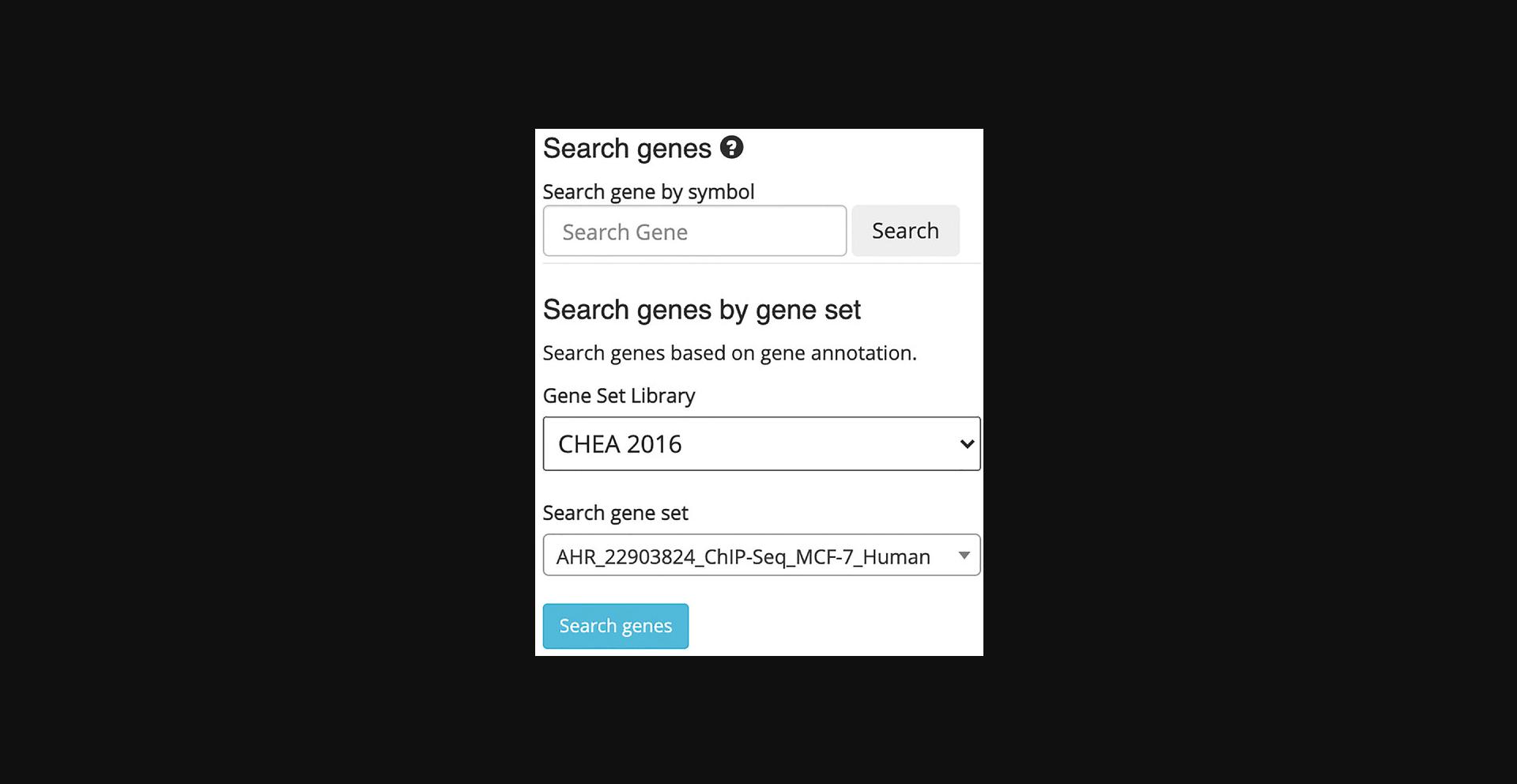
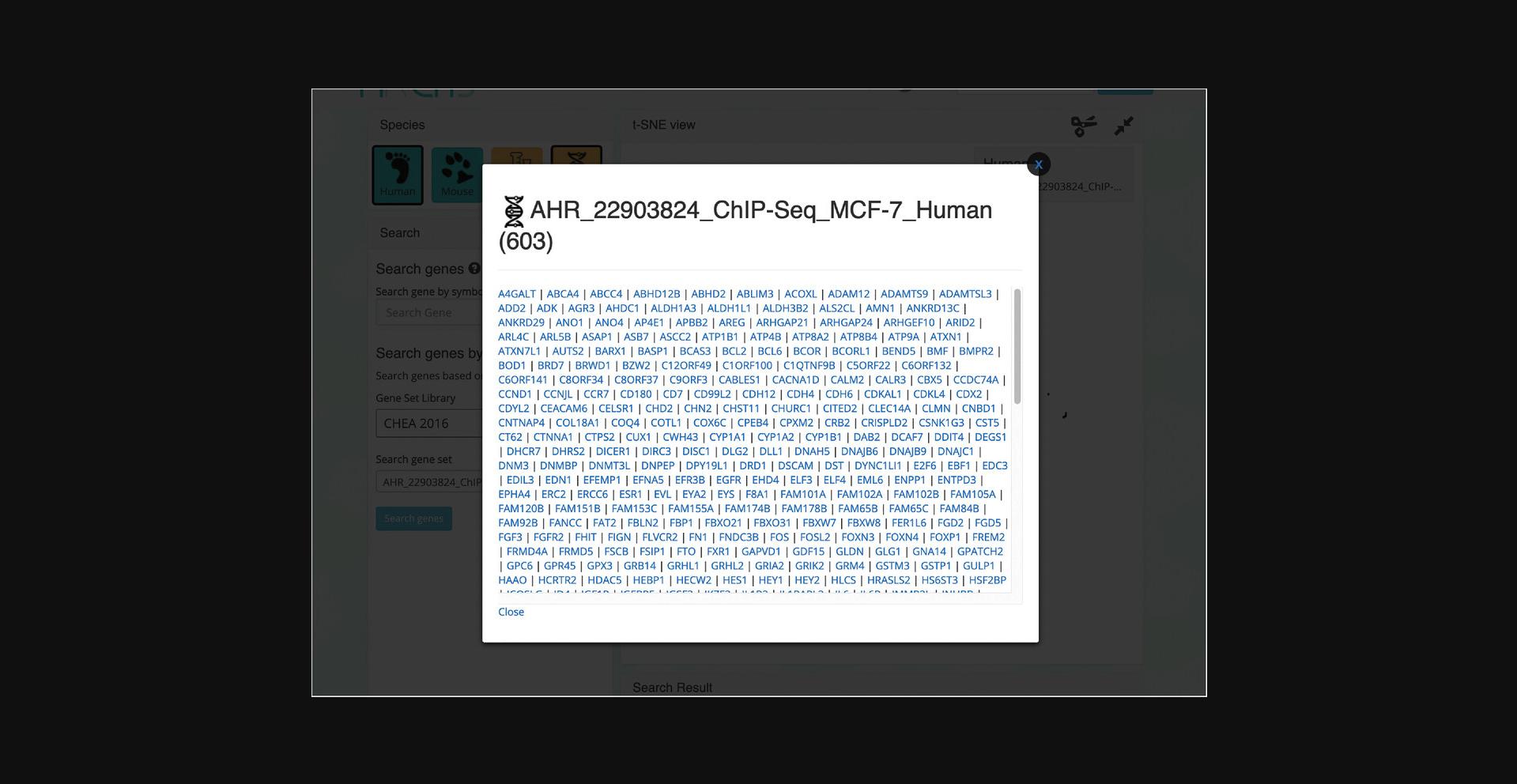
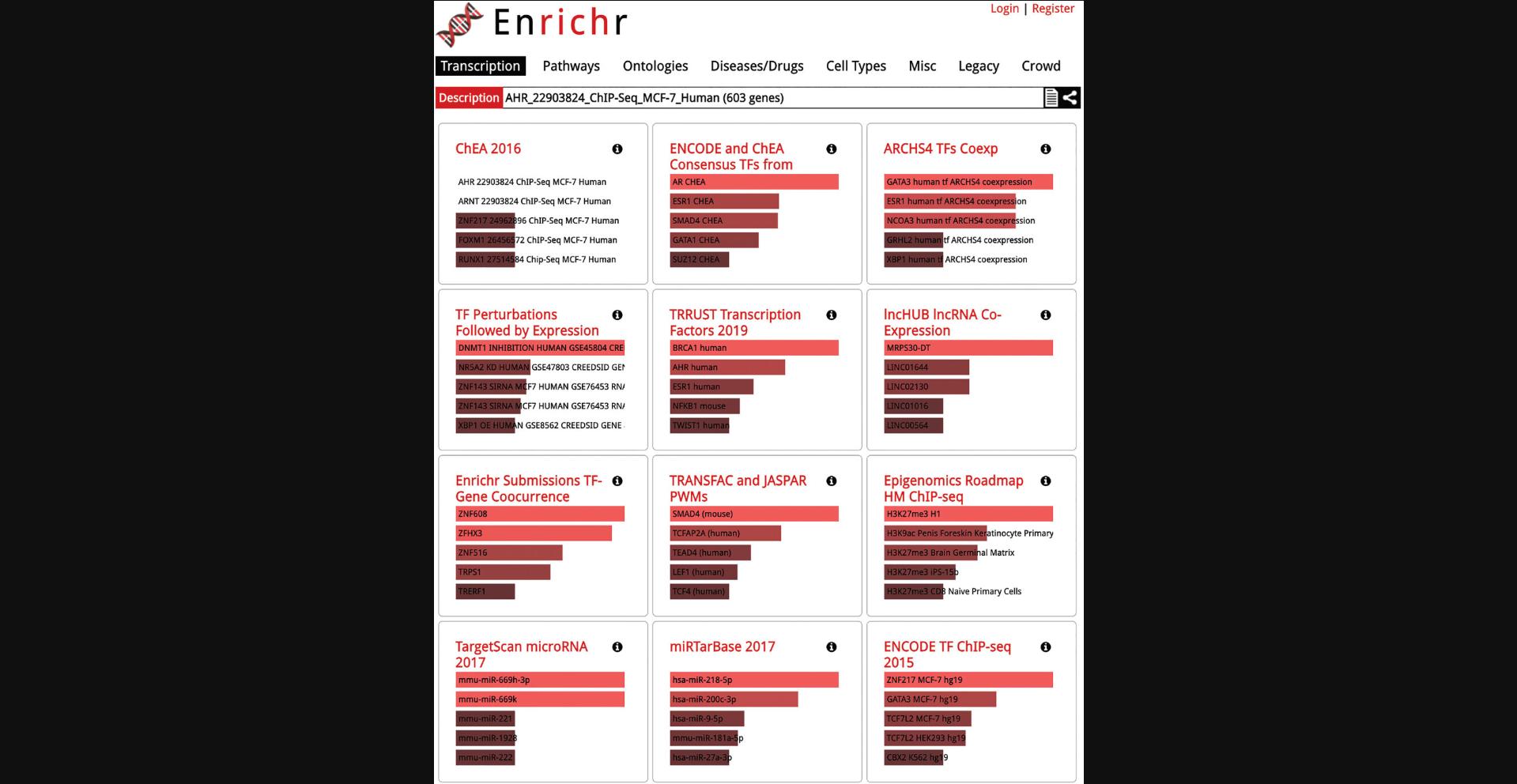
12.The corresponding samples are highlighted within the scatter plot and added to the “Search Result” table under the “Genes” section (Fig. 41). The table includes the number of genes included in the queried gene set which can be clicked to view the gene symbols in the gene set (Fig. 42). Additionally, the gene set can be submitted to Enrichr (Kuleshov et al., 2016) for gene set enrichment analysis by clicking on the Enrichr icon within the table (Fig. 43).
Gene search
13.Single genes can be queried using the autocomplete field within the “Search” field on the left. Input a gene of interest, for example SOX2, and click the search button (Fig. 44).
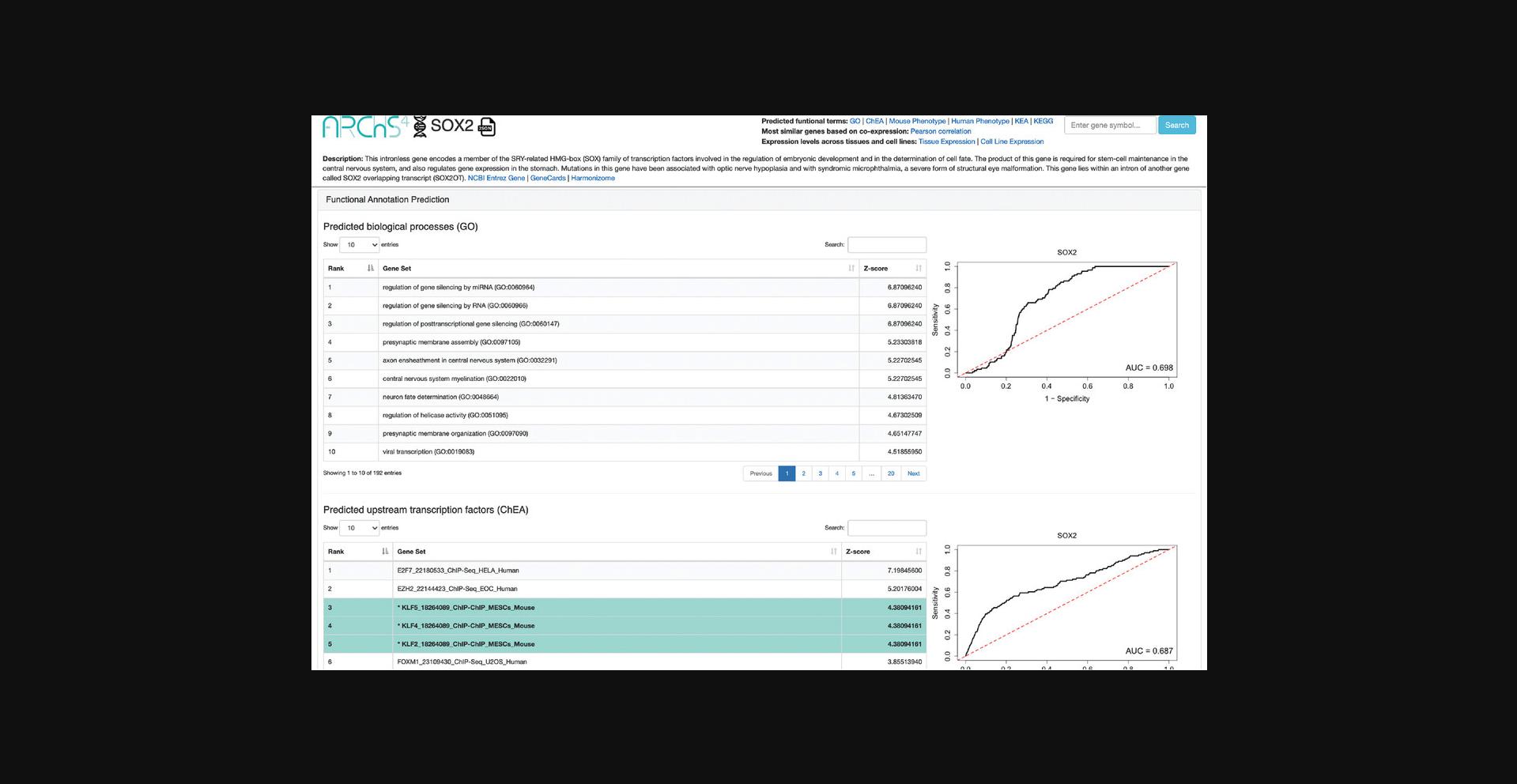
14.A single-gene page is generated for SOX2 (Fig. 45). The top of the page includes a description of the gene and links to other resources with identifying metadata for the gene. The “Functional Annotation Prediction” section contains ROC curves and tables of gene sets from six distinct gene set libraries SOX2 is predicted to be a member of based on co-expression. Known associations are marked in teal.
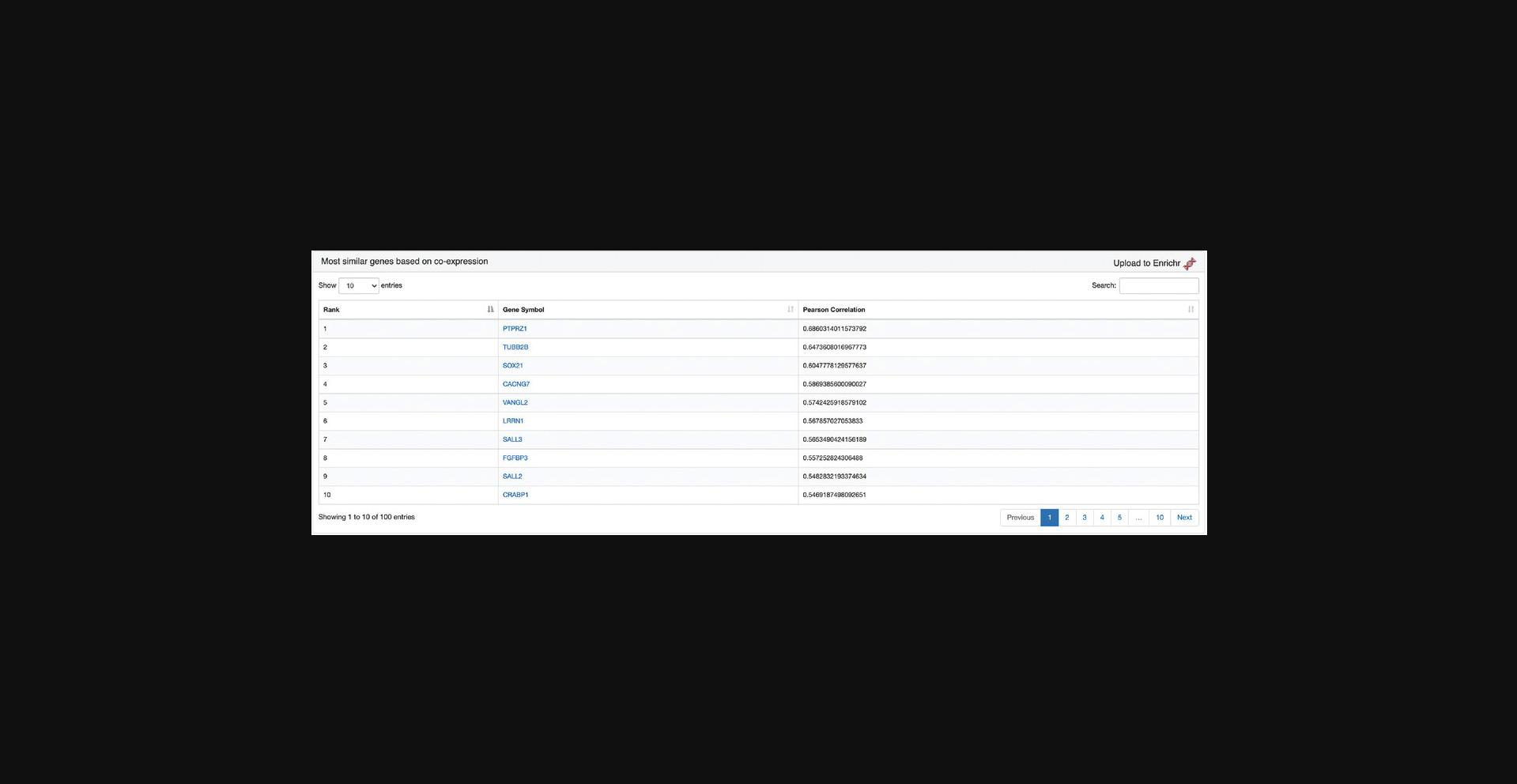
15.The “Most similar genes based on co-expression” section contains a table of the top 100 genes that are most similar to SOX2 based on the Pearson correlation of their expression across all ARCHS4 samples (Fig. 46). The most correlated genes from the table can be submitted to Enrichr by clicking the corresponding link in the top right.
16.The “Tissue Expression” section contains a dendrogram of tissue types divided into organs and cell types. The average expression of SOX2 within a specific tissue or a cell type context is visualized as a collection of box plots (Fig. 47).

17.The “Cell Line Expression” section contains a dendrogram of various cell lines organized by the tissue of origin. The plot visualizes the average expression of SOX2 across the cell lines based on data from ARCHS4 (Fig. 48).

Downloading gene expression data from ARCHS4
18.As described in previous steps, after submitting a search within the data search and visualization page, the “Search Results” table includes a download link to an R script that can be used to retrieve the selected samples. Click the download icon to download the script.
19.Open R Studio and copy and paste the R script from the downloaded R file into R Studio.
20.Ensure that the “rhdf5” library is installed. Open the console in R Studio and input the following:
- if (!requireNamespace("BiocManager", quietly = TRUE))
- install.packages("BiocManager")
- BiocManager::install("rhdf5")
21.Now run the R script downloaded from ARCHS4 to produce an expression matrix for the selected samples that were returned from the search. The expression matrix can be used for further analysis; for example, it can be used to compute the average expression of a gene in a specific disease, cell line, or tissue contexts.
Basic Protocol 4: MAKING PREDICTIONS ABOUT GENE FUNCTION WITH PrismExp
PrismEXP is an Appyter (Clarke et al., 2021; Lachmann et al., 2021) that employs machine learning to predict gene function using gene-gene mRNA co-expression correlations from mRNA-sequencing (RNA-seq) data sourced from ARCHS4, a database composed of human and mouse RNA-seq sample gene counts from GEO (Lachmann et al., 2018). The difference between gene function predictions made by PrismExp and the gene function prediction available from the ARCHS4 website is that the ARCHS4 data is divided first into clusters, and then gene-gene correlations are computed for each cluster. 51 correlation matrices are precomputed and stored in the cloud. At runtime, the correlation data is extracted from the cloud storage and a pretrained Random Forest model is applied on the correlation features to rank the level of association of a single gene to all gene sets from a user-specified gene set library.
Necessary Resources
Hardware
- Desktop or a laptop computer, or a mobile device, with a fast Internet connection
Software
- An up-to-date web browser such as Google Chrome (https://www.google.com/chrome/), Mozilla Firefox (https://www.mozilla.org/en-US/firefox/), Apple Safari (https://www.apple.com/safari/), or Microsoft Edge (https://www.microsoft.com/en-us/edge)
Navigating the input form
1.Navigate to the PrismEXP Appyter (https://appyters.maayanlab.cloud/PrismEXP/).
2.The Appyter input form includes a “Gene Selection” section with a field for inputting a gene symbol of interest for which novel functions will be predicted. Additionally, the “GMT Selection” section includes a field for selecting a GMT file from which predictions will be made (Fig. 49). Click the “Upload” button within the “GMT Selection” section to upload a custom GMT file (Fig. 50).


3.Click submit on the Appyter input form, and a Jupyter Notebook with the input parameters will be launched in the cloud.
Gene function predictions
4.A Jupyter Notebook will begin executing in the cloud once the input form is submitted. The notebook includes an option to download the notebook, toggle display of the code, and run the notebook locally. Additionally, a table of contents exists with clickable elements that link to specific sections within the notebook (Fig. 51).
5.Scroll down to the “Load Gene Correlation” section. The Dataframe displays genes that correlate with your query gene in 51 pre-computed correlation matrices from ARCHS4 (Fig. 52).
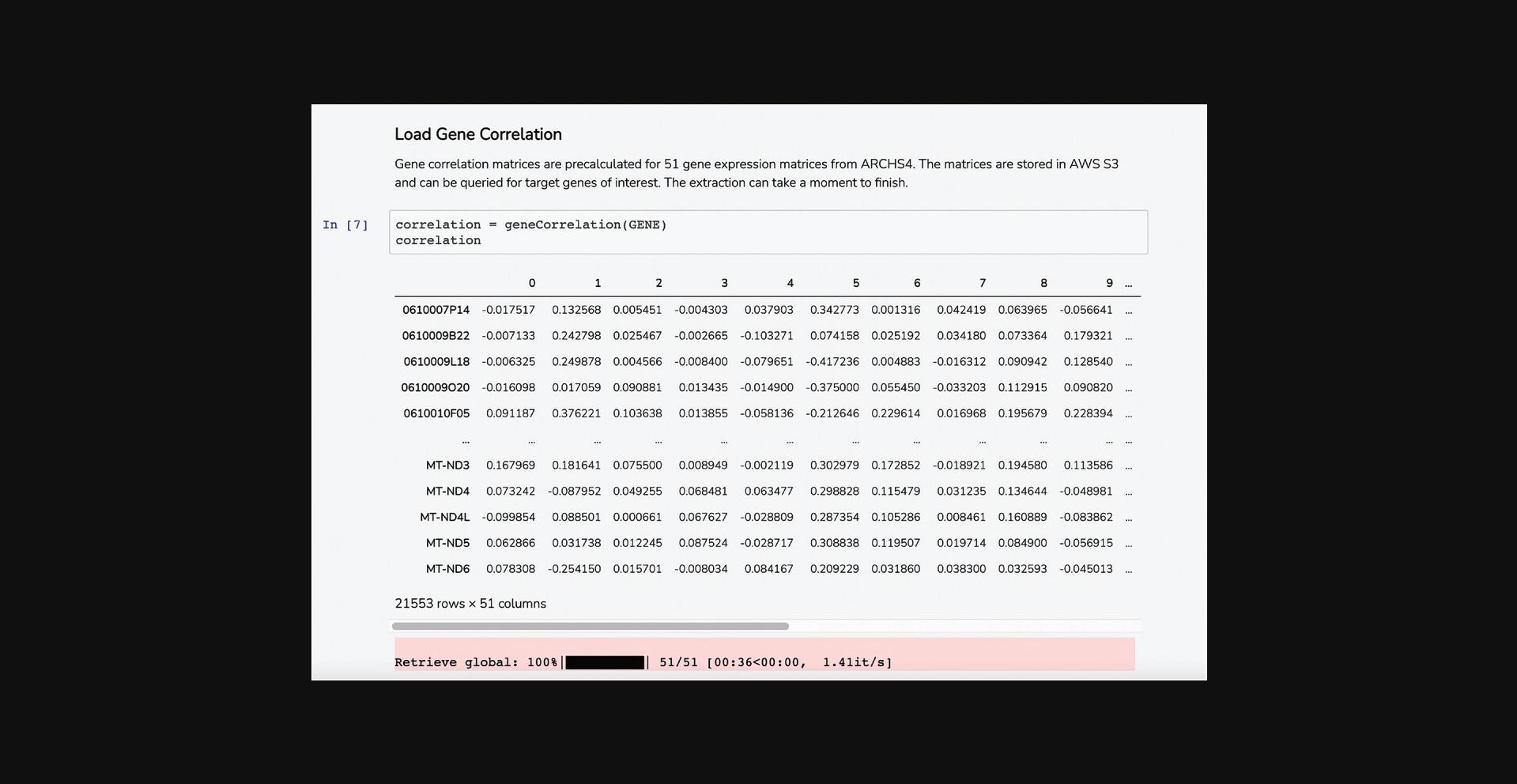
6.Scroll down to the “Avg Correlation Scores” section. This Dataframe displays computed correlation scores to each of the gene set terms from the GMT file based on co-expression values between the query gene and each of the genes included in the gene set (Fig. 53).
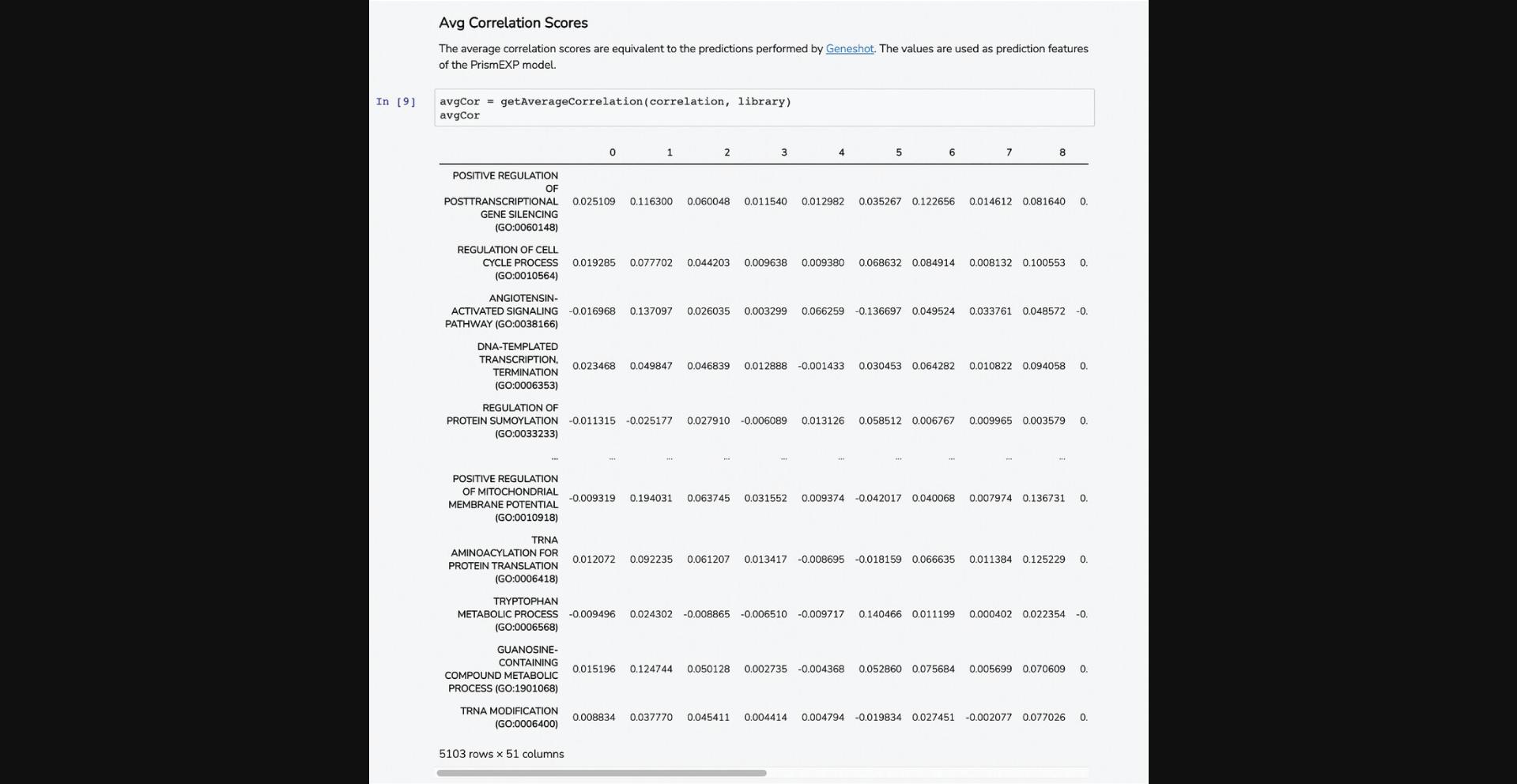
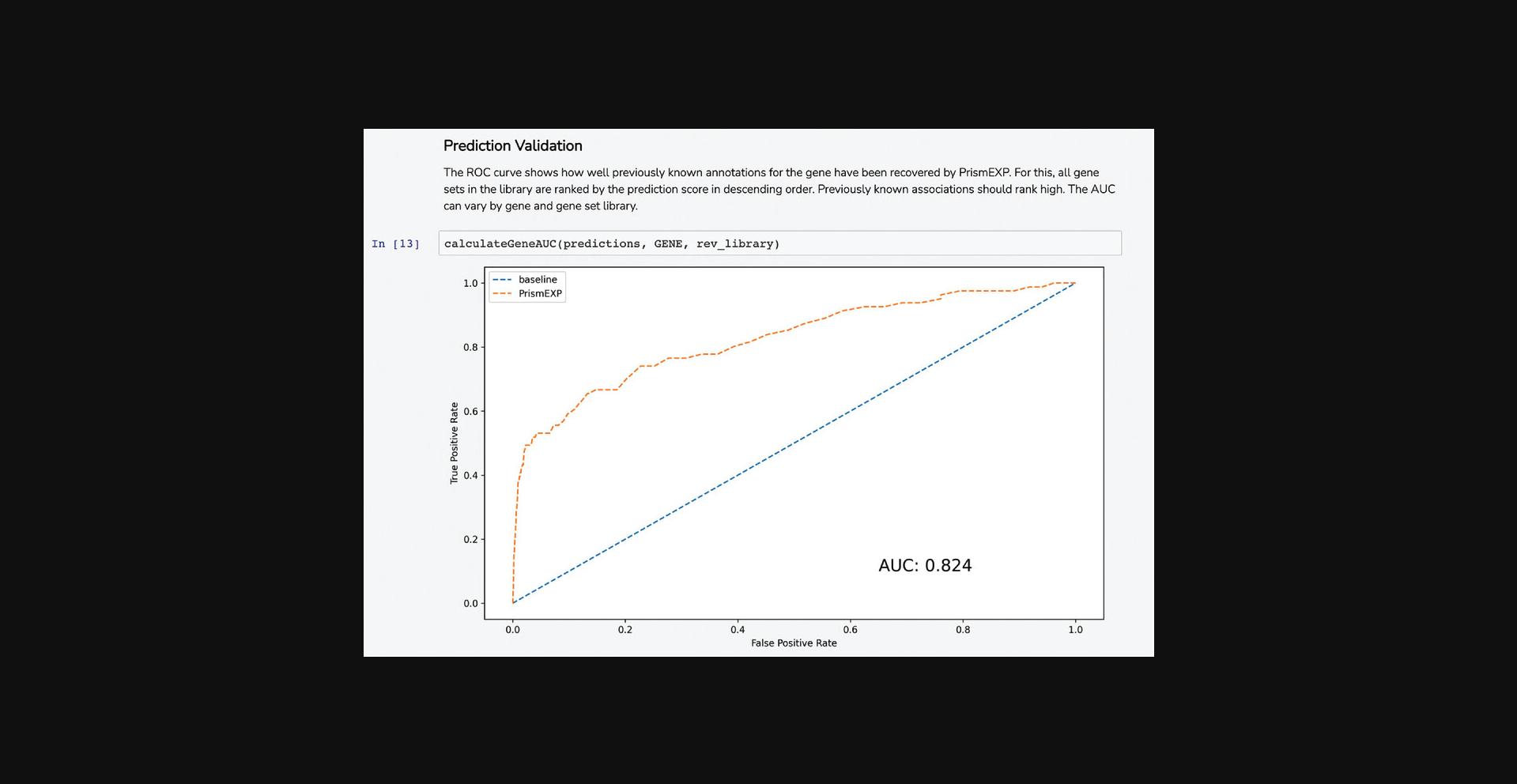
7.The average correlation score matrices are used as the input features for the PrismEXP model. Scroll down to the “Prediction Validation” section. The ROC curve displayed in this section characterizes how well the known annotations for this gene were recovered by the PrismEXP model (Fig. 54).
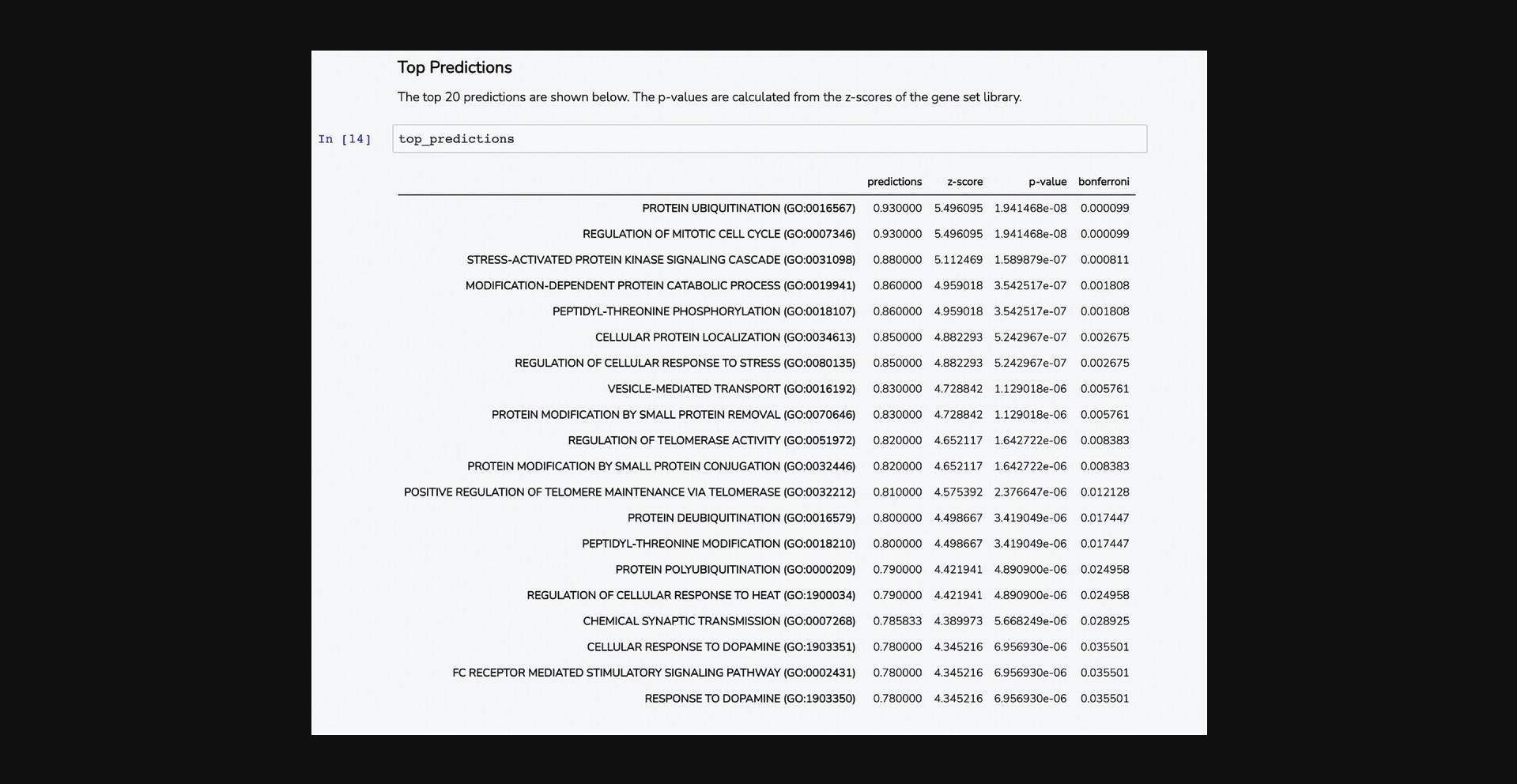
8.Scroll down to the “Top Predictions” section. The Dataframe displays the top 20 gene set terms that the query gene is predicted to be associated with. The table displays the prediction score from the model, z -score, p -value, and Bonferroni corrected p -value (Fig. 55).
9.Scroll down to the “Download Files” section. Click on the appropriate link to download the prediction table or ROC curve in .pdf or .png format (Fig. 56).
Basic Protocol 5: USING GENESHOT TO ILLUMINATE KNOWLEDGE ABOUT UNDER-STUDIED TARGETS
Geneshot is a search engine for querying biomedical terms to retrieve lists of genes most associated with the term from PubMed ID (PMID) co-mentions (Lachmann et al., 2019). To convert search terms to genes, Geneshot uses one of two resources: GeneRIF and AutoRIF. Both GeneRIF and AutoRIF are text files documenting gene-PubMed ID associations. These associations are used to rank genes for a query term based on the number of co-mentions. Geneshot further prioritizes other related genes based on co-occurrence and co-expression matrices with the genes associated with the term from the literature. Additionally, Geneshot includes a gene function prediction feature that prioritizes novel gene set membership for a query gene based on co-occurrence or co-expression.
Necessary Resources
Hardware
- Desktop or a laptop computer, or a mobile device, with a fast Internet connection
Software
- An up-to-date web browser such as Google Chrome (https://www.google.com/chrome/), Mozilla Firefox (https://www.mozilla.org/en-US/firefox/), Apple Safari (https://www.apple.com/safari/), or Microsoft Edge (https://www.microsoft.com/en-us/edge)
- Text editor or development environment of choice, such as Visual Studio (https://visualstudio.microsoft.com/vs/); most updated version of Python (https://www.python.org/downloads/); and Python requests library (https://requests.readthedocs.io/en/master/user/install/)
PubMed query
1.Navigate to the Geneshot homepage (https://maayanlab.cloud/geneshot/).
2.The PubMed Query page includes an input form for submitting search terms (Fig. 57). The top search bar is for terms that the search should include, whereas the lower search bar is for terms that should be omitted from the search. Toggle the size of the gene set that will be used to make further predictions with the “Top Associated Genes to Make Predictions” filter. Use the toggle bar to switch between AutoRIF and GeneRIF (Maglott, Ostell, Pruitt, & Tatusova, 2011) as the underlying databases for gene-PMID associations. Click “Wound Healing” in the example section of the input form to launch a search (Fig. 58).
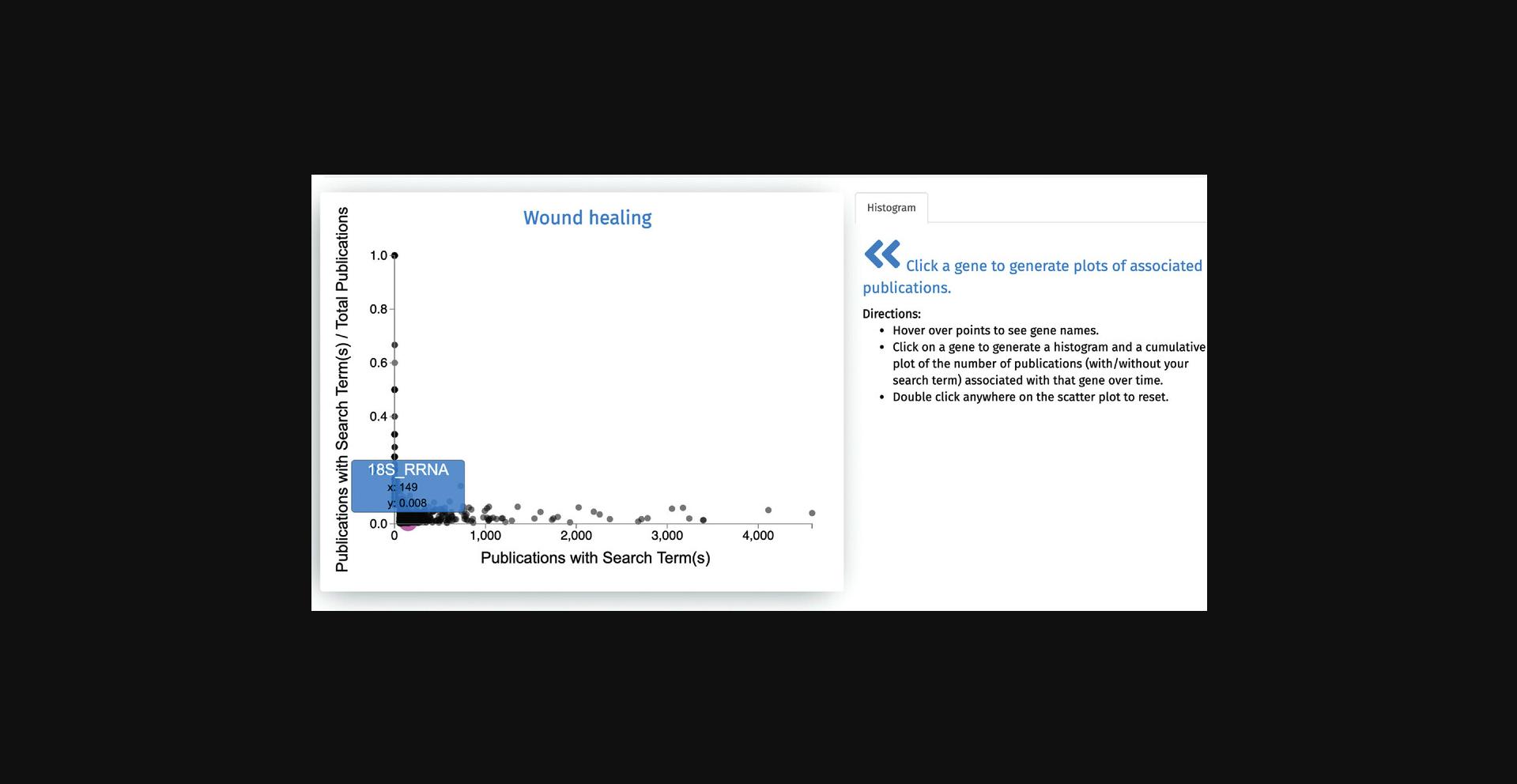
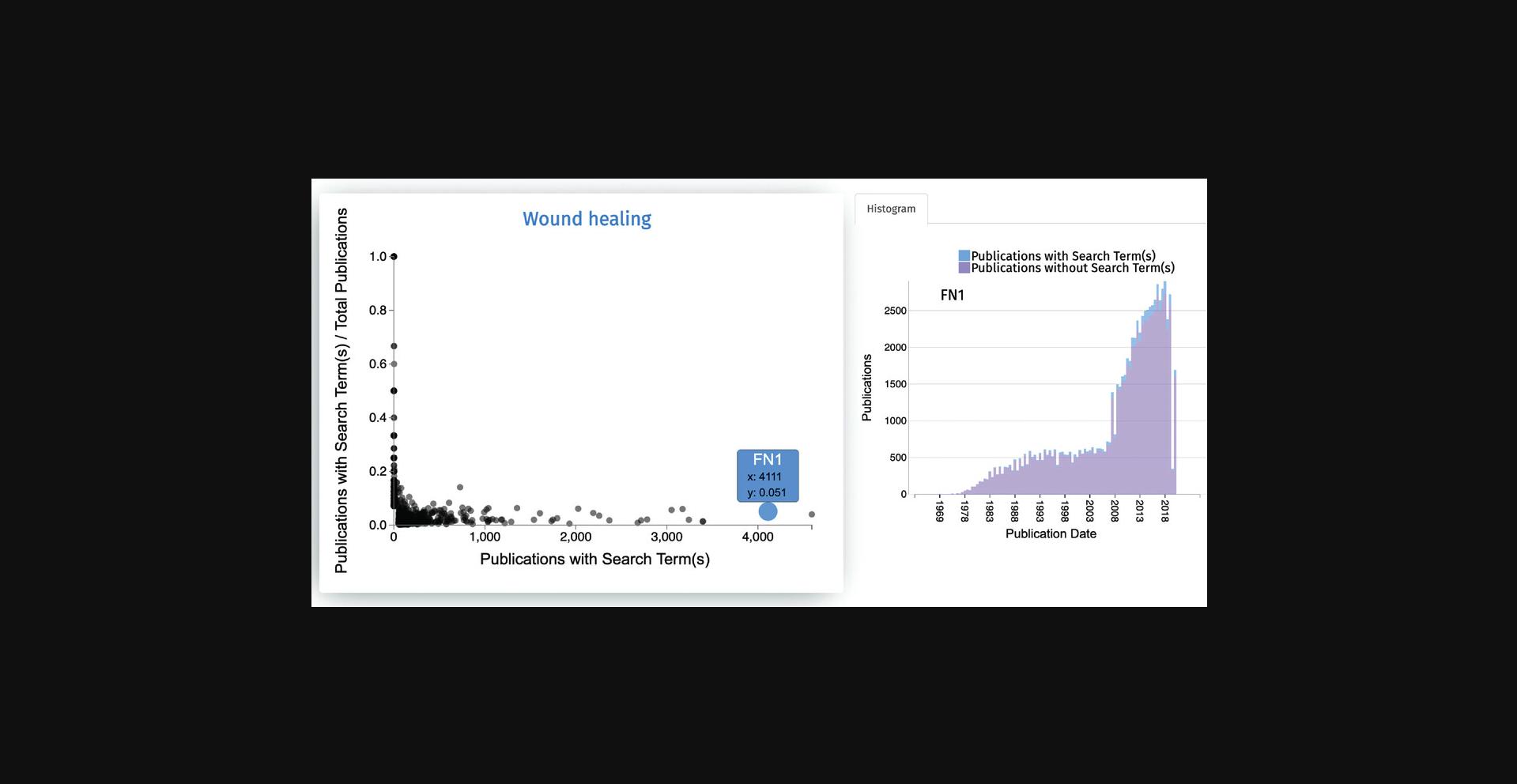
3.The first output from the search is a scatter plot of all genes associated with “wound healing” (Fig. 59). The x -axis of the scatter plot displays the counts of Publications with Search Term, and the y -axis shows the fraction of Publications with Search Term/Total Publications. Hover over any point on this plot to display the gene name and its corresponding x and y values.
4.Clicking on any of the points in the scatter plot generates a histogram displaying the association of the gene with the search terms based on literature co-mentions over time (Fig. 60). The number of publications for the selected gene that do not match the search term is displayed as pink bars, while the number of publications matching the search term and the gene is displayed as blue bars.
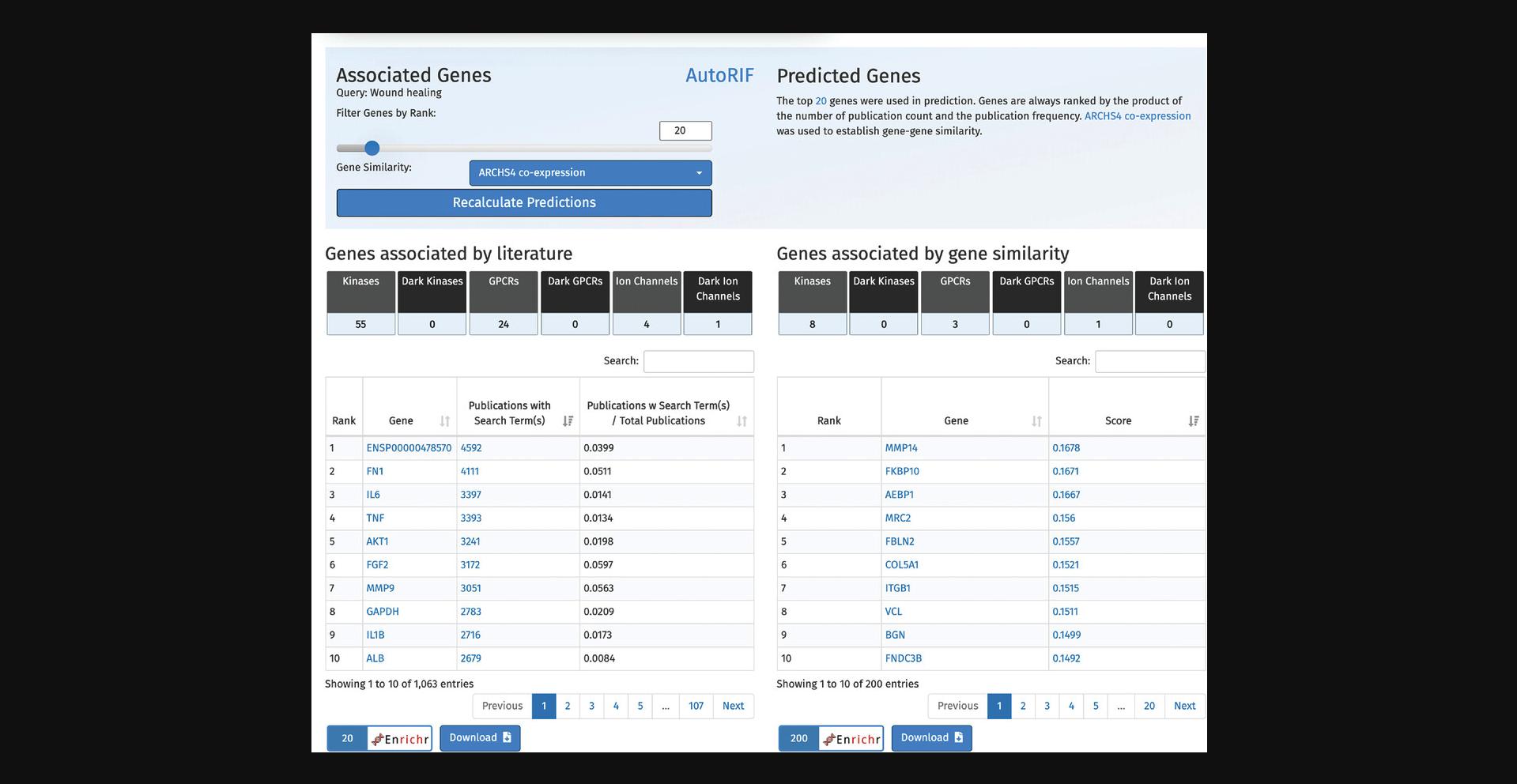
5.Scroll down to view the tables of associated genes and predicted genes (Fig. 61). The left table includes the top genes associated with “wound healing” ranked by number of PubMed ID co-mentions. The right table shows the top 200 genes predicted to be associated with “wound healing” based on co-expression with the top 20 genes from the associated table. Each of the tables include a row of buttons that, when clicked, filter the genes from each table into a specific gene family. Additionally, the genes from each table can be submitted to Enrichr for gene set enrichment analysis, and each table itself can be downloaded.
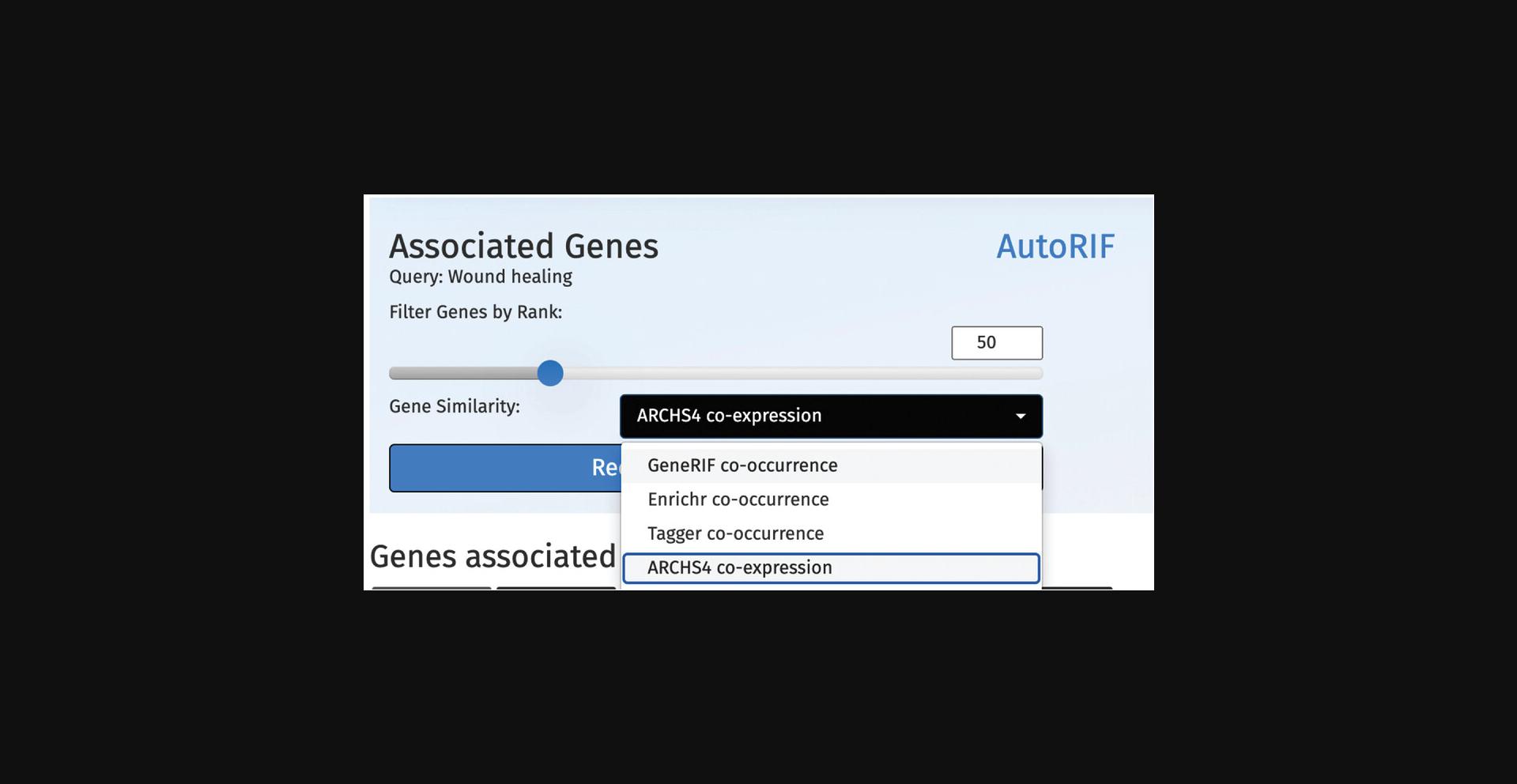
6.To recalculate the predictions, use the drop-down menu above the associated table to select a new gene-gene similarity matrix and increase or decrease the associated gene set size using the scroll bar. Click the “Recalculate Predictions” button to update the prediction table (Fig. 62).
Gene function predictions
7.Navigate to the Gene Function Prediction page by clicking the corresponding link within the navigation bar at the top of the page. This page includes an input form for selecting a gene of interest, Enrichr gene set library from which gene functions will be sourced from, and a gene-gene similarity matrix from which predictions will be calculated (Fig. 63). By using functional prediction by association, the input gene can be predicted to be a member of gene sets. Click the example to launch a query.
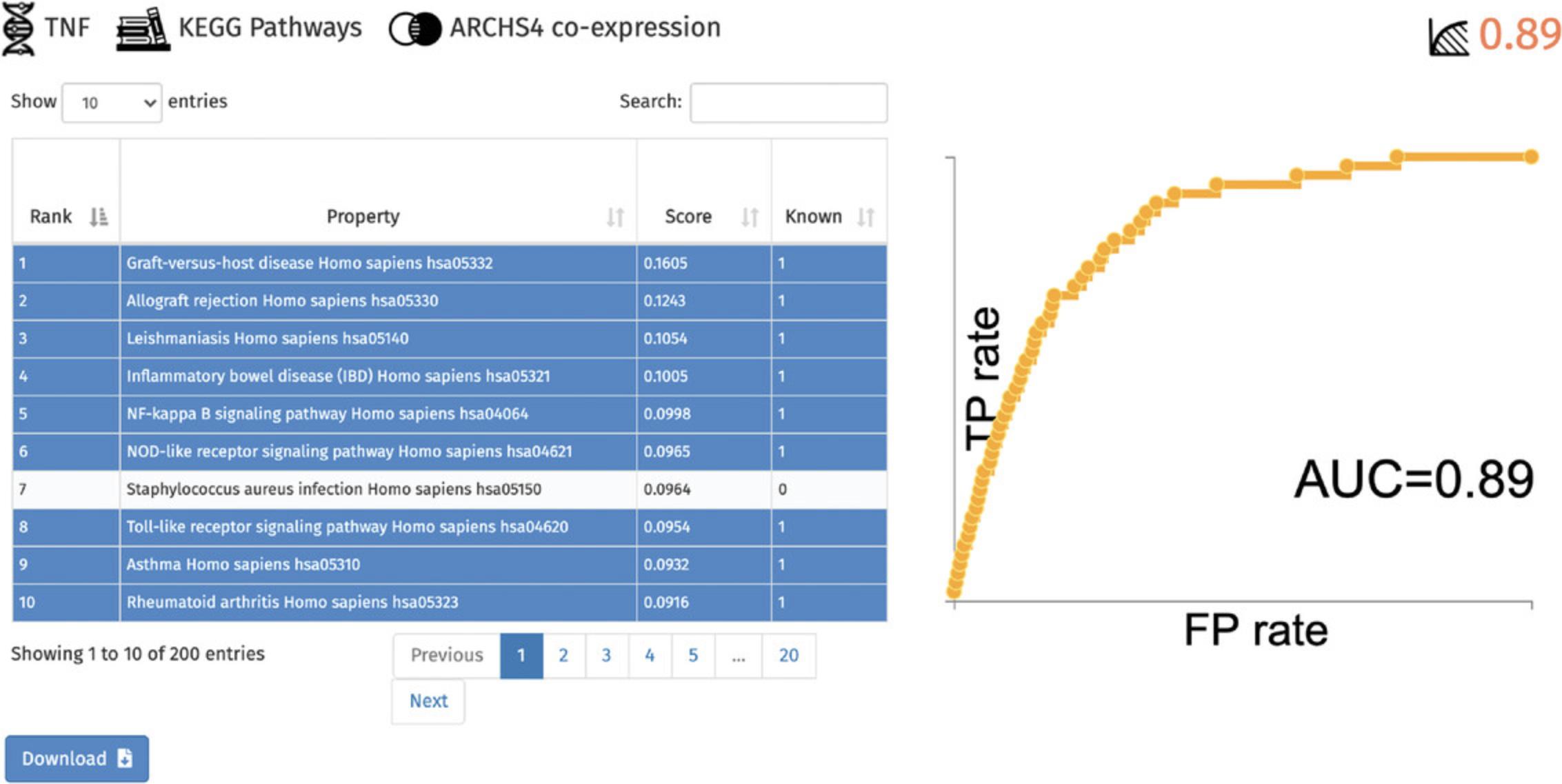
8.A table of the top predicted functions and ROC curve of prediction performance are generated (Fig. 64). Known associations within the table are highlighted in blue, whereas previously unknown associations are not highlighted. The table is available for download.
Gene set augmentation
9.Navigate to the Gene Set Augmentation page by clicking the corresponding link within the navigation bar at the top of the page. The input form on this page includes a text box for pasting a gene set for augmentation, a drop-down menu of gene-gene similarity matrices from which predictions will be calculated, and a toggle bar for switching between GeneRIF and AutoRIF for retrieving publication counts for each gene (Fig. 65).
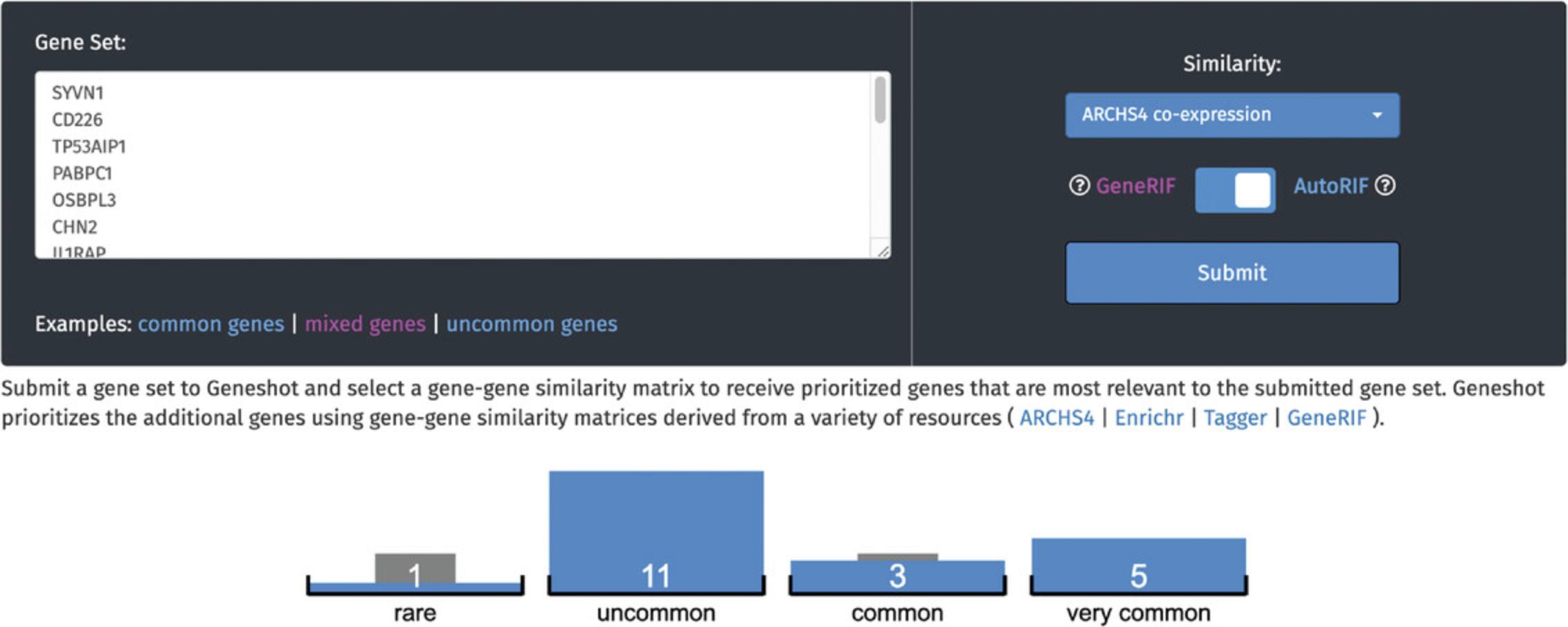
10.Click on the “mixed genes” example to submit a query. The input genes are first sorted into quantiles based on their novelty in the literature (Fig. 66).
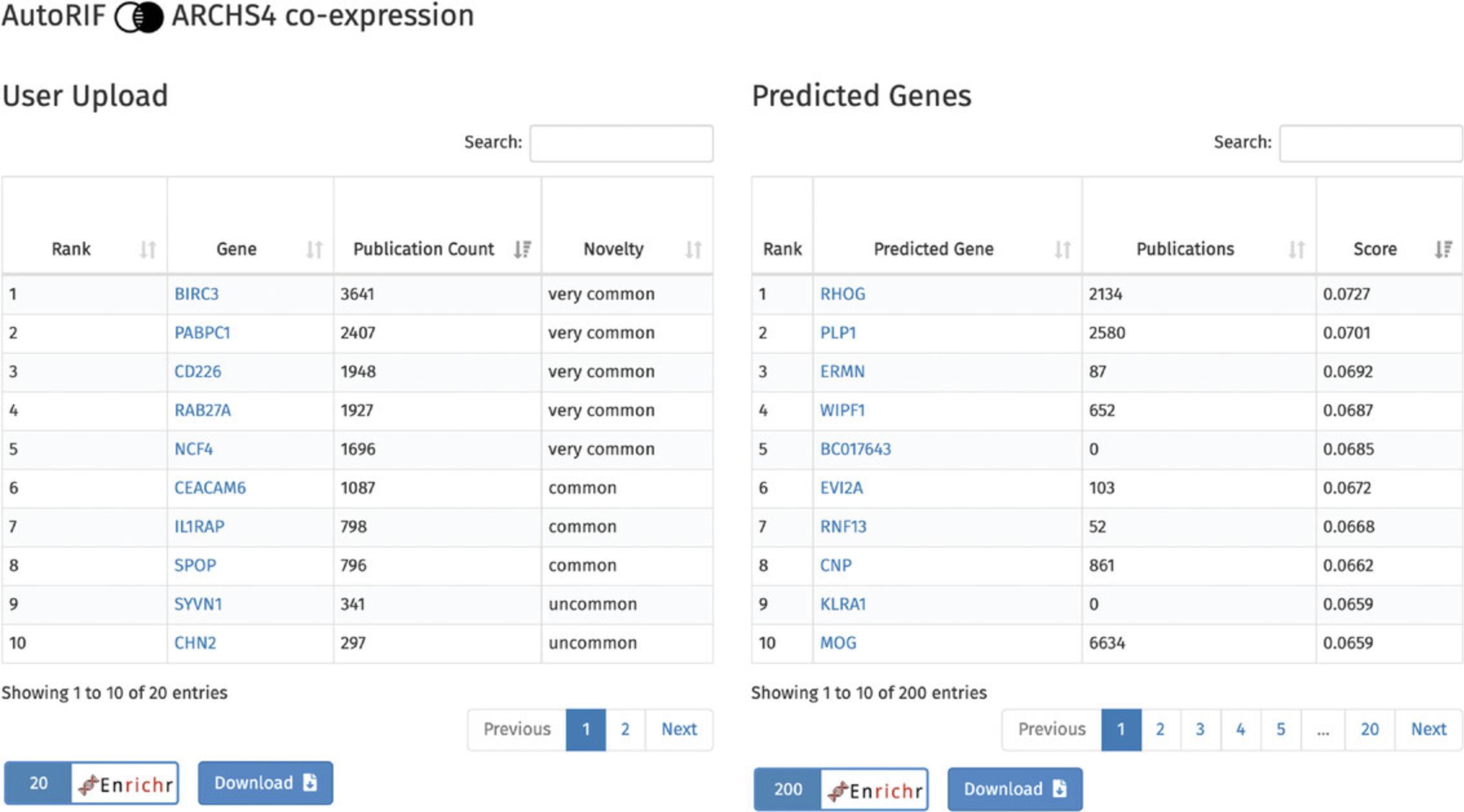
11.Scroll to the bottom of the page where there is a table with the submitted genes on the left, and a table of genes predicted to be associated with the input genes based on the selected gene-gene similarity matrix, in this case ARCHS4 co-expression, on the right (Fig. 67). The “user upload” table ranks the genes by the amount of PubMed abstracts they are mentioned in, along with their novelty. The predicted genes table ranks genes by their similarity score with the input gene set. Genes from both tables can be submitted to Enrichr for gene set enrichment analysis, and each table can be downloaded.
Geneshot API example
12.Open a new or existing Python code file. Import the JSON and requests libraries at the top of the file as follows.
- import json
- import requests
13.Call the requests.post method to send a POST request to the URL. The payload variable contains the parameters that are sent to the API endpoint specified in GENESHOT_URL. In this case the endpoint is /search and the parameters are rif, which specifies whether AutoRIF or GeneRIF is used as the association file, and term, which specifies the query term for the search.
- GENESHOT_URL = ˈhttps://maayanlab.cloud/geneshot/api/searchˈ
- payload = {"rif": "generif", "term": "hair loss"}
- response = requests.post(GENESHOT_URL, json=payload)
- data = json.loads(response.text)
- print(data)
14.Use the json.loads method to view the response as a JSON object containing all genes related to the query term.
- {
- "PubMedID_count": 34412,
- "gene_count": {
- "ABCC6P2": [
- 1,
- 0.25
- ],
- "ABI3": [
- 2,
- 0.125
- ],
- ...
- },
- "query_time": 1.121943712234497,
- "return_size": 298,
- "search_term": "hair loss"
- }
For more information on using the various Geneshot API endpoints, please refer to the API documentation (https://maayanlab.cloud/geneshot/api.html).
Basic Protocol 6: EXPLORING UNDER-STUDIED TARGETS WITH TIN-X
TIN-X (Target Importance and Novelty eXplorer; Cannon et al., 2017) is an informatics workflow, REST API, and web application used to identify, visualize, and explore protein-disease associations. TIN-X is based on text mining data processed from scientific literature. TIN-X visualizations plot information for protein-disease associations along two axes, specifically “novelty” and “importance.” Briefly, Novelty is used to estimate the scarcity of publications about a protein target, whereas Importance estimates the strength of the association between that protein and a specific disease.
Necessary Resources
Hardware
- Desktop or a laptop computer, or a mobile device, with a fast Internet connection
Software
- An up-to-date web browser such as Google Chrome (https://www.google.com/chrome/), Mozilla Firefox (https://www.mozilla.org/en-US/firefox/), Apple Safari (https://www.apple.com/safari/), or Microsoft Edge (https://www.microsoft.com/en-us/edge)
Browse diseases
1.Navigate to the TIN-X web app (https://www.newdrugtargets.org/).
2.The default TIN-X mode, “Browse Diseases,” (upper-left) starts with the Disease Ontology (DO; Schriml et al., 2019; see Internet Resources). The DO hierarchy can then be navigated using the left panel (Fig. 68). Given this hierarchical nature, a larger number of target-disease associations can be text-mined from biomedical literature for higher-level terms (e.g., N = 13405 for “nervous system disease”), as opposed to child terms (e.g., N = 9733 for “neurodegenerative disease,” N = 4587 for “Synucleinopathy,” N = 4587 for “Parkinson's Disease”) or leaf terms (e.g., N = 227 for “Early Onset Parkinson's Disease”).
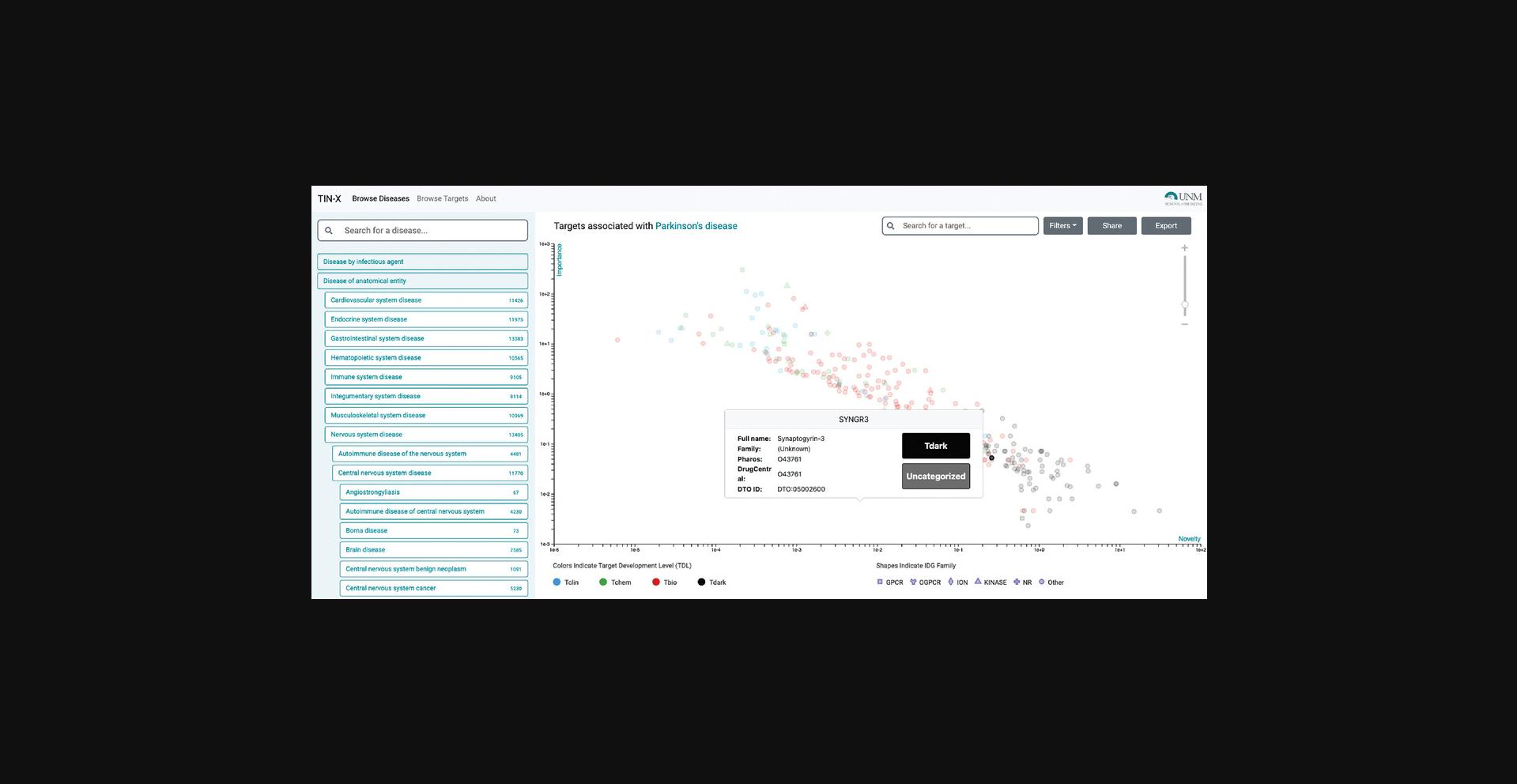
3.Searching by disease name is also supported. Targets with stronger associations (higher Importance) are in the upper part of the plot, while targets with a higher number of publications (lower Novelty) are located on the left side of the plot. Points situated in the upper-right area of the plot (if any) are most likely to be of interest, as they are located at the Pareto frontier, i.e., targets for which a large number of published papers mentioning that target also mention the selected disease.
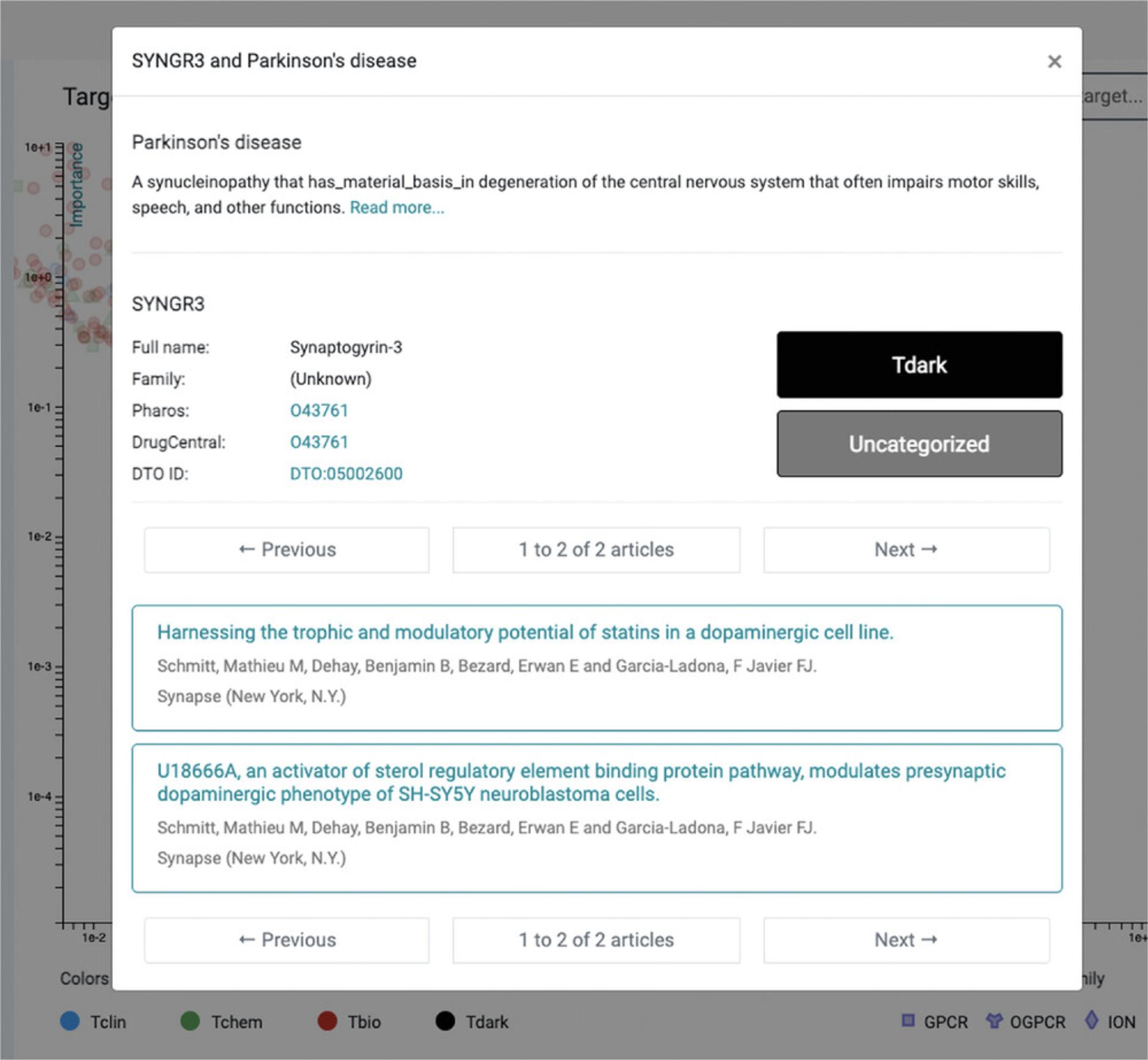
4.Targets are colored by Target Development Levels, and can be filtered as such (Tclin/Tchem/Tbio/Tdark). They can also be filtered by protein superfamily (e.g., kinases). Upon selecting a protein, links to both Pharos and DrugCentral are provided for that protein (Fig. 69); selecting the titles allows the user to navigate through abstracts or to examine the document of interest in PubMed (additional clicks are required).
5.Once the desired level of granularity for diseases is reached, the user can examine target-disease associations, which are plotted along the Novelty-Importance axes in log-log format. To reach “Parkinson's Disease,” one must click Disease of anatomical entity → Nervous System Disease → Neurodegenerative disease → Synucleinopathy → Parkinson's Disease.
6.A highly-ranked gene associated with Parkinson's Disease is “Synaptogyrin-3” (SYNGR3) and is classified as Tdark (Fig. 69). While the exact function of SYNGR3 is unknown, there is recently published evidence that SYNGR3 encodes for a synaptic vesicle protein that interacts with a dopamine transporter (Egaña et al., 2009). The most novel association (lowest Importance) is for “Tripartite motif-containing protein 10” (TRIM10), which is supported by one genome-wide association study (Witoelar et al., 2017) focused on the overlap between Parkinson's Disease and autoimmune diseases.
7.Both the “Browse Diseases” and the “Browse Targets” exploratory modes support an interactive way to manipulate the number of points displayed on the scatter plot. To change the number of plotted points, simply go to the top right side of the panel, where a vertical bar is placed between a “+” and a “-” sign. Sliding this bar up or down increases or decreases the number of visible points within the plot. By default, 300 or fewer points are plotted. Thresholds are defined by non-dominated solution (NDS) ranking, a.k.a. Pareto frontier, meaning that all hidden points are inferior to those visible in one or both variables.
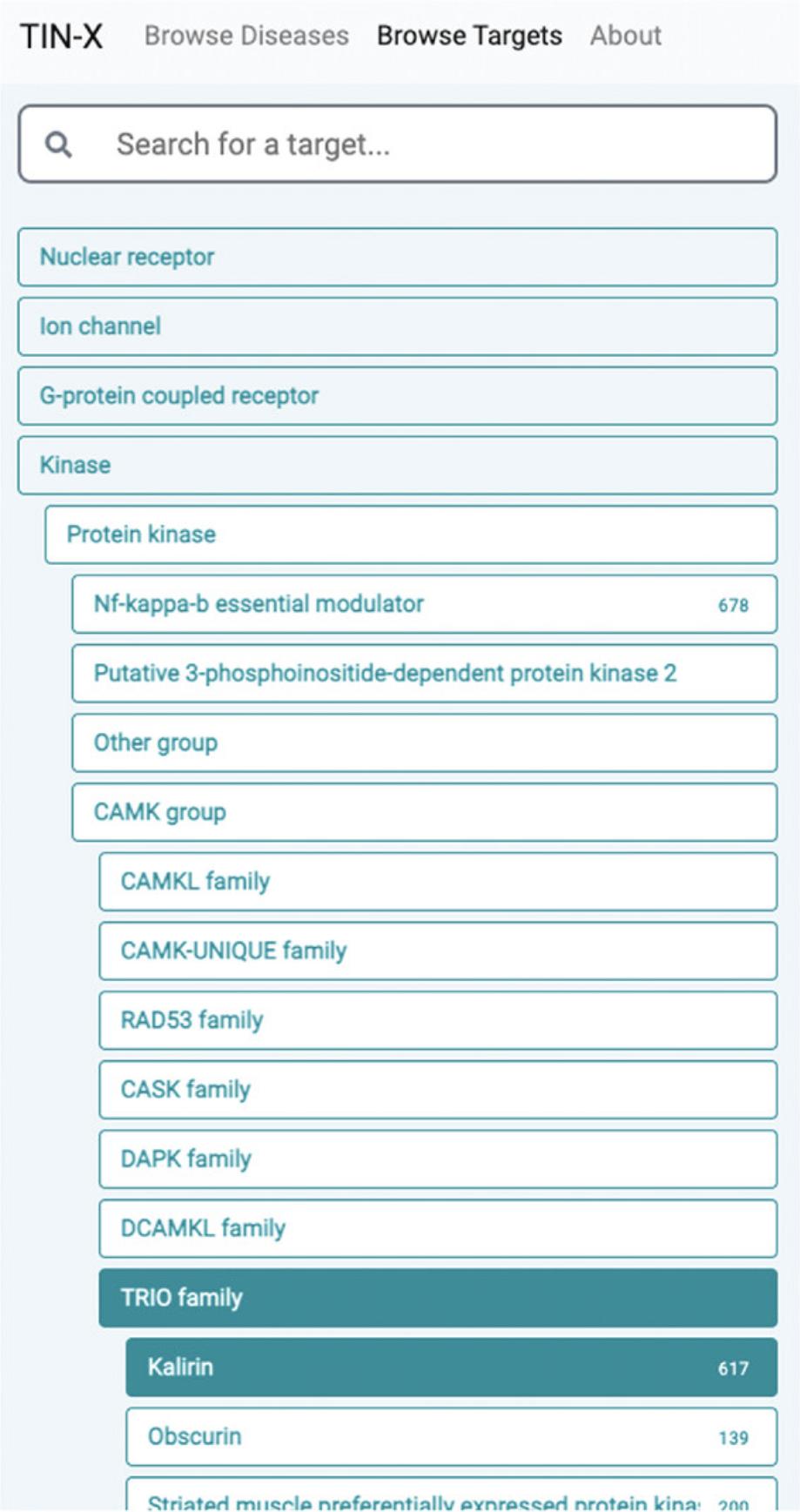
Browse targets
8.From the upper left menu, “Browse Targets” can be selected. The Drug Target Ontology (Lin et al., 2017) hierarchy becomes visible, and can be navigated from the left panel (Fig. 70). For each protein, Diseases are plotted with log–log Importance–Novelty axes and color-coded according to the top hierarchical Disease Ontology term (e.g., diseases of anatomical entity, diseases of metabolism, etc.).
9.Searching by target name is supported. Diseases with stronger associations (higher Importance) are in the upper part of the plot, while diseases with a higher number of publications (lower Novelty) are on the left side of the plot. Diseases that are likely of most interest are plotted in the upper-right area of the plot (Fig. 71).
10.The plot, however, remains target-centric. Upon clicking on a point, the disease name and protein name are displayed, with appropriate links to Pharos and DrugCentral (Fig. 72).
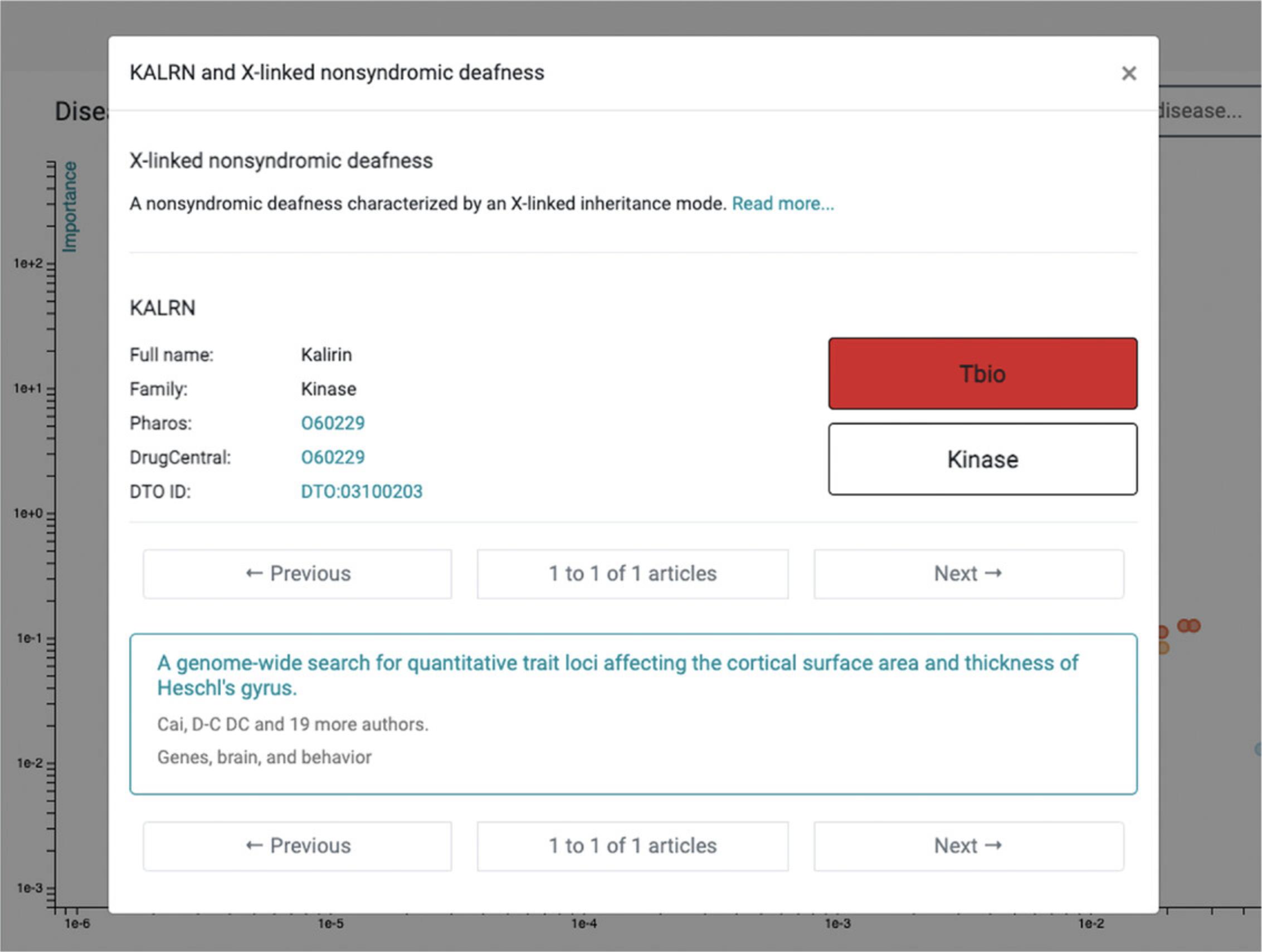
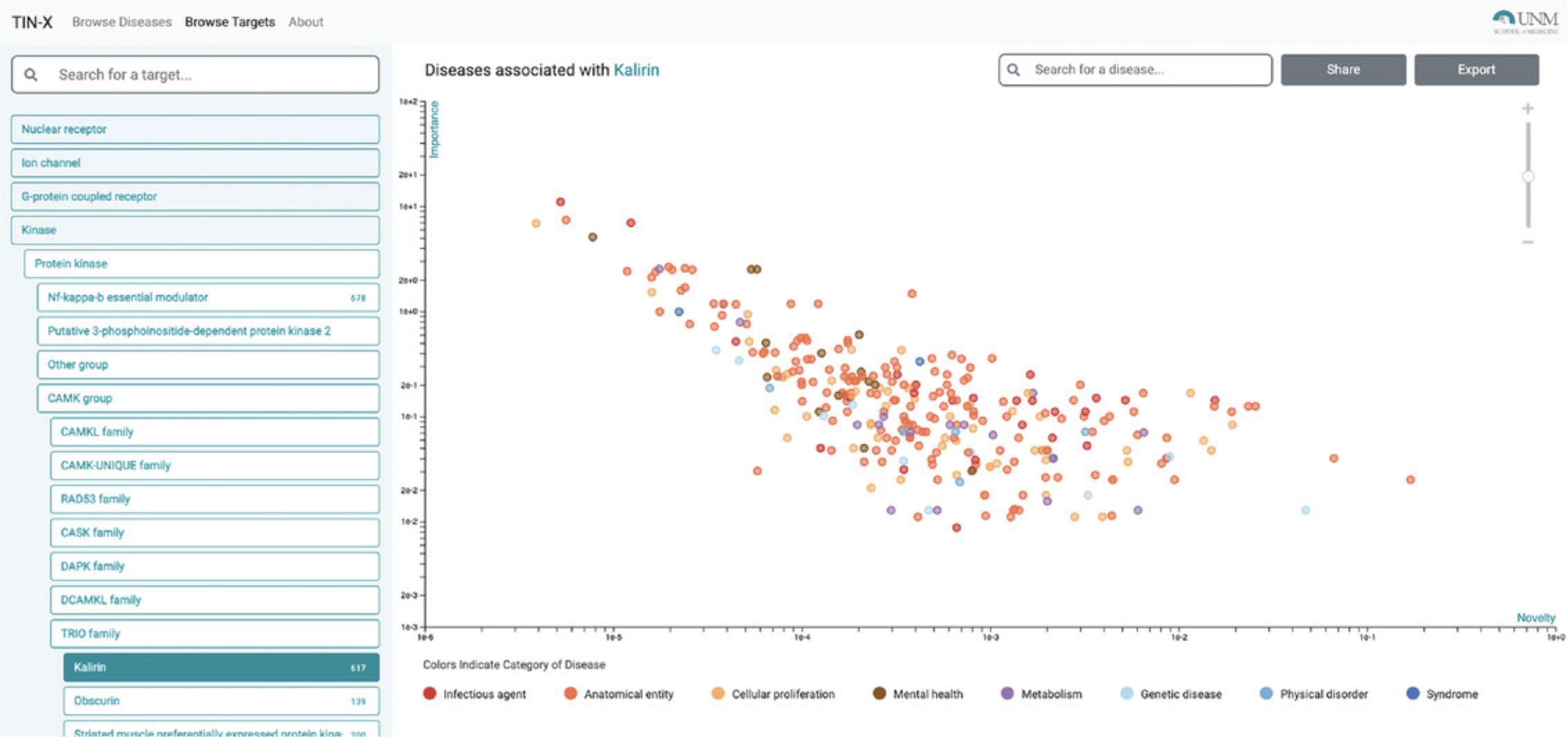
11.When selecting a target family (e.g., kinase), the user can drill down to the desired level of granularity before examining disease associations for a specific protein. Starting from Kinase, for example, the user must click Protein kinase → CAMK group → TRIO family → Kalirin, before diseases associated with Kalirin (KALRN) are displayed (Fig. 70).
12.The top disease (highest Importance, lowest Novelty) associated with KALRN is “disease by infectious agent,” followed by “psychotic disorder.” We recommend repeated scrolling before identifying a leaf term corresponding to the Disease Ontology (see Internet Resources). For example, next to “psychotic disorder” is “schizophrenia” (a child term); this association is supported by 26 publications, including Miller et al. (2017). The most novel association (lowest Importance) is for “X-linked nonsyndromic deafness” (Fig. 72), supported by Cai et al. (2014). This association is genuine, as the gene name (KALRN) is mentioned in the abstract, in relation to the rs333332 SNP.
Sharing and downloading data
13.Whether in “Browse Diseases” or “Browse Targets” mode, the user can share data in two ways. First, for any given plot, the specific URL (universal resource locator) for that visualization can be copied and shared with third-party users. This can be done by clicking on the “Share” button. Second, the data can be exported (in comma-separated value format), and thus archived or post-processed with third-party software. Exported data includes Novelty and Importance scores, in addition to Disease names and identifiers in the “Browse Targets” mode, as well as Target names and identifiers in the “Browse Diseases” mode, respectively.
Basic Protocol 7: INTERACTING WITH THE DrugCentral USER INTERFACE
DrugCentral is an online compendium (Ursu et al., 2017) centered on “active pharmaceutical ingredients” and their link to “pharmaceutical products.” DrugCentral distills relevant information from “pharmaceutical product” (or formulation) package inserts; while these are frequently referred to as “drugs” by patients and medical practitioners, herein we reserve the term “drugs” for “active pharmaceutical ingredients.” All data, including downloads, related to DrugCentral can be accessed at its designated web portal (https://drugcentral.org/). DrugCentral provides information on active ingredients, chemical entities, pharmaceutical products, drug mode of action, medical uses (indications, contra-indications and off-label uses), and pharmacologic action, as well as adverse events (Ursu et al., 2019). As of 2021, DrugCentral (Avram et al., 2021) separately stores adverse events for women and men, and provides regulatory information extracted from the FDA Orange Book (see Internet Resources). DrugCentral is current (as of the date of the release) with regulatory approvals from the United States (US FDA), the European Union (EMA), Japan (PDMA) and, more recently, some drugs approved in China and Russia. Limited information on drugs that have been discontinued or withdrawn is available, particularly for drugs approved outside the U.S. when package inserts and relevant information are not in English.
Necessary Resources
Hardware
- Desktop or a laptop computer, or a mobile device, with a 100 Mbps or higher (fast) Internet connection
Software
- An up-to-date web browser such as Google Chrome (https://www.google.com/chrome/), Mozilla Firefox (https://www.mozilla.org/en-US/firefox/), Apple Safari (https://www.apple.com/safari/), or Microsoft Edge (https://www.microsoft.com/en-us/edge)
Queries supported by DrugCentral
1.Navigate to the DrugCentral portal (https://drugcentral.org/).
2.The main DrugCentral search bar supports three types of queries: drug, target, and disease. Each of these will filter and prioritize results according to a four-level ranking system ordered from highest to lowest, as follows:
-
Query term matching drug name (or synonyms) mechanism of action target, or drug indication (see below).
-
Query term matching disease term in drug contraindications or off-label uses, targets listed in drug bioactivity profiles (not MoA targets), or pharmacologic action descriptions.
-
Query term matching the short drug description text.
-
Query term matching full text in the FDA drug labels processed from DailyMed (Fig.73).
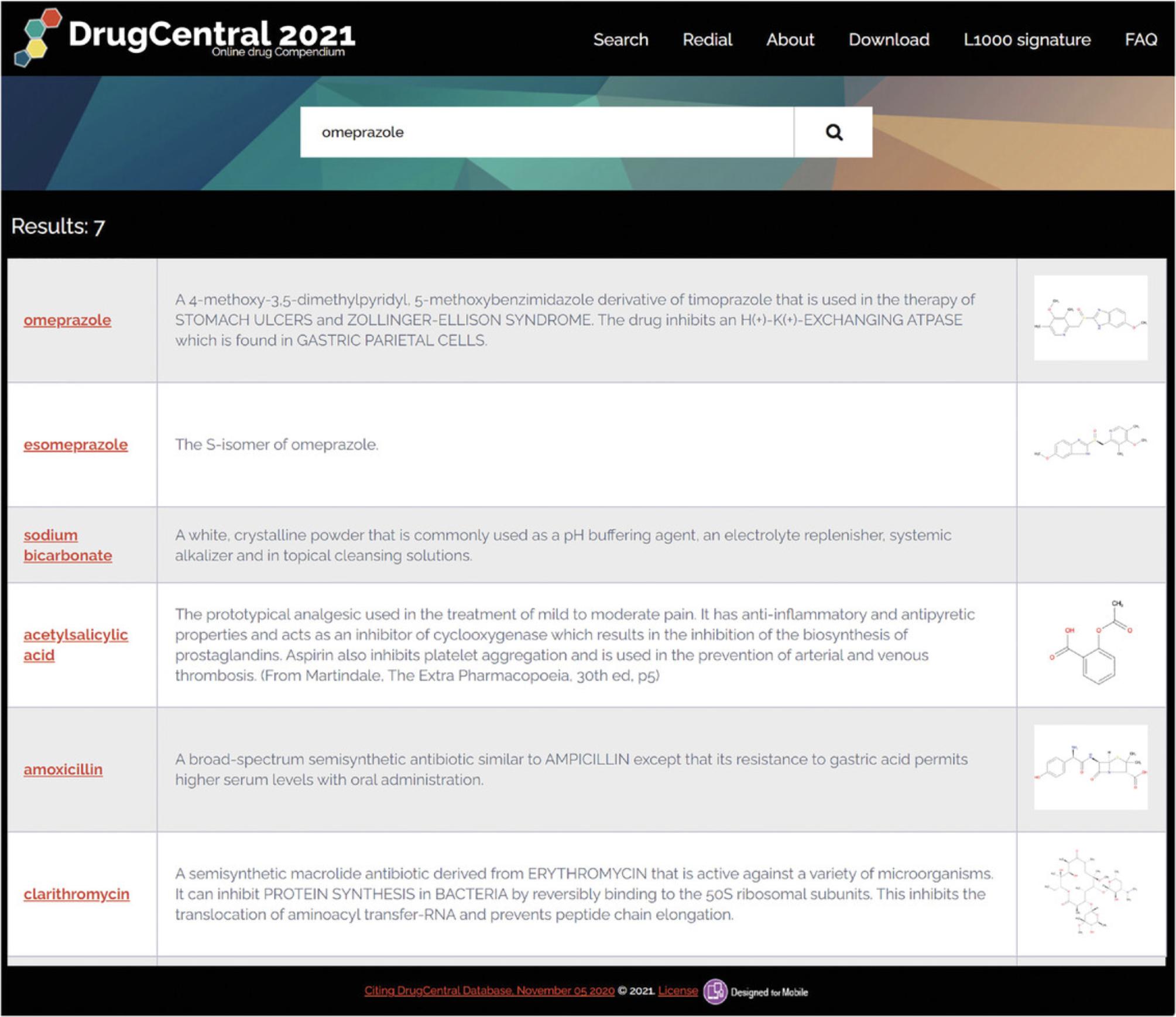
3.For example, drug query results are sorted to display active ingredients first (e.g., omeprazole), followed by related ingredients (e.g., esomeprazole) and by other active ingredients that are co-formulated with the queried substance into pharmaceutical products. A query by brand name (e.g., Prilosec) includes other antacids such as sodium bicarbonate, antibiotics such as amoxicillin and clarithromycin (co-prescribed with omeprazole to treat stomach ulcers caused by Helicobacter pylori), as well as acetyl-salicylic acid, which is combined with omeprazole for the prevention of stroke. (Fig. 74)
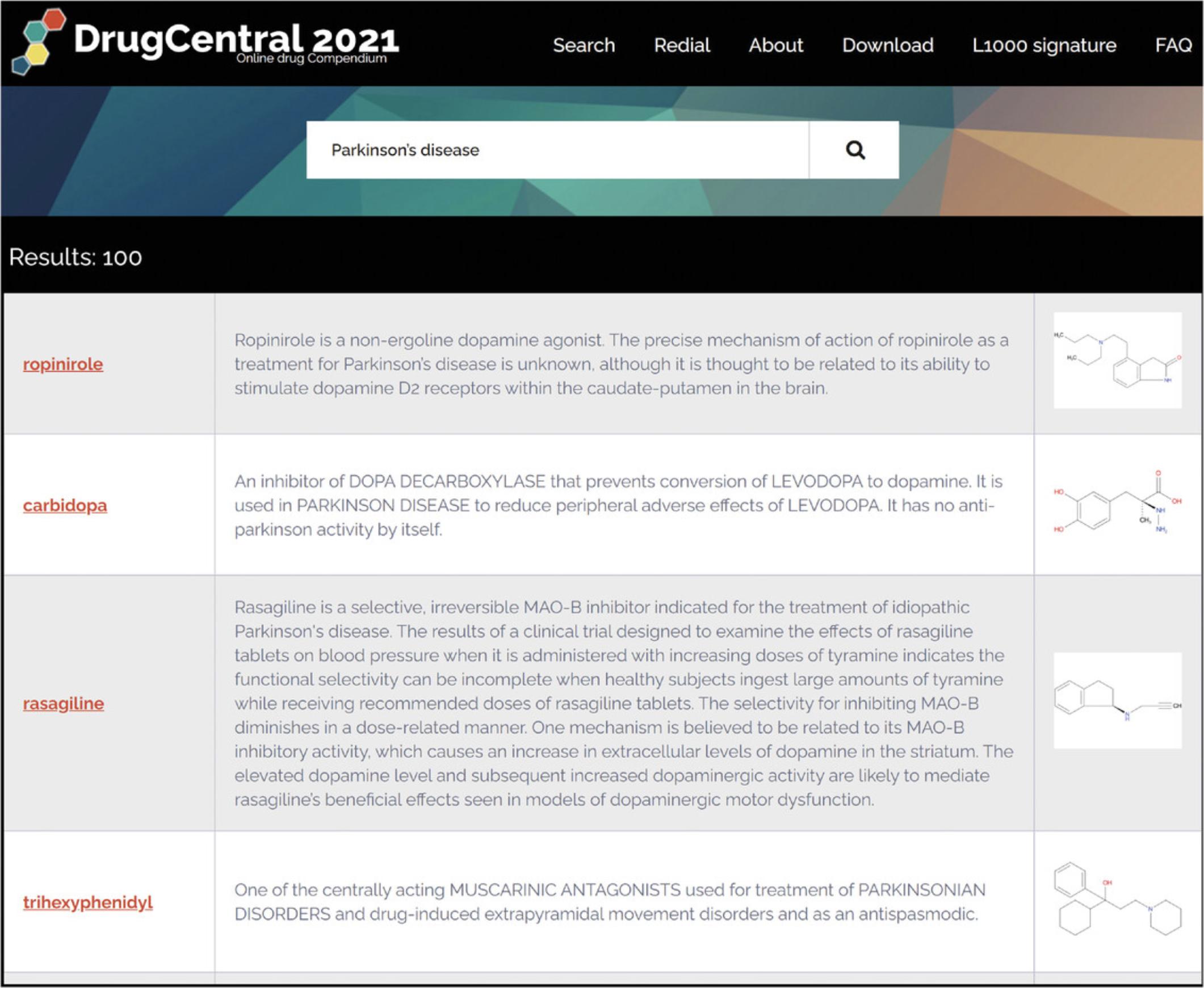
4.Disease names are mappable to multiple terminologies such as Disease Ontology, MeSH, SNOMED-CT, and MedDRA. Disease term queries first retrieve indications, followed by off-label and contraindications, then other sections (e.g., side effects) that contain medical/disease terms. For example, the query “Parkinson's disease” (PD) first lists drugs indicated for PD (e.g., ropinirole), followed by drugs indicated in complications of PD (e.g., fludrocortisone is indicated for the PD-associated orthostatic hypotension), then by drugs that list PD as side-effect (e.g., dimenhydrinate) (Fig. 75).
5.Target name queries support input as text (e.g., “muscarinic m1”), gene symbol (CHRM1), or UniProt (P11229) and SwissProt (ACM1_HUMAN) identifiers. It is recommended to use the exact target names adopted by UniProt, though gene/protein identifiers are preferred.
Queries supported by DrugCentral: REDIAL
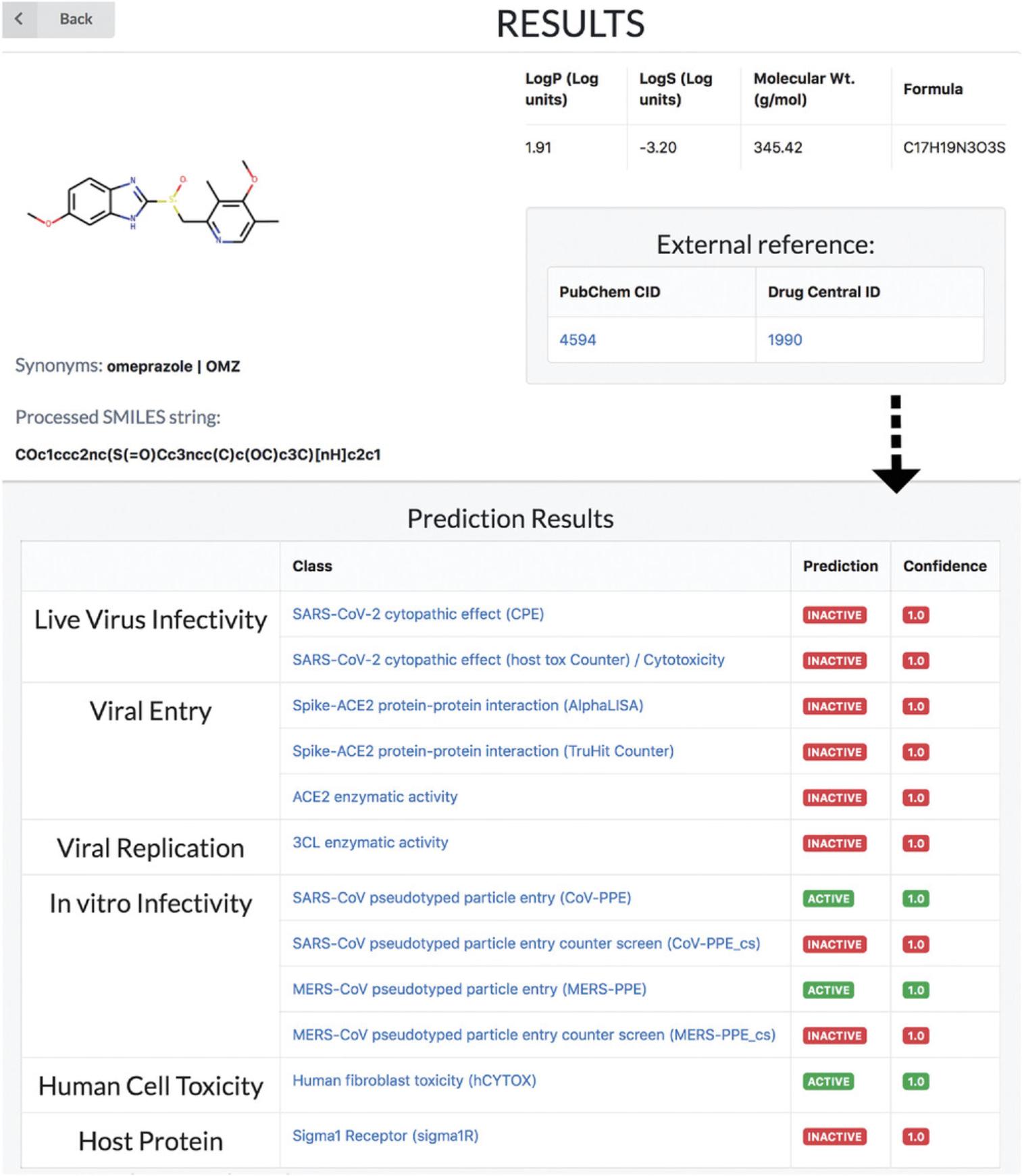
6.Given its basic science focus, the machine-learning-based REDIAL-2020 platform (KC et al., 2021), which is also part of DrugCentral, supports queries by drug name (e.g., omeprazole), by PubChem compound identifier (e.g., 4594), or by chemical structure in the SMILES (Weininger, 1988) format (e.g., COc1ccc2nc(S(=O)Cc3ncc(C)c(OC)c3C)[nH]c2c1). Regardless of format, all input queries for REDIAL-2020 are converted to SMILES format in order to predict anti-viral properties (Fig. 76).
Queries supported by DrugCentral: L1000
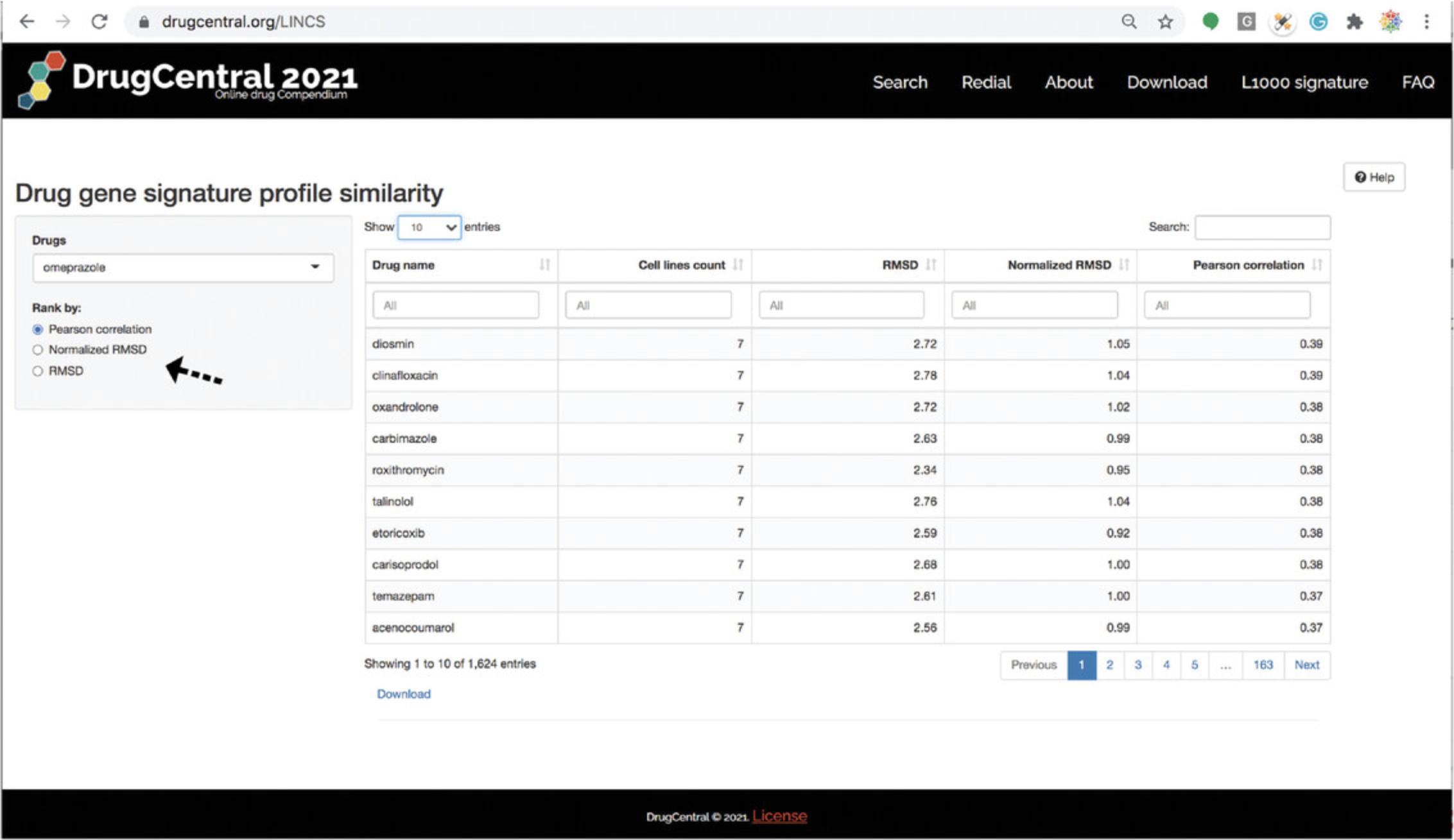
7.The other search interface available in DrugCentral, implemented in R-Shiny (https://shiny.rstudio.com/), supports browsing and searching for drug names for which gene perturbation profiles were recorded across one more of the 81 cell lines collected during the LINCS (Library of Integrated Cellular Signatures) project. Based on the L1000 perturbation profiles for 1613 drugs, the L1000 DrugCentral app allows users to query (via drug names) which drugs have the most similar gene perturbation profiles, ranked by cell lines (Fig. 77).
DrugCentral Drugcards: A step-by-step content guide
8.At its core, DrugCentral is a drug-centric resource. Thus, all queries are likely to provide information that is displayed in the form of “drug cards.” Data elements identified when searching a drug by name would be thus retrieved in a similar manner when searching by target or by disease, as both queries result in lists of drug cards.
9.Each drug card can be directly accessed (linked out) by observing the following (specific) format:
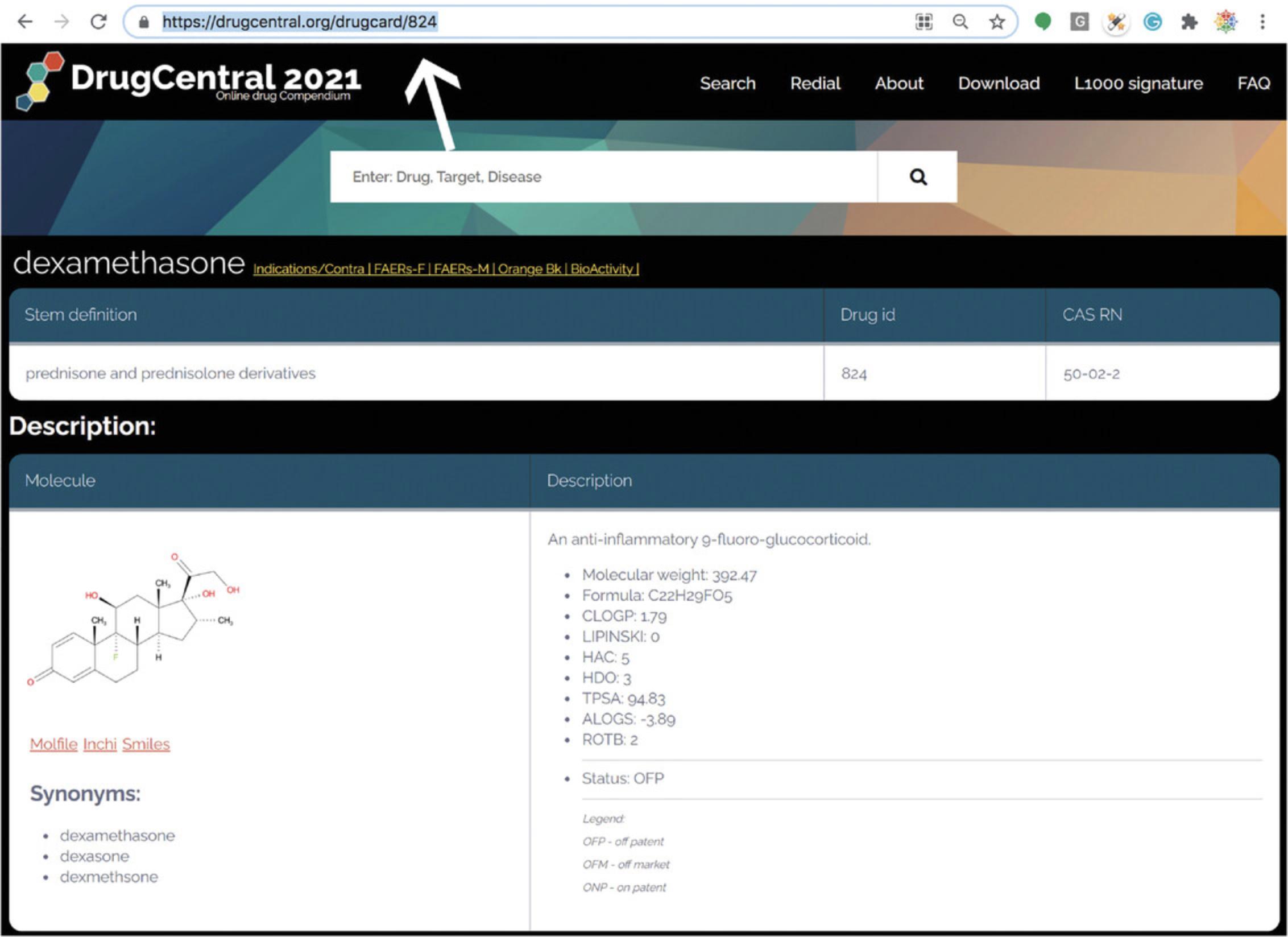
- https://drugcentral.org/drugcard/<DrugcentralStruct.ID>
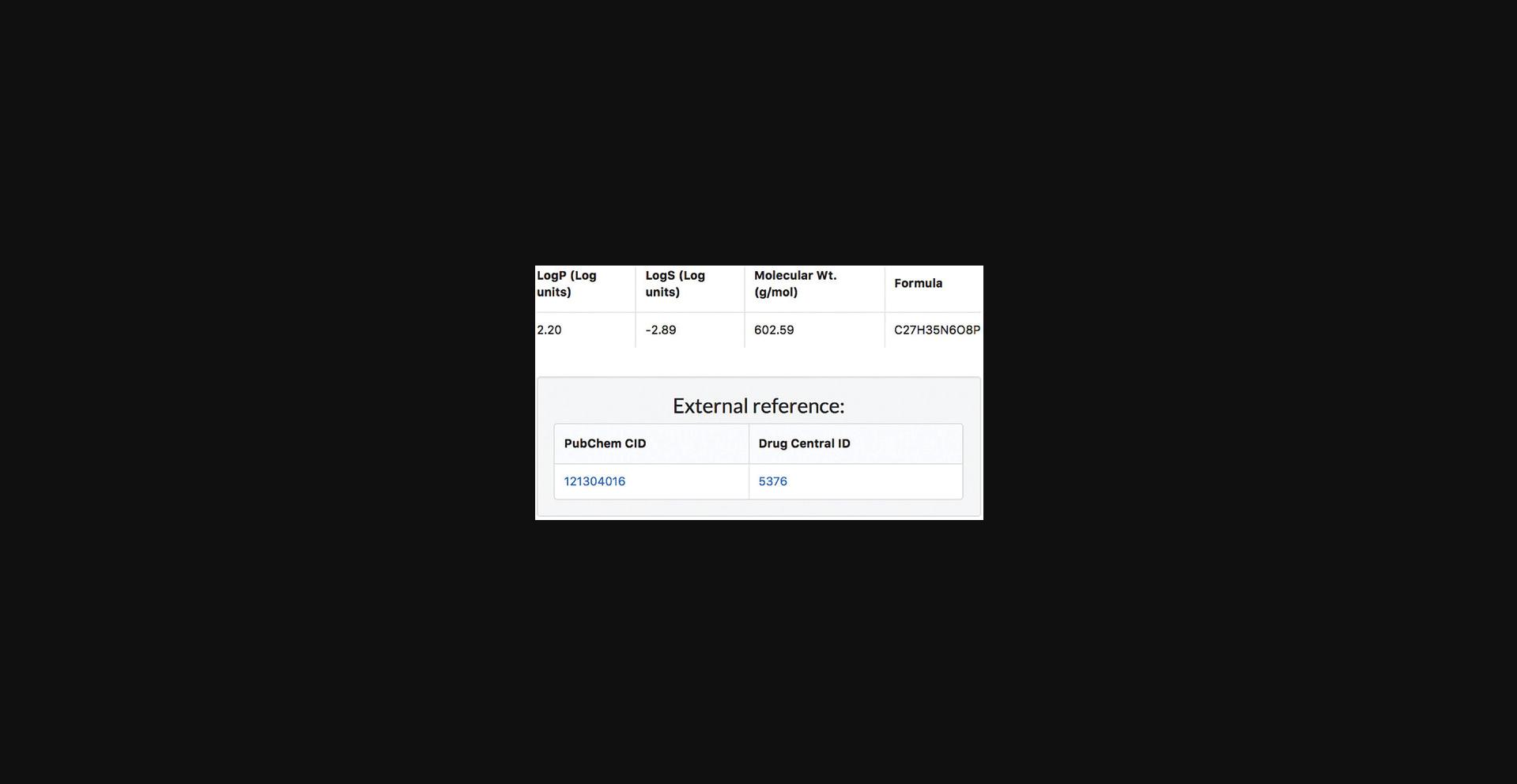
where “DrugcentralStruct.ID” is the DrugCentral structure ID number. For example, DrugcentralStruct.ID=824 resolves to dexamethasone. This manner of mining drug cards is not intended for casual users. Rather, this format is intended for programmatic access to DrugCentral content (Fig. 78).
10.What follows is a “section by section” guide to drug card content, shown by section title. These are not intended as comprehensive explanations, but rather as brief illustrations of the diverse content available through DrugCentral.
11.“Stem definition” displays International Nonproprietary Names (INN), which are associated with “pharmacologically related groups”; that section also displays Chemical Abstract Services (CAS) registry numbers, in addition to DrugCentral IDs.
12.“Description” depicts the two-dimensional chemical structure (as well as three separate chemical structure file formats), a number of synonyms, and computed chemical descriptors such as Lipinski's “rule of 5” (Lipinski et al., 2001). The intellectual property/regulatory status of the drug (if available) is also shown under “Status,” with one of three options—OFP: off patent; OFM: off market; and ONP; on patent—respectively (Avram, Curpan, Halip, Bora, & Oprea, 2020).
13.“Drug dosage” provides a sample (typically, the “maximum dose strength”) of the dosages available for oral/non-oral formulations of the drug.
14.“ADMET Properties” (Absorption, Distribution, Metabolism, Excretion, and Toxicity) provides experimental ADMET values, when available. These properties are half-life, systemic clearance, volume of distribution at steady state and fraction unbound, all intravenous pharmacokinetic parameters (Lombardo, Berellini, & Obach, 2018), the fraction excreted unchanged in urine (extent of metabolism), water solubility, and their composite parameter BDDCS (Biopharmaceutical Drug Disposition Classification System), as discussed elsewhere (Benet, Broccatelli, & Oprea, 2011), and MRTD, Maximum Recommended Therapeutic Daily Dose (Contrera, Matthews, Kruhlak, & Benz, 2004).
15.“Approvals” shows the date of approval by regulatory agencies (if available).
16.“FDA adverse event reporting system (Female),” followed by “FDA Adverse Event Reporting System (Male)” lists adverse events, separated by sex, in the decreasing order of the likelihood ratio (Huang, Zalkikar, & Tiwari, 2011).
17.“Pharmacologic action” highlights the drug annotations corresponding to (sometimes multiple) ATC (Anatomical, Therapeutic, and Chemical) classification system codes available at WHOCC (see Internet Resources); chemical ontology information from ChEBI (EBI Web Team; see Internet Resources); and MeSH (Medical Subject Headings; see Internet Resources) terms from the MeSH Browser.
18.“Drug use” lists indications, off-label use, and contra-indications, mapped to SNOMED-CT (Bhattacharyya, 2016) and DOID (Disease Ontology: Institute for Genome Sciences at the University of Maryland), where available. Drug indications and contra-indications are mined from package inserts (drug labels), whereas off-label uses are from literature.
19.“Acid dissociation constants calculated using MoKa v3.0.0” shows calculated acid/base dissociation constants, as calculated with the MoKa software (Milletti et al., 2010).
20.“Orange Book patent data (new drug applications)” and “Orange Book exclusivity data (new drug applications)” complement DrugCentral information on marketed pharmaceutical formulations by adding FDA Orange Book (Orange Book: Approved drug products with therapeutic equivalence evaluations; see Internet Resources) for patents, as well as exclusivity data, for new drug applications.
21.“Bioactivity Summary” distills information from multiple bioactivity databases, e.g., ChEMBL (Mendez et al., 2019) and the IUPHAR Guide to Pharmacology (Armstrong et al., 2019), in addition to scientific literature and information from drug labels. Numeric information is converted to the negative log molar of the effective drug concentration at measurement. Mechanism-of-action drug targets (Santos et al., 2017) are marked separately.
22.The “External reference” section contains drug identifiers used by other on-line resources. This section includes identifiers used in medical practice, such as the Veterans Health Administration (e.g., VHA unique identifier, VUID), the National Drug File reference terminology (NDFRT; see Internet Resources) and RxNorm (see Internet Resources), as well as identifiers used by PubChem, ChEBI, DrugBank, etc.
23.Last but not least, the “Pharmaceutical products” section provides direct links to DailyMed (https://dailymed.nlm.nih.gov/dailymed/), while incorporating simple meta-data descriptors such as “category” (e.g., prescription vs. over-the-counter), number of ingredients, administration route, etc. This section also includes a clickable container that captures the full text (no images) of the FDA approved package insert.
DrugCentral Target Cards: A step-by-step content guide
24.In Addition to DrugCentral's Drugcards, a set of Target Cards can be directly accessed by observing the following (URL) syntax:
25.For example, https://drugcentral.org/target/P23975/ resolves to Sodium-dependent noradrenaline transporter. This method of mining Target Cards is not intended for casual users. Rather, this format is intended for programmatic access to machine readable Target metadata (Fig. 79).
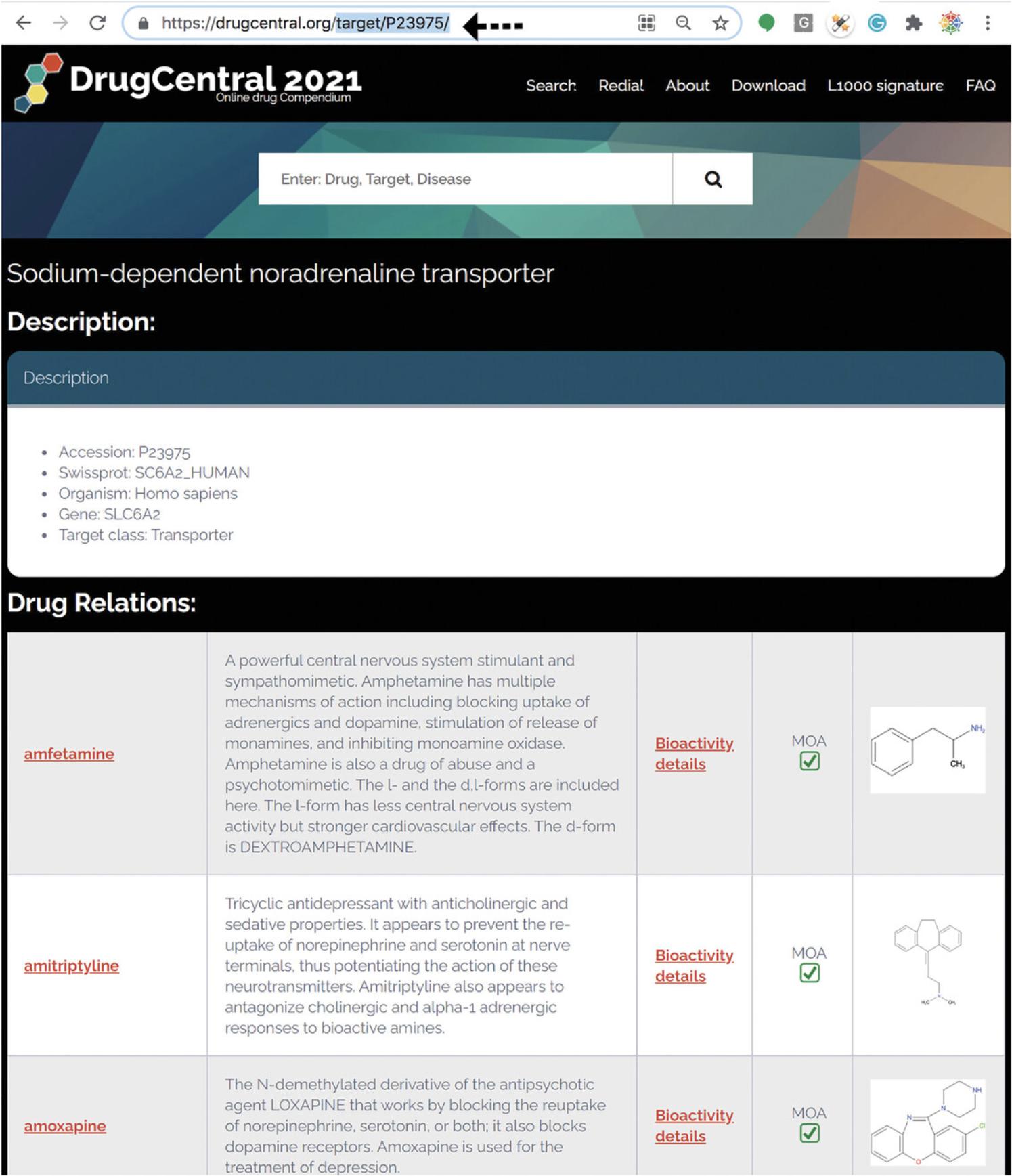
26.What follows is a “section by section” guide to Target card content and target metadata.
27.“Description” depicts the Accession ,Swissprot, Organism, and Gene & Target class, followed by Drug relations where the Drugs Bioactivity mechanism-of-actions are identified and marked.
28.To retrieve all cross-referenced Drug Central Targetcards mapped to Uniprot Accession Ids, use the following (machine readable) URL syntax (Fig. 80):
https://drugcentral.org/static/Drugcentral_uniprot_Mapping.txt
Additional information
29.The “Download Database dump 9/18/2020 (Postgres v10.12)” option contains all the information stored in DrugCentral. It requires a new or existing Postgres database setup. Users are directed to consult the Postgresql documentation on how to install, configure, and load database contents. This is also available via public instance at drugcentral:unmtid-dbs.net: 5433, username="drugman", password="dosage", with responsiveness depending on user load.
30.Example queries to extract subsets of data from DrugCentral : These require a local instance of DrugCentral loaded into a PostgreSQL database. To load the DrugCentral database dump assuming PostgreSQL is up and running and the user has admin privileges, run the following in PostgreSQL:
- #create database drugcentral and then run using the OS shell
- $gunzip -c drugcentral.dump.06212018.sql.gz | psql drugcentral#Example 1: Select Off-patent drugs that bind to “Mast/stem cell growth factor #receptor Kit” as mode-of-action target” in DrugCentral's Postgres Db.
- -select
- distinct(structures.name) as drug_name
- from
- structures
- join act_table_full on structures.id = act_table_full.struct_id
- Where
- structures.status =ˈOFPˈ and
- act_table_full.moa = 1 and
- act_table_full.target_name = ˈMast/stem cell growth factor receptor Kitˈ
- #Example 2: Select drugs indicated for seasonal allergic rhinitis that have #the lowest LLR for somnolence in males.
- -select
-
- distinct(structures.name) as drug_name,
- faers_male.*
- from
- structures
- join struct2atc on structures.id = struct2atc.struct_id
- join atc on struct2atc.atc_code = atc.code
- join faers_male on structures.id=faers_male.struct_id
- Where
- atc.l2_name = 'ANTIHISTAMINES FOR SYSTEMIC USE' and
- faers_male.meddra_name = ˈSomnolenceˈ and
- faers_male.llr <= 2*faers_male.llr_threshold
- order by
- faers_male.llr asc
31.To download additional example SQL queries for extracting subsets of data from DrugCentral, use the following URL: https://unmtid-shinyapps.net/download/example_query.sql.
Basic Protocol 8: ESTIMATING ANTI-SARS-CoV-2 ACTIVITIES WITH DrugCentral REDIAL-2020
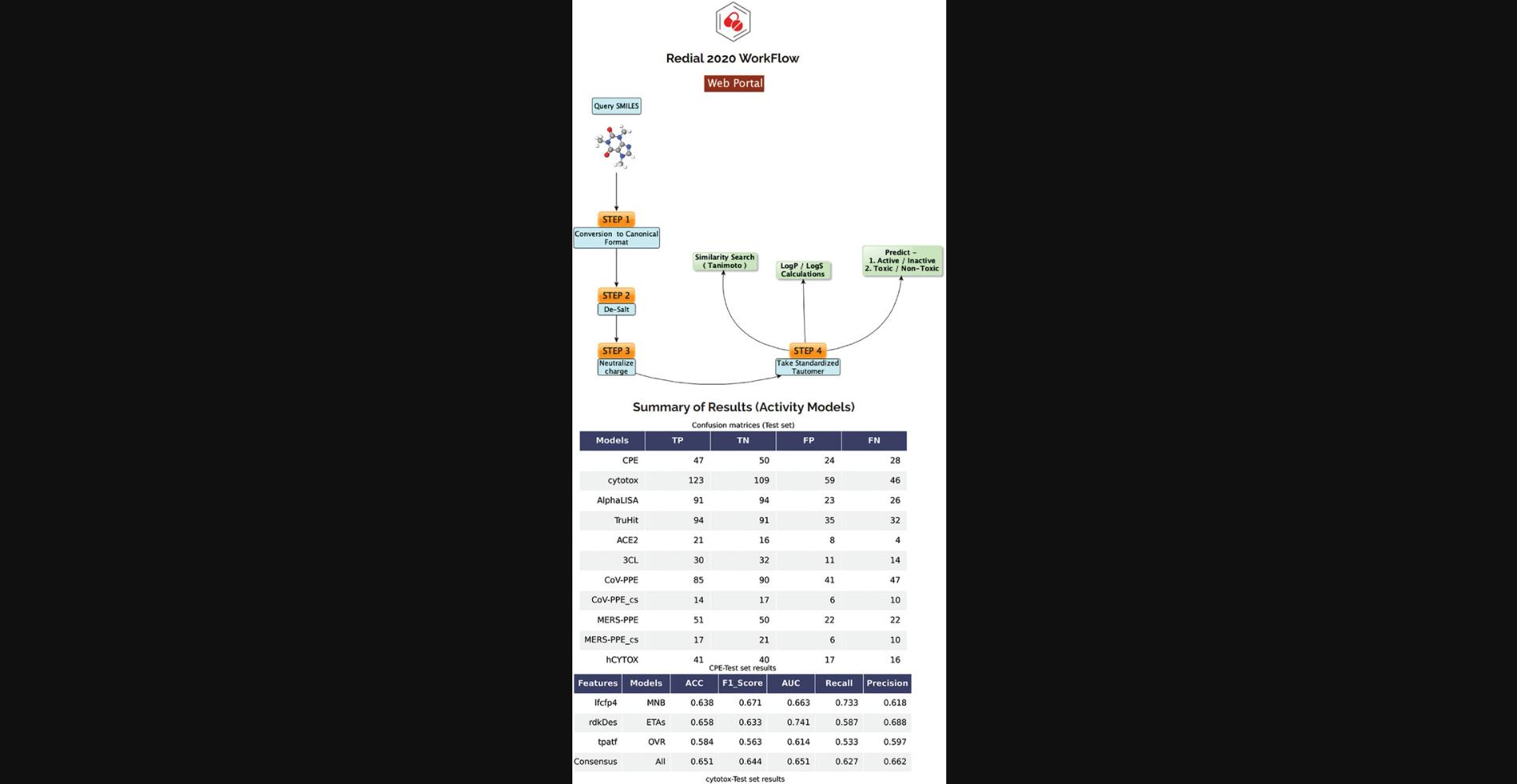
There is currently an urgent need to find effective drugs for treating coronavirus disease 2019 (COVID-19). DrugCentral REDIAL-2020 (KC et al., 2020) is a suite of machine learning models that forecast activities for live viral infectivity, viral entry, and viral replication specifically for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), in vitro infectivity, and human cell toxicity. This application serves the scientific community when prioritizing compounds for in vitro screening and may ultimately accelerate identifying novel drug candidates for COVID-19 treatment. REDIAL-2020 consists of eleven independently trained machine learning models using high-throughput screening data from the NCATS COVID19 portal (https://opendata.ncats.nih.gov/covid19/index.html) and includes a similarity search module that queries the underlying experimental dataset for similar compounds. These models were developed using experimental data generated by the following assays: the SARS-CoV-2 cytopathic effect (CPE) assay and its host cell cytotoxicity counterscreen; the Spike–ACE2 protein–protein interaction (AlphaLISA) assay and its TruHit counterscreen, the angiotensin-converting enzyme 2 (ACE2) enzymatic activity assay; the 3C-like (3CL) proteinase enzymatic activity assay; the SARS-CoV pseudotyped particle entry (CoV-PPE) assay and its counterscreen (CoV-PPE_cs); the Middle East respiratory syndrome coronavirus (MERS-CoV) pseudotyped particle entry assay (MERS-PPE) and its counterscreen (MERS-PPE_cs); and the human fibroblast toxicity (hCYTOX) assay (Fig. 81).
Necessary Resources
Hardware
- Desktop or a laptop computer, or a mobile device, with a 100 Mbps or higher (fast) Internet connection
Software
- An up-to-date web browser such as Google Chrome (https://www.google.com/chrome/), Mozilla Firefox (https://www.mozilla.org/en-US/firefox/), Apple Safari (https://www.apple.com/safari/), or Microsoft Edge (https://www.microsoft.com/en-us/edge)
REDIAL: A step-by-step content guide
1.By accessing REDIAL-2020 (http://drugcentral.org/Redial) from any web browser, including mobile devices, the submission page is displayed.
2.The web server accepts SMILES, drug names or PubChem CIDs as input. Regardless of input, the protocol converts drug names (from DrugCentral) or PubChem CIDs into SMILES.
3.The user interface provides a summary of the models, such as model type, which descriptor categories were used for training, and the evaluation scores.
4.The user interface depicts the processes of cleaning the chemical structures (encoded as SMILES) before training the machine learning models (Fig. 82).
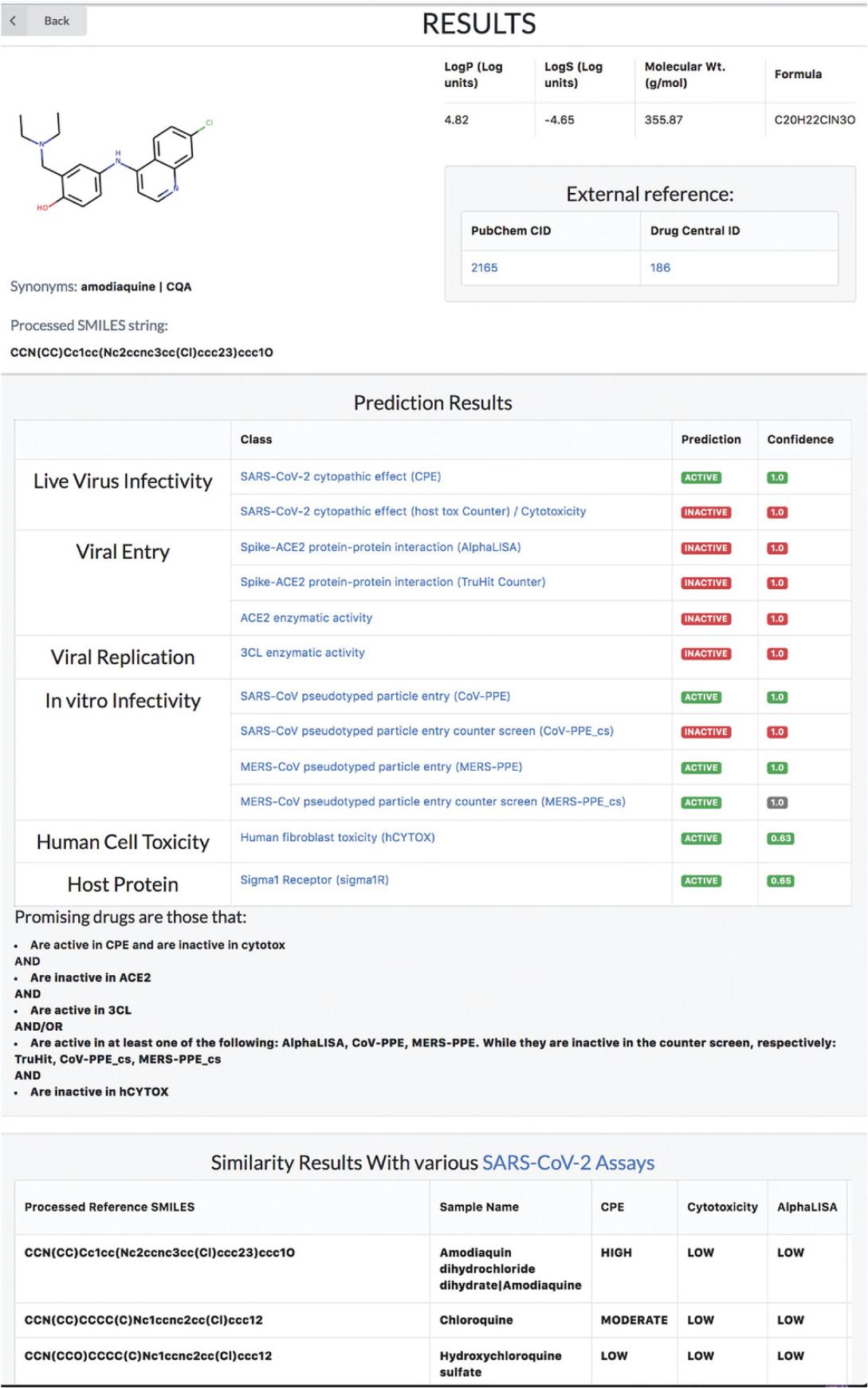
5.As an example, amodiaquine has been shown to have promising anti-SARS-CoV-2 behavior in several papers (Bocci et al., 2020; Si et al., 2021), but its mechanism of action has not been well established yet. When given as an input to REDIAL, the webapp opens a new window with the predicted activities.
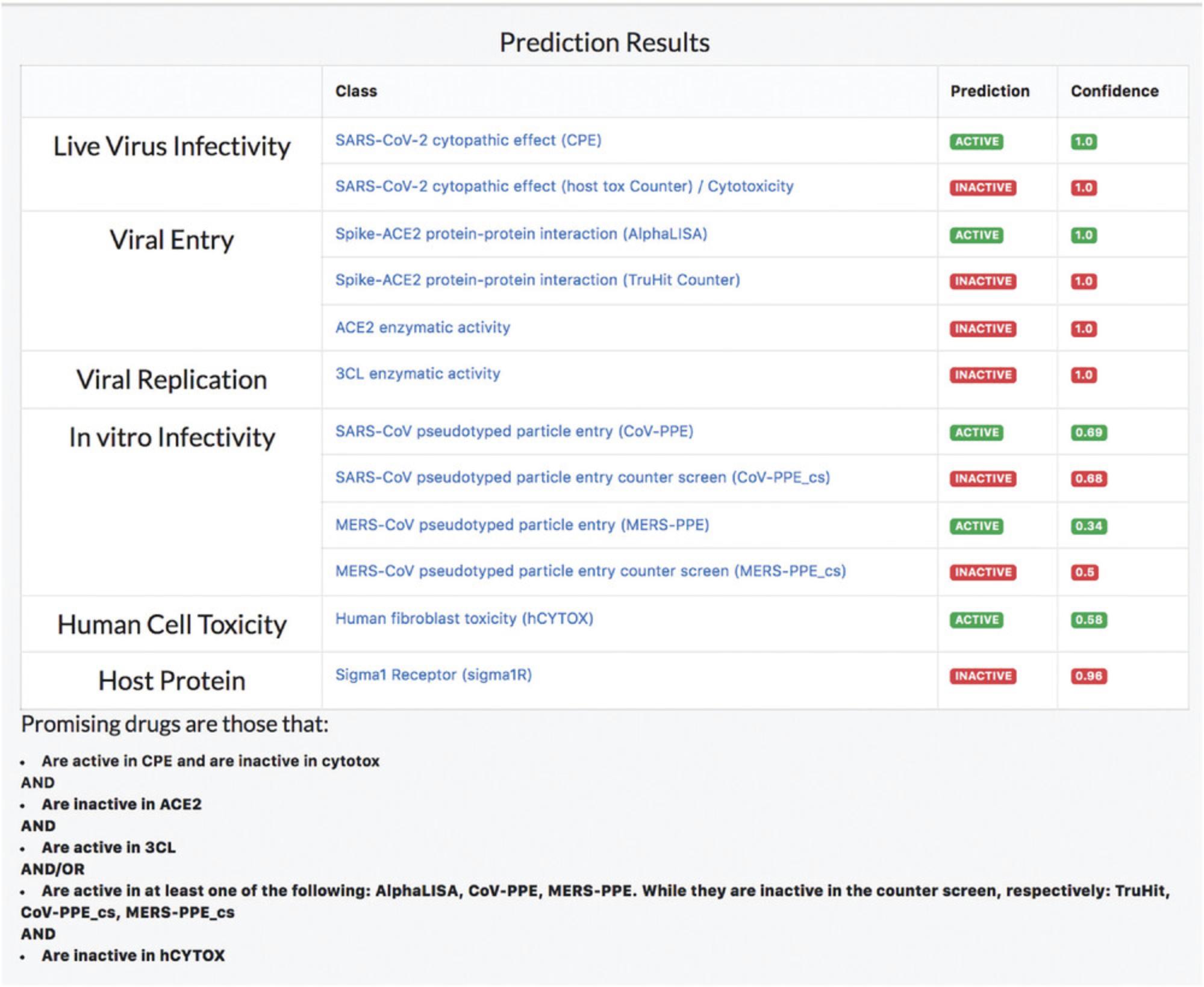
6.The prediction results table shows that amodiaquine is predicted to be active in cytopathic effect experiments, while there are no clues on its mechanism (inactive in AlphaLISA, ACE2, 3CL assays) (Fig. 83).
7.REDIAL-2020 links directly to DrugCentral for approved drugs and to PubChem for chemicals (where available), enabling easy access to further information on the query molecule (Fig. 84).
8.Using REDIAL-2020 estimates, promising anti-SARS-CoV-2 compounds would ideally be active in the CPE assay while inactive in cytotox and in hCYTOX.
Queries supported by REDIAL
9.Input queries such as drug name and PubChem CID are converted to SMILES before processing. Each SMILES string input is subject to four different steps, namely, converting the SMILES into canonical SMILES, removing salts (if present), neutralizing formal charges (except permanent ones), and standardizing tautomers. REDIAL-2020 predicts input compound activity across all eleven assays: CPE, cytotox, AlphaLISA, TruHit, ACE2, 3CL, CoV-PPE, CoV-PPE_cs, MERS-PPE, MERS-PPE_cs, and hCYTOX (Fig. 85).
Additional information
10.All of the codes and the trained models are available from: https://doi.org/10.5281/zenodo.4606720.
11.The source code and specific models are available through Github at: https://github.com/sirimullalab/redial-2020, or via Docker Hub (https://hub.docker.com/r/sirimullalab/redial-2020) for users preferring a containerized version. All the pre-ML processing and “data cleaning” scripts are here: https://github.com/sirimullalab/redial-2020/tree/master/data-cleaning
12.All workflows and procedures were performed using the KNIME platform 10.The NCATS data associated with the aforementioned assays were downloaded from the COVID-19 portal. https://opendata.ncats.nih.gov/covid19/assays.
Basic Protocol 9: DRUG SET ENRICHMENT ANALYSIS USING DRUGMONIZOME
Drugmonizome (Kropiwnicki et al., 2021) serves processed data extracted from drug and small molecule databases available from a variety of online repositories and data portals. The processed data is provided in the form of drug set libraries which serve as the underlying database for drug set enrichment analysis. Drugmonizome enables users to submit lists of drugs and small molecules as the input query. These drug sets are compared against various drug set libraries that contain known associations between drugs and their attributes, for example, side effects, indications, targets, pathways, induced gene expression signatures, and other attributes. Additionally, Drugmonizome provides options for querying metadata associated with drug sets to find relevant drugs, small molecules, and drug sets for a given free-text query.
Necessary Resources
Hardware
- Desktop or a laptop computer, or a mobile device, with a fast Internet connection
Software
- An up-to-date web browser such as Google Chrome (https://www.google.com/chrome/), Mozilla Firefox (https://www.mozilla.org/en-US/firefox/), Apple Safari (https://www.apple.com/safari/), or Microsoft Edge (https://www.microsoft.com/en-us/edge)
Metadata search
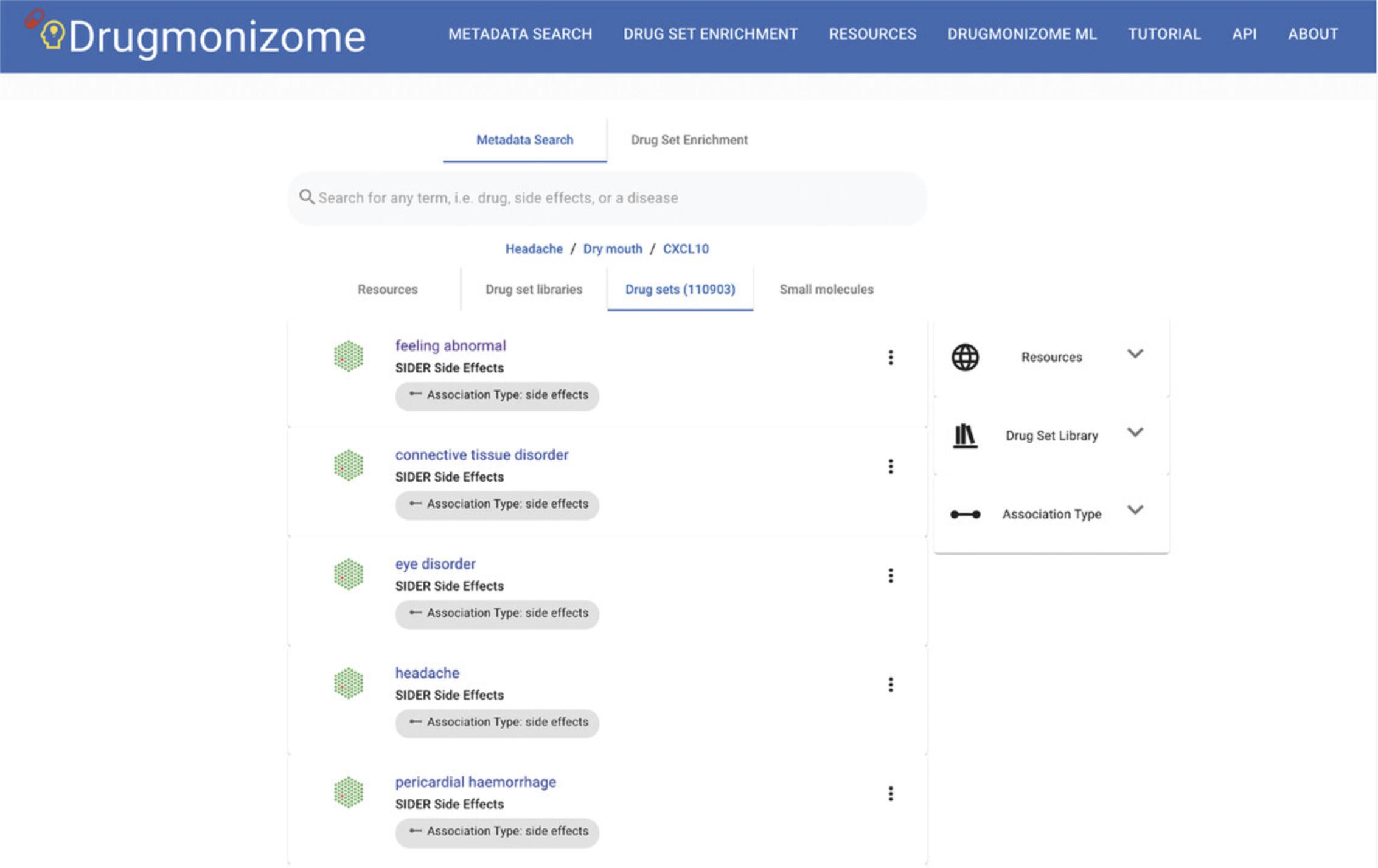
1.Navigate to the Drugmonizome homepage (https://maayanlab.cloud/drugmonizome/). The metadata search is displayed by default. Using the search bar, users can submit query terms of interest to identify resources, drug set libraries, drug sets, and small molecules contained in Drugmonizome. Example terms are suggested for each type of metadata search (Fig. 86).
2.Alternate between resource, drug set library, drug set, and small molecule metadata searches by clicking the corresponding tab. When performing metadata searches for drug sets, use the filter table to query terms within specific resources, drug set libraries, and association types.
3.Upon submitting a term of interest using the search bar, a list of results that match the term is displayed (Fig. 87).
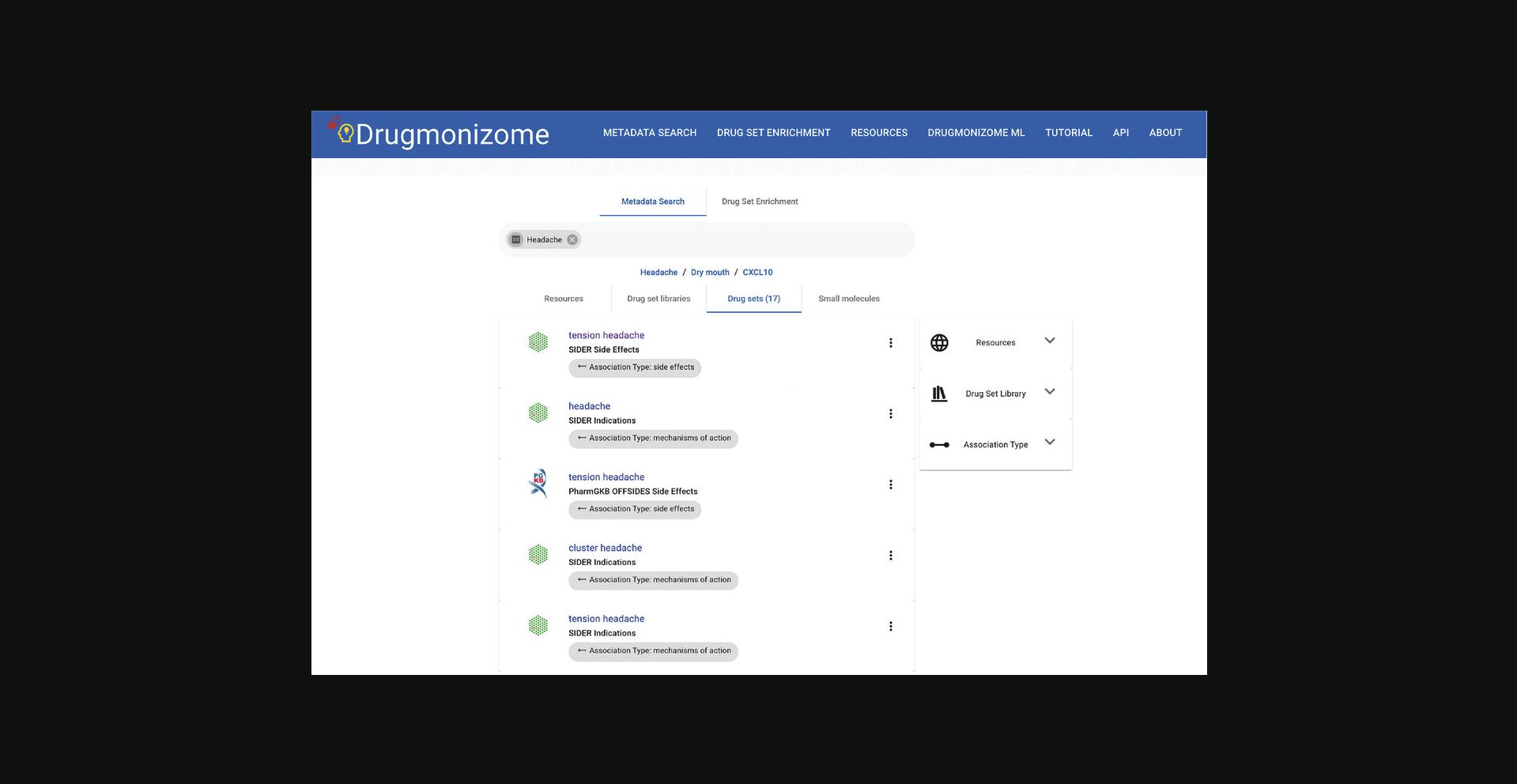
4.Clicking on any term displays a page with identifying metadata for the resource, drug set library, drug set, or small molecule. When perusing drug set metadata, a search bar exists for querying specific small molecules of interest within the set (Fig. 88).
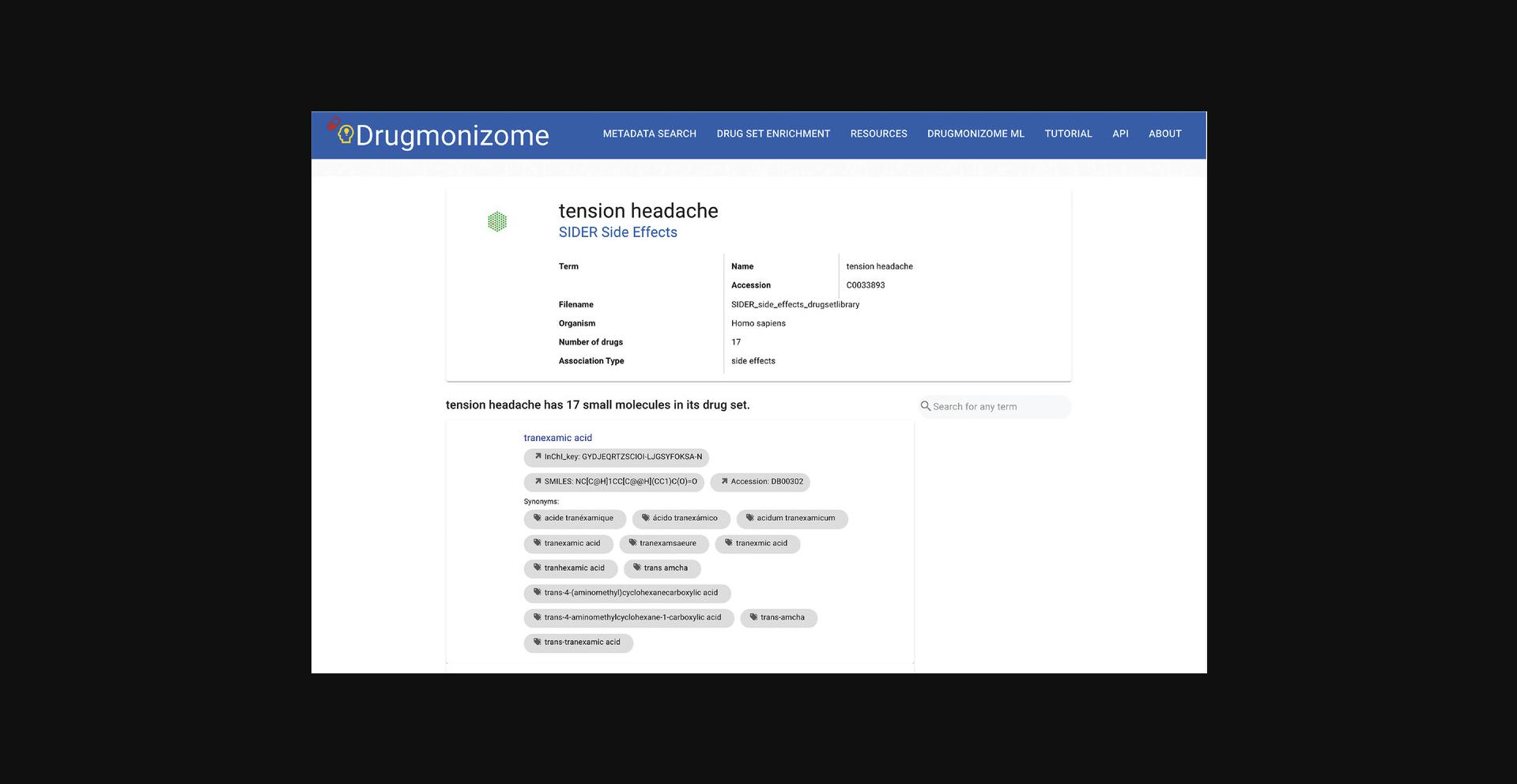
Drug set enrichment
5.Navigate to the drug set enrichment page by clicking the corresponding tab on the website header. The drug set enrichment page includes a search box where a list of drugs and small molecules can be pasted. The page also includes several example drug sets that are pasted into the box when clicked (Fig. 89). As an example, click the “69 in vitro COVID-19 hits from a drug screen by Ellinger et al.” link to populate the search box with a small molecule set.
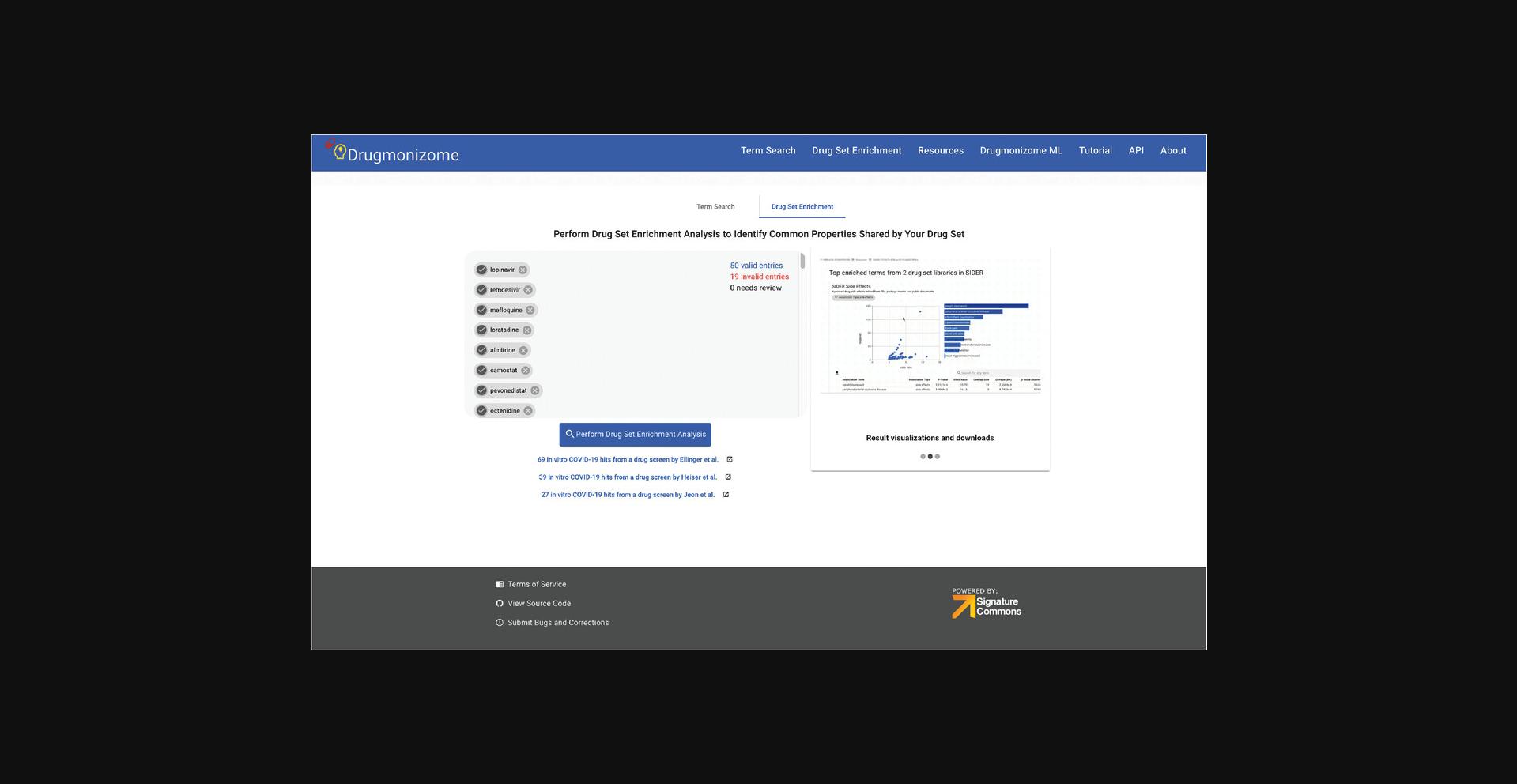
6.Click the “Perform Drug Set Enrichment Analysis” button and a results page of all resources with enriched terms is returned. Each of the resources with enriched drug set libraries are represented as an icon with the number of enriched terms for each resource (Fig. 90).
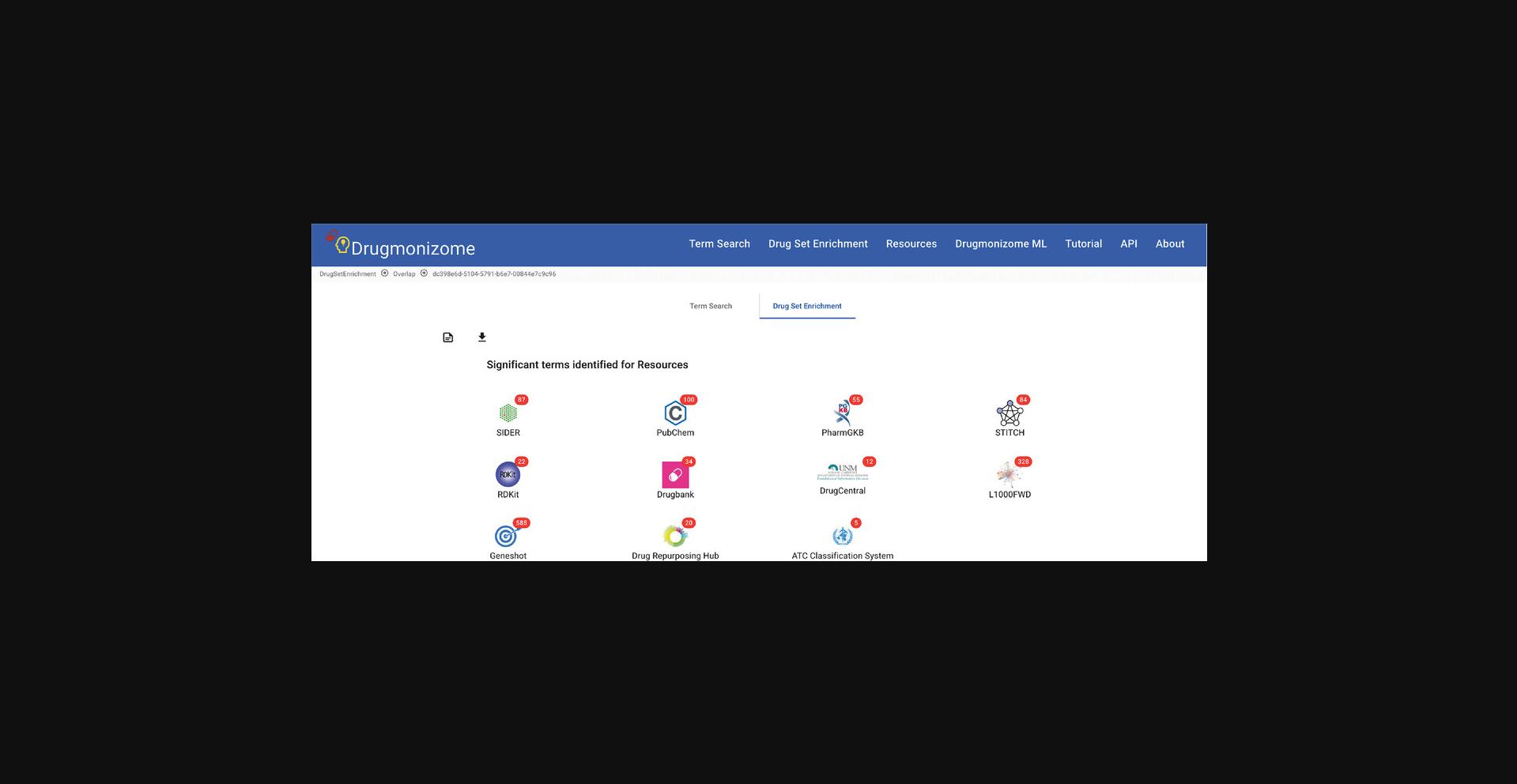
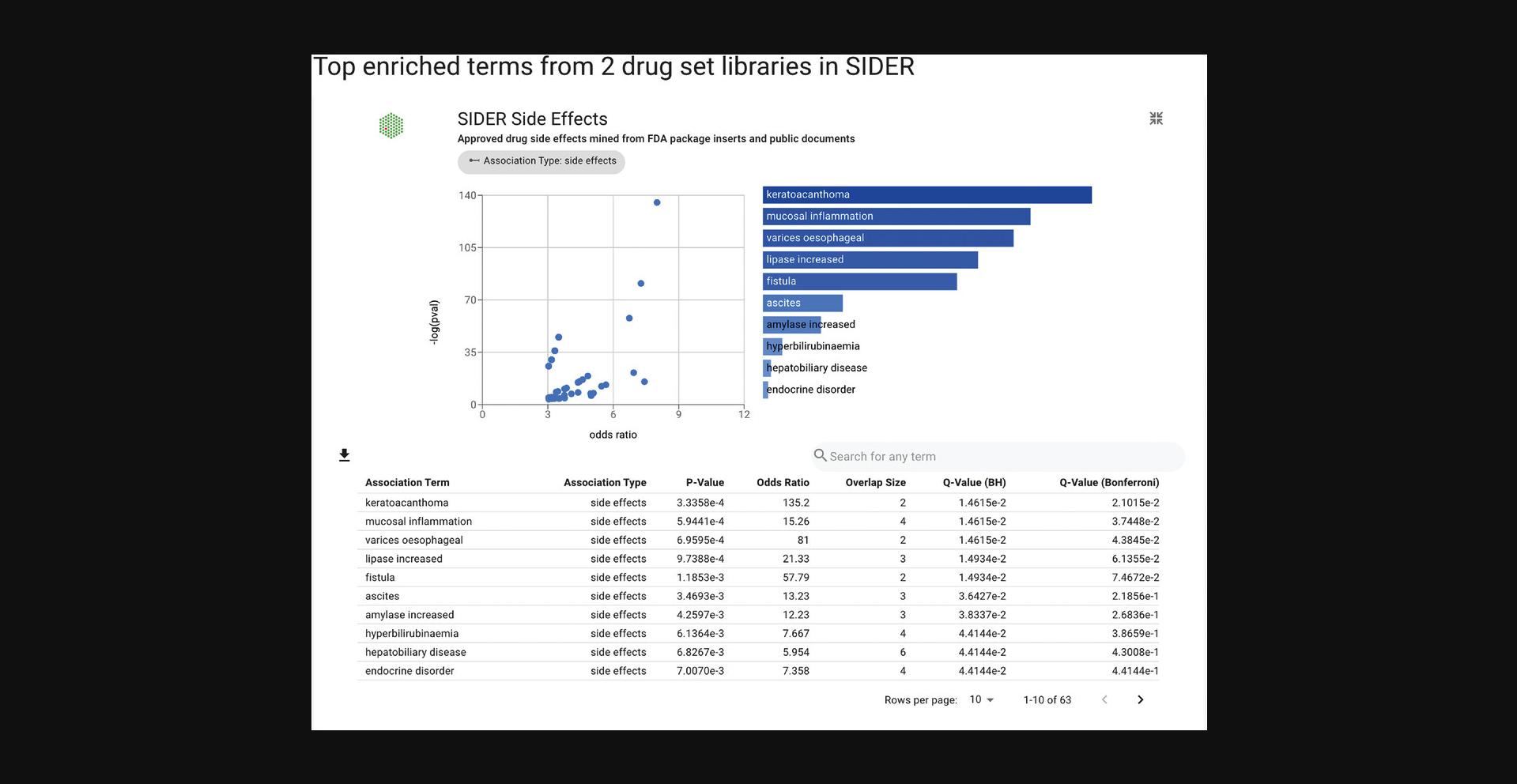
7.Click on any of the resource icons to be redirected to a page with the top enrichment results for each drug set library represented by a toggleable bar graph or scatter plot. The drug set library enrichment results can be expanded by clicking the corresponding button (Fig. 91).
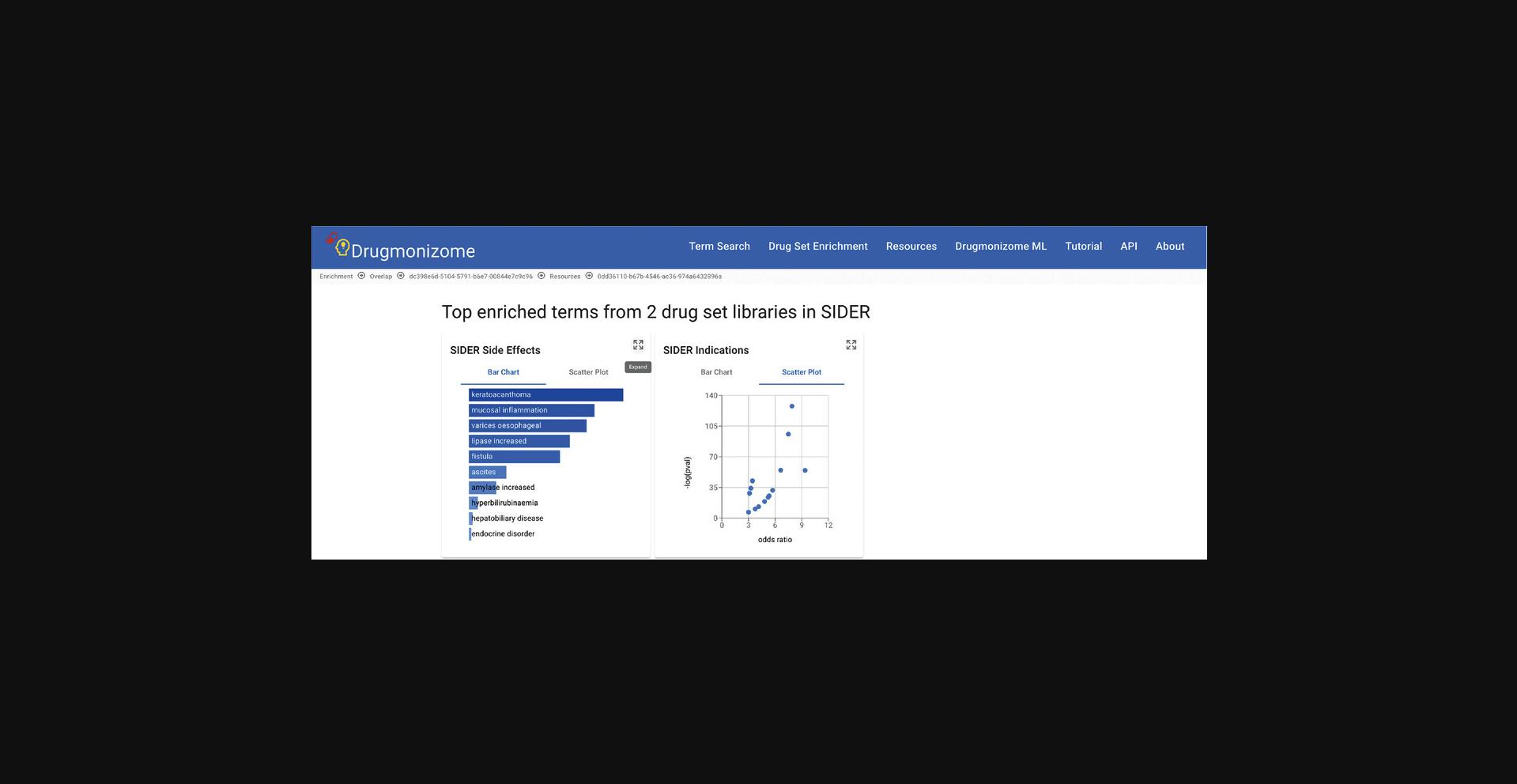
8.The expanded page includes the scatter plot, bar graph, and table view of the top enriched terms. The table representation displays the top enriched terms and their p -values, odds ratio, and corrected q -values. Terms of interest can be queried using the search bar above the table. The table is also available for download as a .TSV file (Fig. 92).
Resources pages
9.Navigate to the resources page by clicking the corresponding tab on the website navigation bar (Fig. 93).
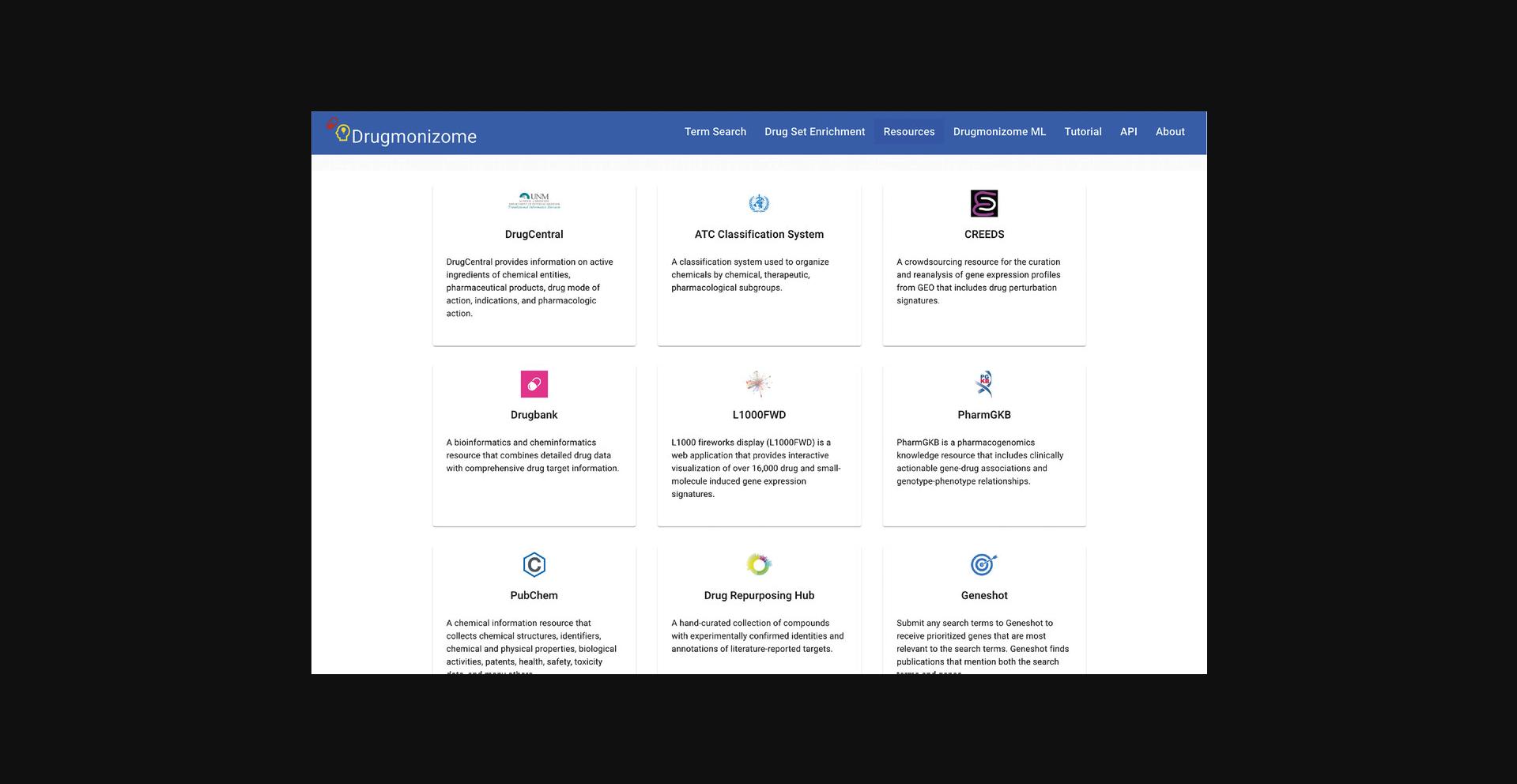
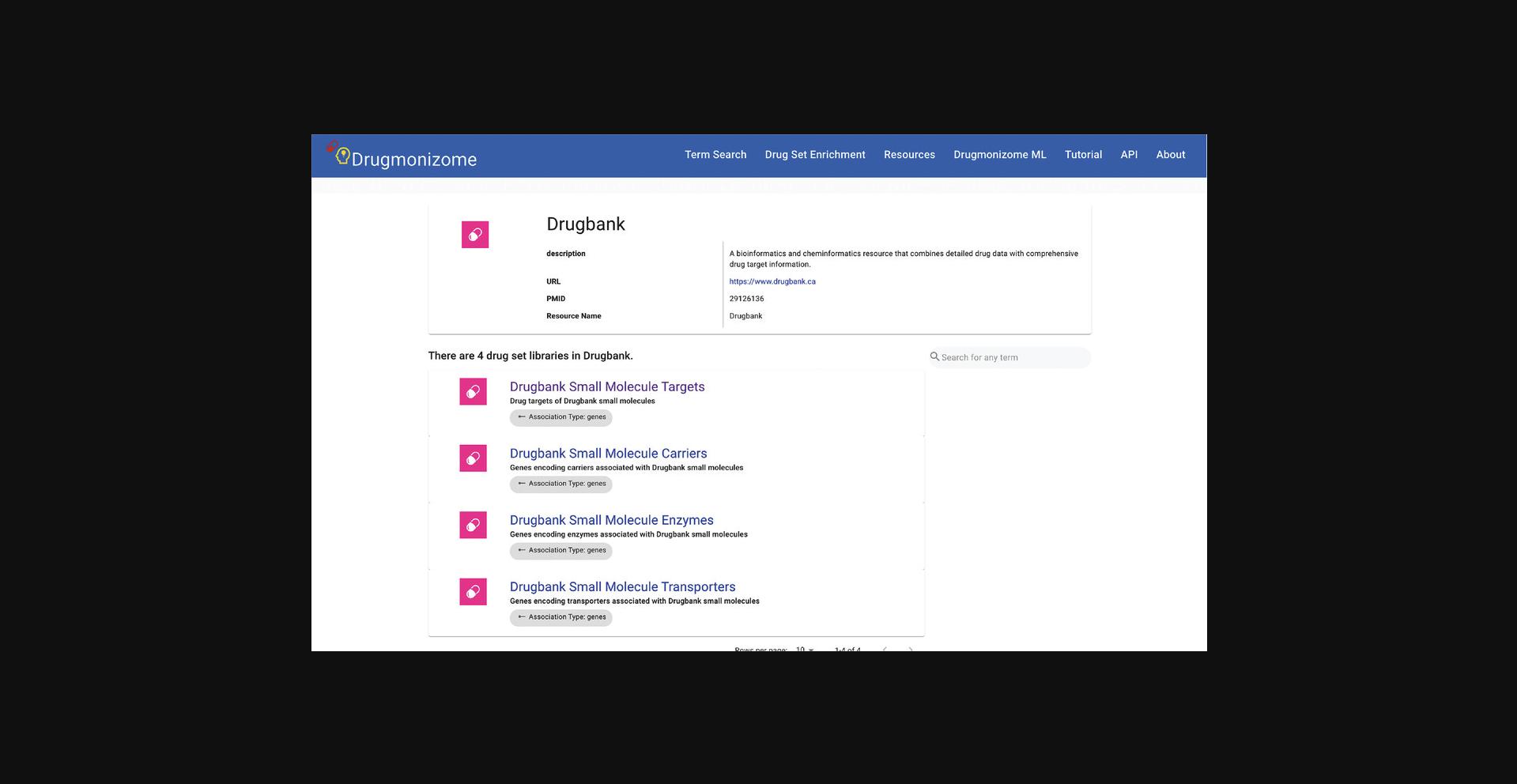
10.Each of the drug data resources used to create drug set libraries is cataloged on this page. Click on the DrugBank resource card to view metadata specific to DrugBank, as well as drug set libraries curated from DrugBank (Fig. 94).
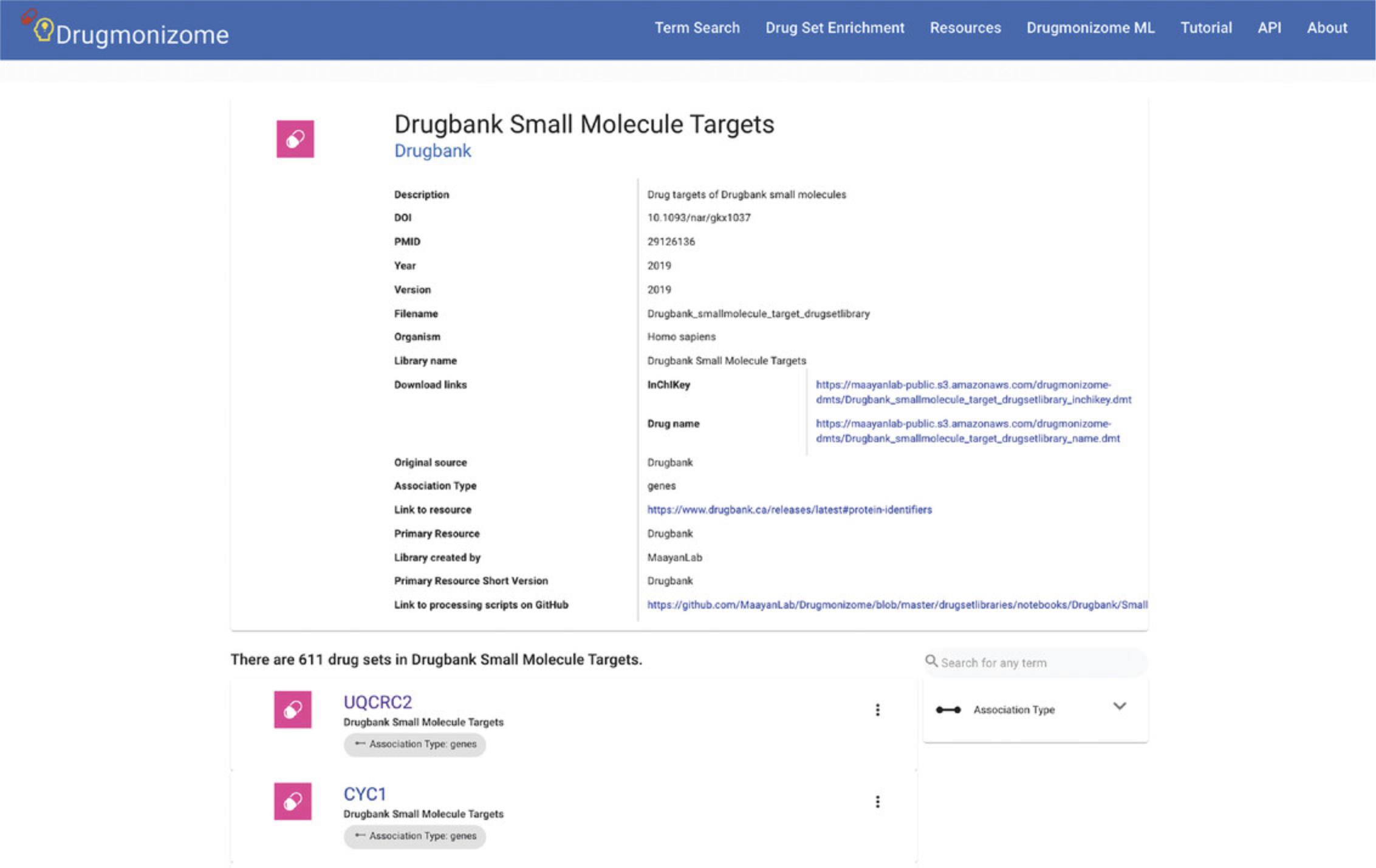
11.Click on the “DrugBank Small Molecule Targets” library to be redirected to a page with identifying metadata for the drug set library. The metadata for the drug set library includes download links for the .DMT files in drug name or InChIKey format (Fig. 95). Additionally, each of the drug sets included in this library are listed below. Clicking on any drug set name redirects to a page with metadata specific to the drug set, as well as the set of associated small molecules.
Basic Protocol 10: THE DRUGMONIZOME-ML APPYTER
A wealth of data from a multitude of sources is readily available for thousands of bioactive small molecules in Drugmonizome (Kropiwnicki et al., 2021). The information in Drugmonizome can be harnessed to develop machine learning models that utilize such data to predict the properties of small molecules that are poorly annotated. The Drugmonizome database draws upon a variety of publicly available resources to label each small molecule by its associations with pathways, protein targets, induced gene expression profiles, chemical features, and other attributes. Drugmonizome-ML is an Appyter (Clarke et al., 2021) that executes a machine learning pipeline as a Jupyter notebook using the data curated for creating Drugmonizome. Drugmonizome-ML can be used to make predictions for indications and other attributes such as drug targets or side effects for poorly annotated pre-clinical bioactive small molecules.
Necessary Resources
Hardware
- Desktop or a laptop computer, or a mobile device, with a fast Internet connection
Software
- An up-to-date web browser such as Google Chrome (https://www.google.com/chrome/), Mozilla Firefox (https://www.mozilla.org/en-US/firefox/), Apple Safari (https://www.apple.com/safari/), or Microsoft Edge (https://www.microsoft.com/en-us/edge)
Input dataset selection
1.Navigate to the Drugmonizome-ML Appyter (https://appyters.maayanlab.cloud/Drugmonizome_ML/). The input form is divided into three sections: input dataset selection, target label selection, and machine learning pipeline.
2.Select datasets from Drugmonizome and SEP-L1000 (Kropiwnicki et al., 2021; Wang, Clark, & Ma'ayan, 2016) to populate the feature matrix that will be used for learning and classification. Each of the datasets’ contents are described using tooltips (Fig. 96). For the demonstration, select the “LINCS Gene Expression Signatures” from the “Transcriptomic and Imaging Datasets” subfield and “Morgan Fingerprints” from the “Chemical Fingerprints Generated for Compounds from SEP-L1000” subfield.
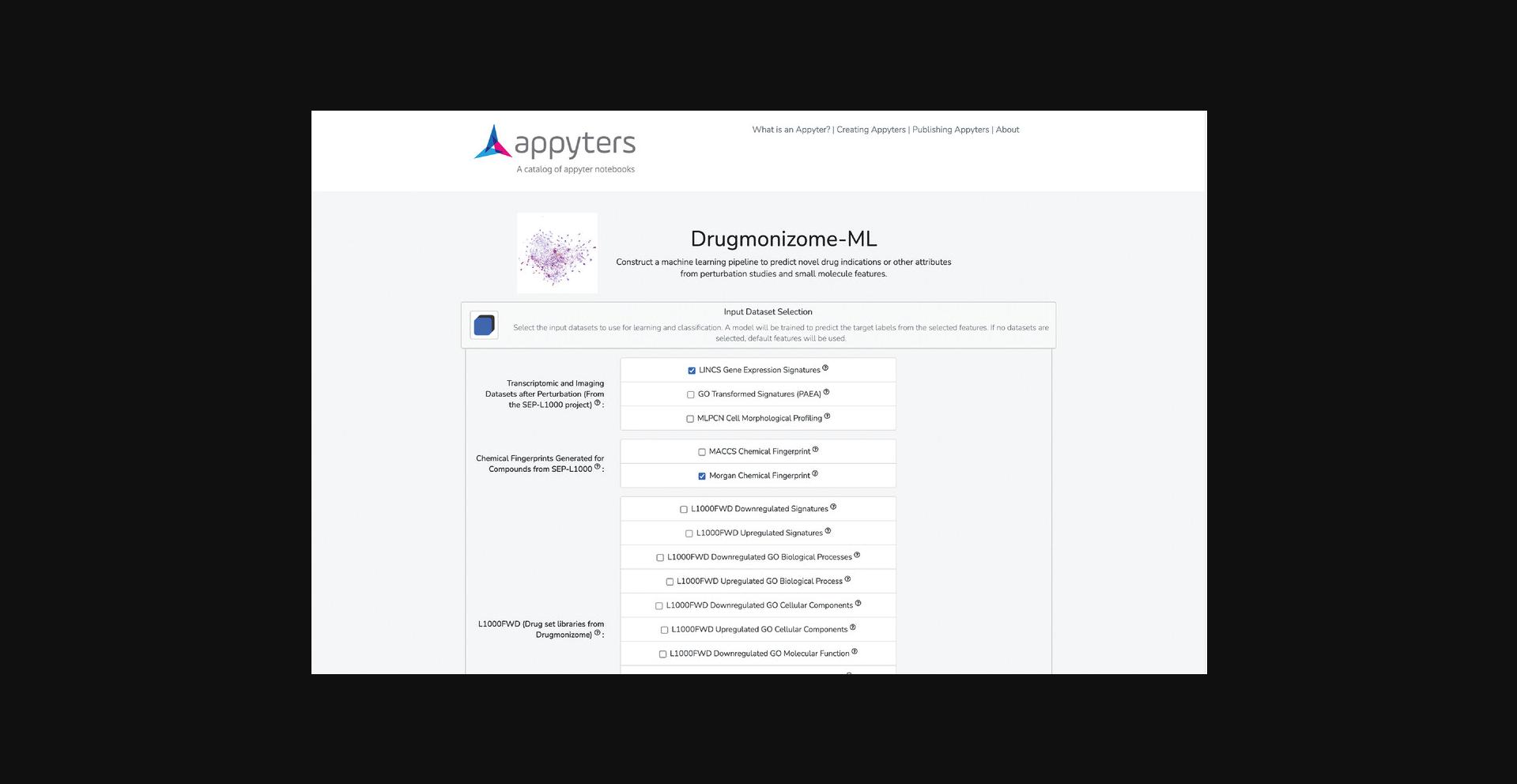
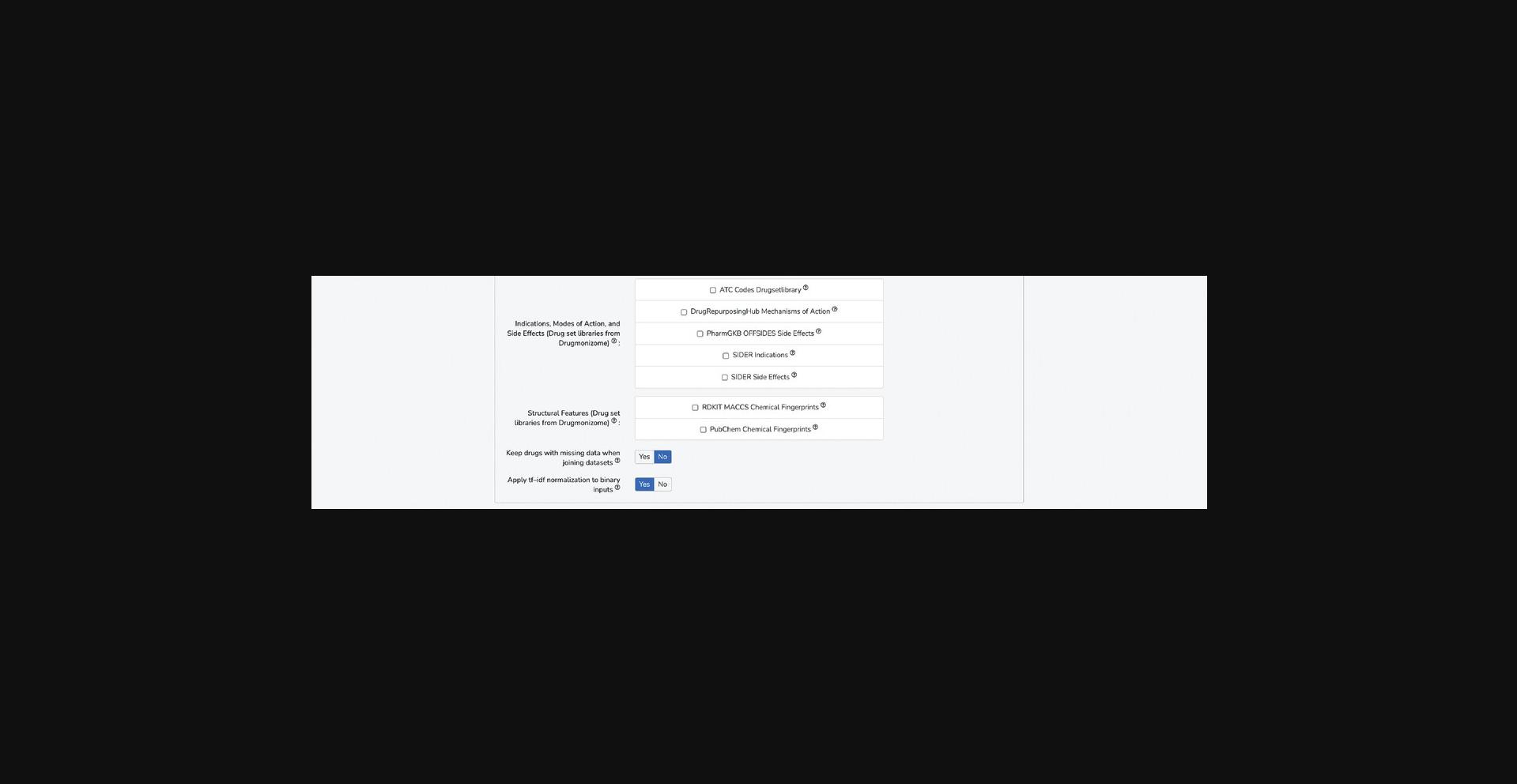
3.Additional options for pre-processing the feature matrix are available. If selecting features from various data sources, it is likely that not all compounds will be included across all feature sets; therefore, a toggleable option decides whether drugs with missing data are retained or dropped from the feature matrix. Additionally, because some of the available feature sets are binary association matrices, there is the option to apply TF-IDF normalization to account for frequency of common and rare features among the small molecules (Fig. 97). In general, the default settings for these options are recommended.
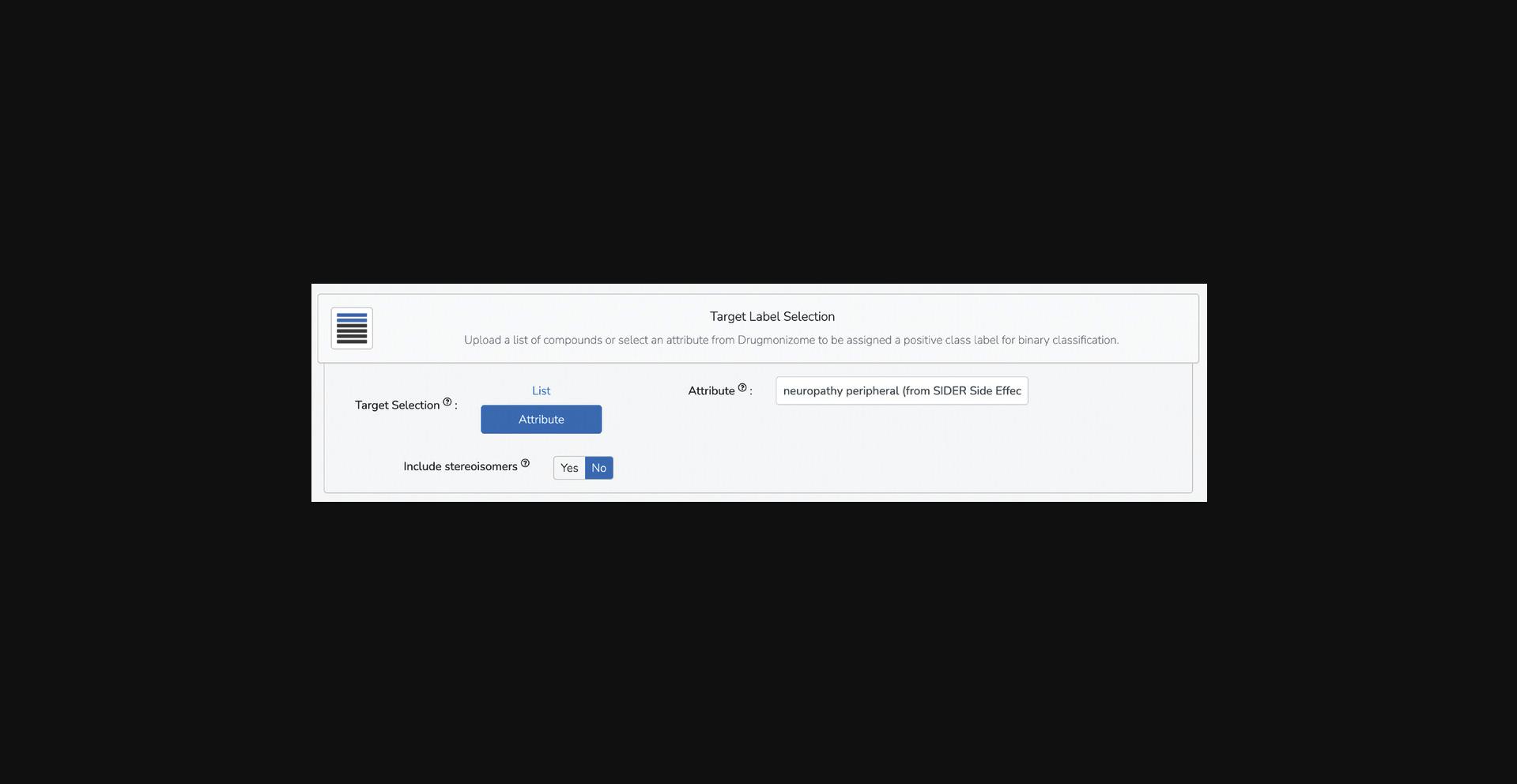
Target label selection
4.In this section, select the positive class label for a binary classification problem. There is the option to select an attribute from any of the Drugmonizome drug set libraries in an autocomplete field where relevant drug-set labels from Drugmonizome are offered as potential class labels (Fig. 98). Type any characters into the autocomplete field and matching drug-set labels will be displayed. For the demonstration, type “neuropathy peripheral (from SIDER Side Effects)” into the autocomplete field.
5.Alternatively, upload a newline-separated .txt file of compounds to be used as positive examples of a class to predict by selecting the “List” option in the “Target Label Selection” section. Example .txt files are available for download to understand the structure of the file (Fig. 99). Choose the drug identifier format (drug name or InChI key in which small molecules within the text file are described. InChI Keys are the recommended format.
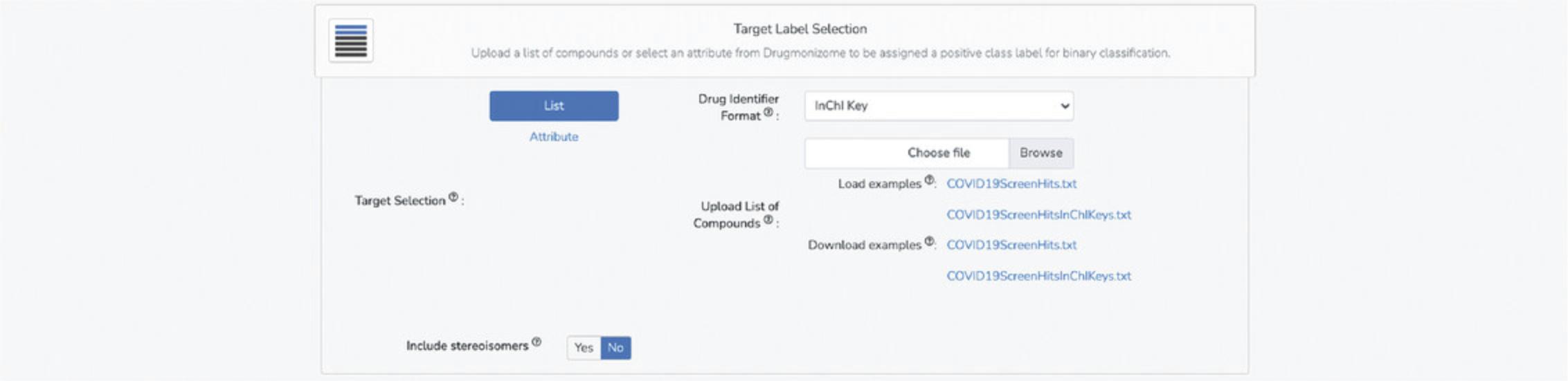
6.The “Include stereoisomers” option decides whether to match compounds from the feature matrix to the target vector using the first 14 characters of the InChIKey (which encodes chemical connectivity), thus including stereoisomers of a particular small molecule, or whether to consider only one form of a molecule and match by the whole InChIKey.
Machine learning pipeline
7.In this section, select data visualization options, machine learning classifiers, machine learning hyperparameters, and methods to evaluate the classifier (Fig. 100).
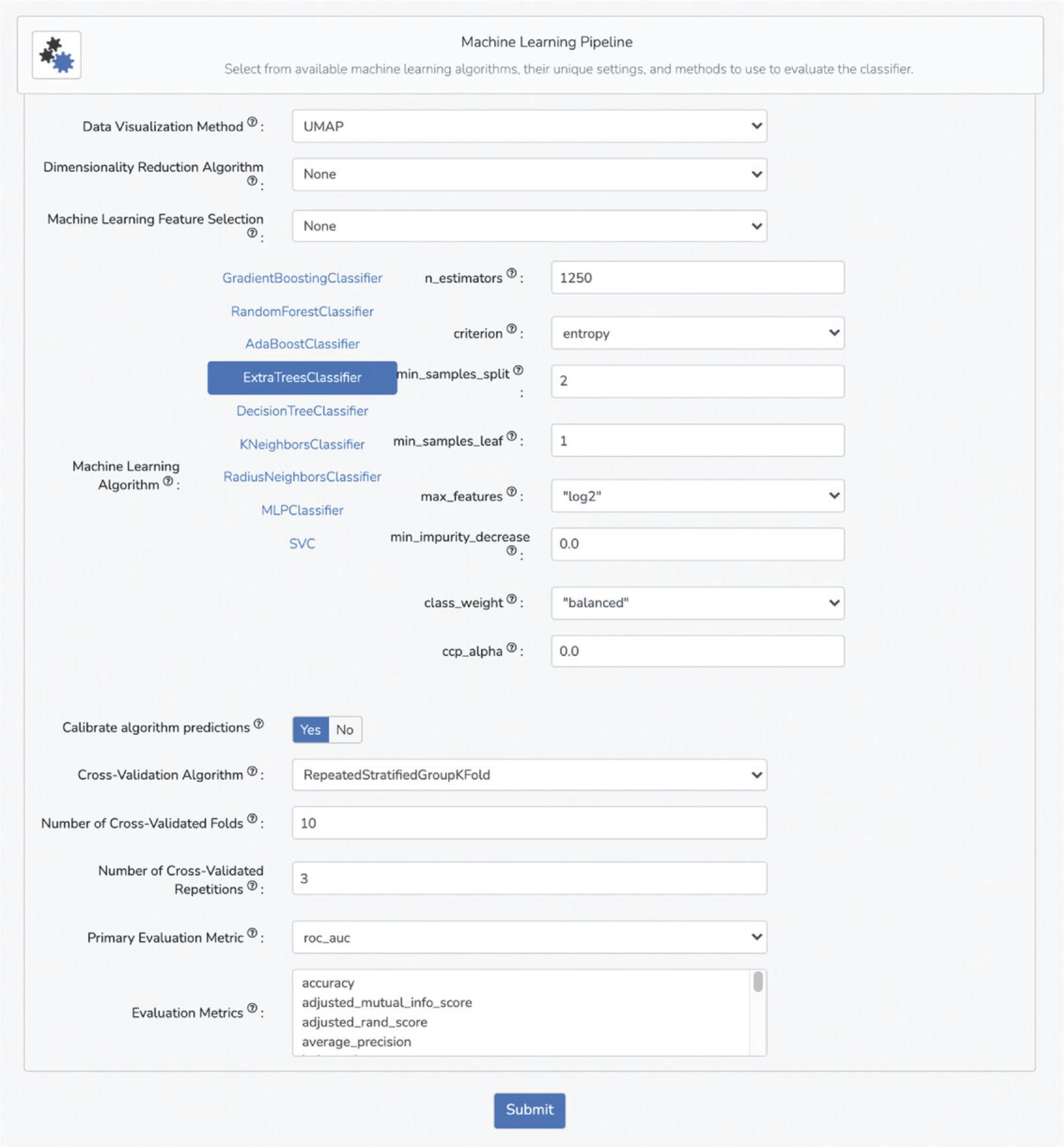
8.Select your preferred data visualization method from the drop-down menu under the “Data Visualization Method” field. The default and recommended method is UMAP.
9.If applicable, select a dimensionality-reduction algorithm from the drop-down menu under the “Dimensionality Reduction Algorithm” field.
10.If applicable, select a feature-selection method from the drop-down menu under the “Machine Learning Feature Selection” field.
11.The “Machine Learning Algorithm” section includes 9 distinct classifiers that can be chosen by clicking on the corresponding classifier name. Furthermore, each classifier has hyperparameter fields that can be modified. For example, select the “Extra Trees classifier”. Input “1250” in the “n_estimators” field. Select “entropy” in the “criterion” drop-down menu. Select “log2” in the “max_features” drop-down menu. All other hyperparameters can be kept as default.
12.Select whether to calibrate algorithm predictions by selecting the appropriate choice in the “Calibrate algorithm predictions” field. This setting will calibrate the predictions output by the chosen model, eliminating model-imparted bias. It is recommended to keep this setting as default.
13.Select a cross-validation method from the drop-down menu under the “Cross-Validation Algorithm” field. The recommended option is Repeated Stratified Group K-Fold because this cross-validation method will maintain class ratios across train and validation splits. Furthermore, choose the number of cross-validation folds and cross-validation repetitions in the subsequent fields. For the demonstration, input “10” into the “Number of Cross-Validation Folds” field and “3” into the “Number of Cross-Validated Repetitions” field.
14.Choose the primary evaluation metric for assessing the performance of the model from the drop-down menu under the “Primary Evaluation Metric” field. The default and recommended metric is “roc_auc”.
15.Choose any additional evaluation metrics from the drop-down menu under the “Evaluation Metrics” field, and these metrics will also be reported for the trained model.
16.Click “Submit” at the bottom of the input form.
Navigating the Drugmonizome-ML Appyter Notebook
17.A Jupyter Notebook will begin executing in the cloud once the input form is submitted. The notebook includes an option to download the notebook, toggle displaying the code, and run the notebook locally. Additionally, a table of contents exists with clickable elements that link to specific sections within the notebook (Fig. 101).
18.Scroll down to the “Select Input Datasets and Target Classes” section or click on the corresponding section from the table of contents. The feature matrix that was generated based on the selected features from the input form is displayed. The feature matrix is composed of 19,898 compounds and 3026 features from LINCS Gene Expression Profiles and TF-IDF normalized Morgan Fingerprints (Fig. 102).
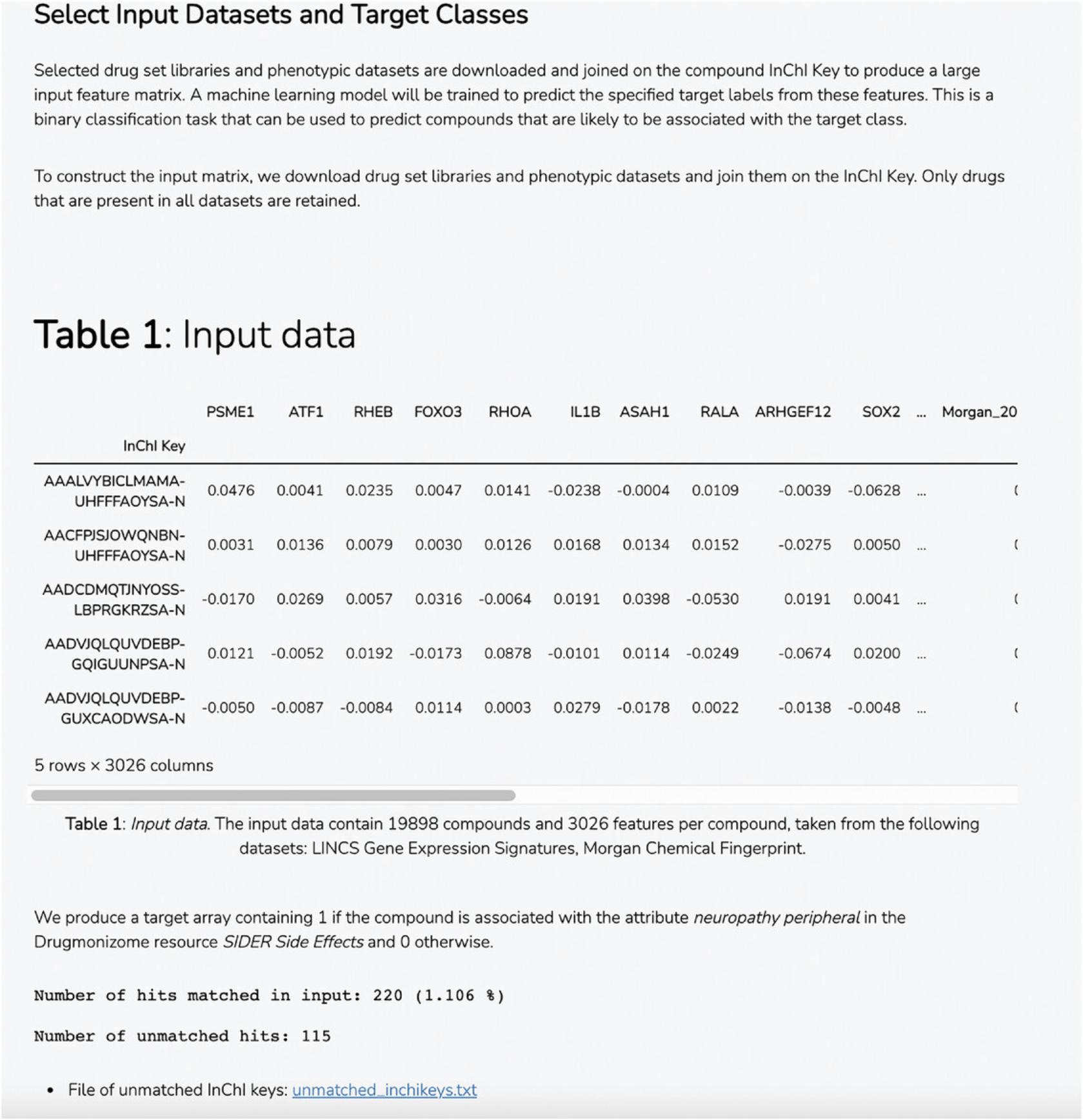
19.Additionally, information is displayed about how the target array is constructed, how many compounds from the target array are included in the feature matrix, and how many compounds were discarded because they were not included in the feature matrix. Unmatched compounds are available for download.
20.Navigate to the “Dimensionality Reduction and Visualization” section to view the input feature space using the dimensionality reduction and visualization methods that were selected in the input form. Positive class labels are labeled within the visualization to demonstrate how the class of interest is clustered in the feature space (Fig. 103).
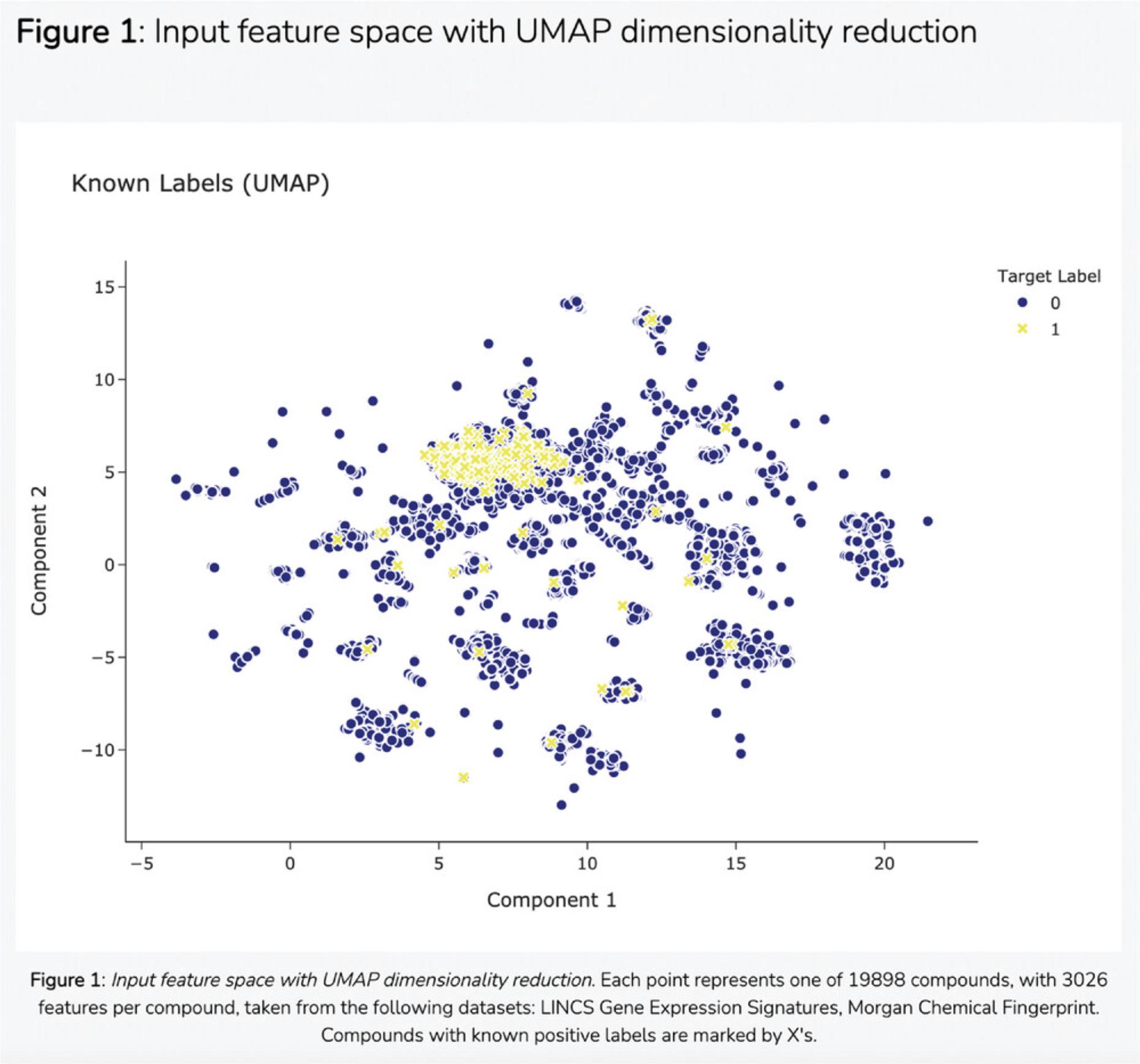
21.Navigate to the “Machine Learning” section to view the trained classifier and evaluations of the classifier's performance. The receiver operating characteristic curve (Fig. 104), precision-recall curve (Fig. 105), and confusion matrix (Fig. 106) are displayed. Click the hyperlinks in the figure headers to download the figures.
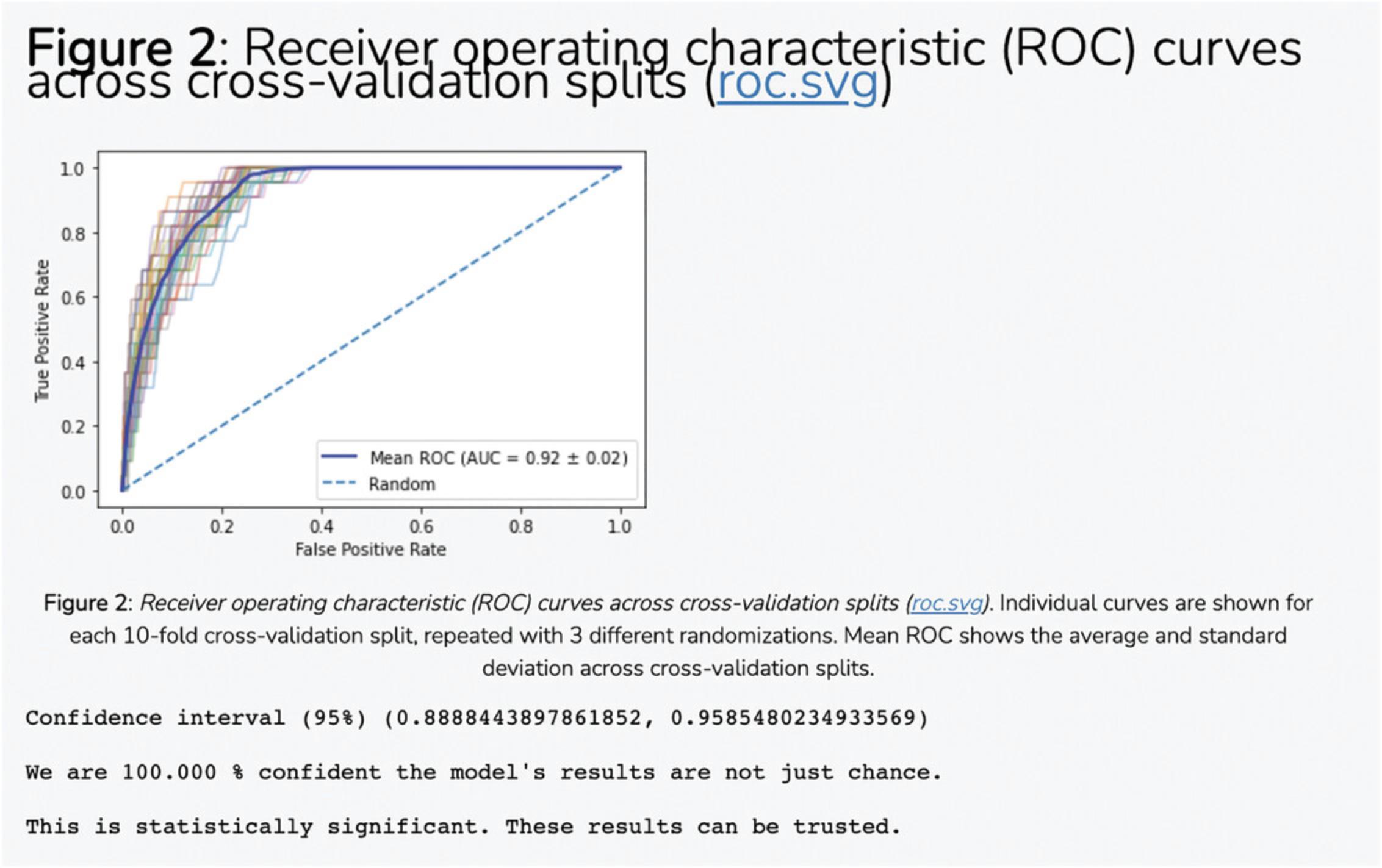
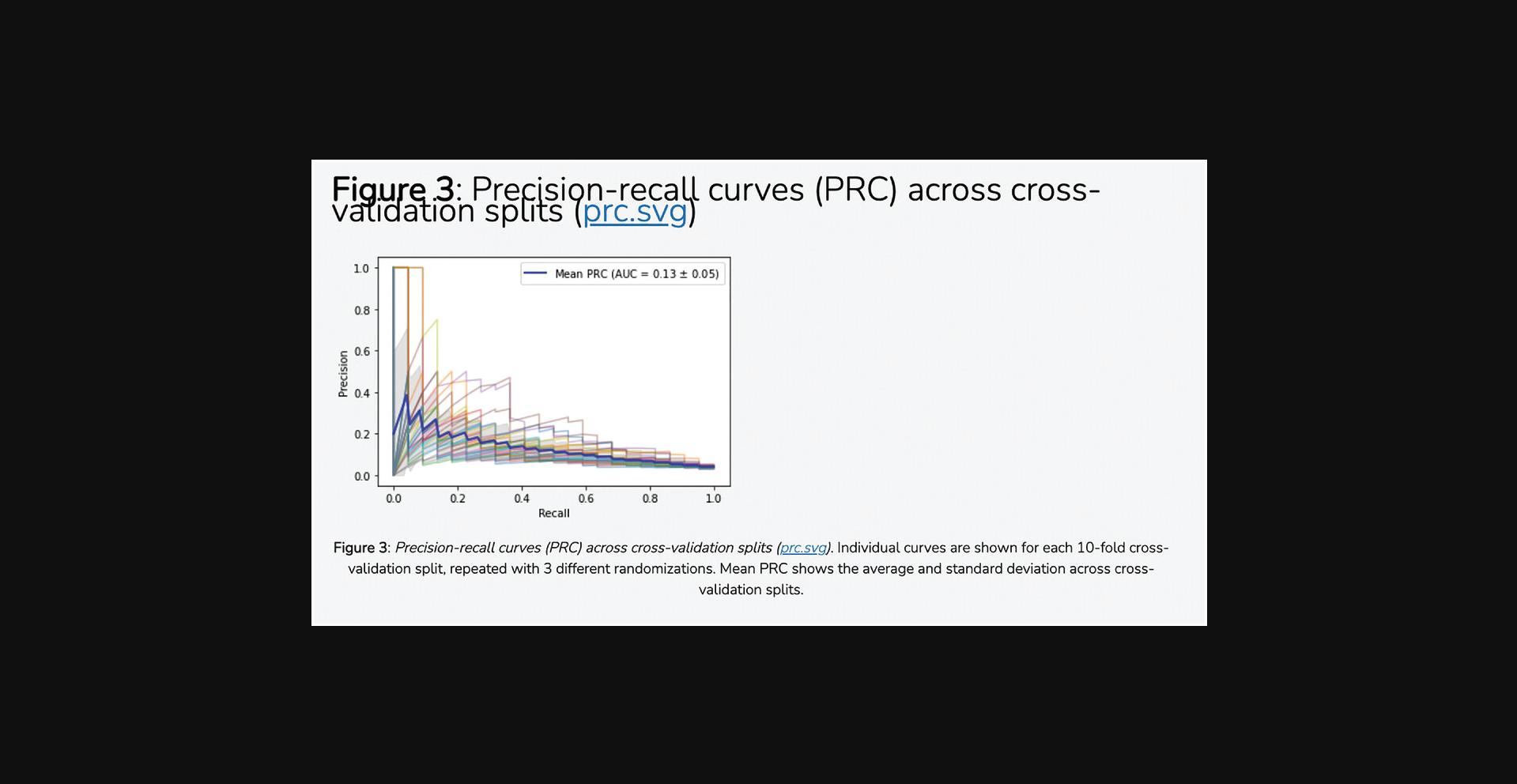
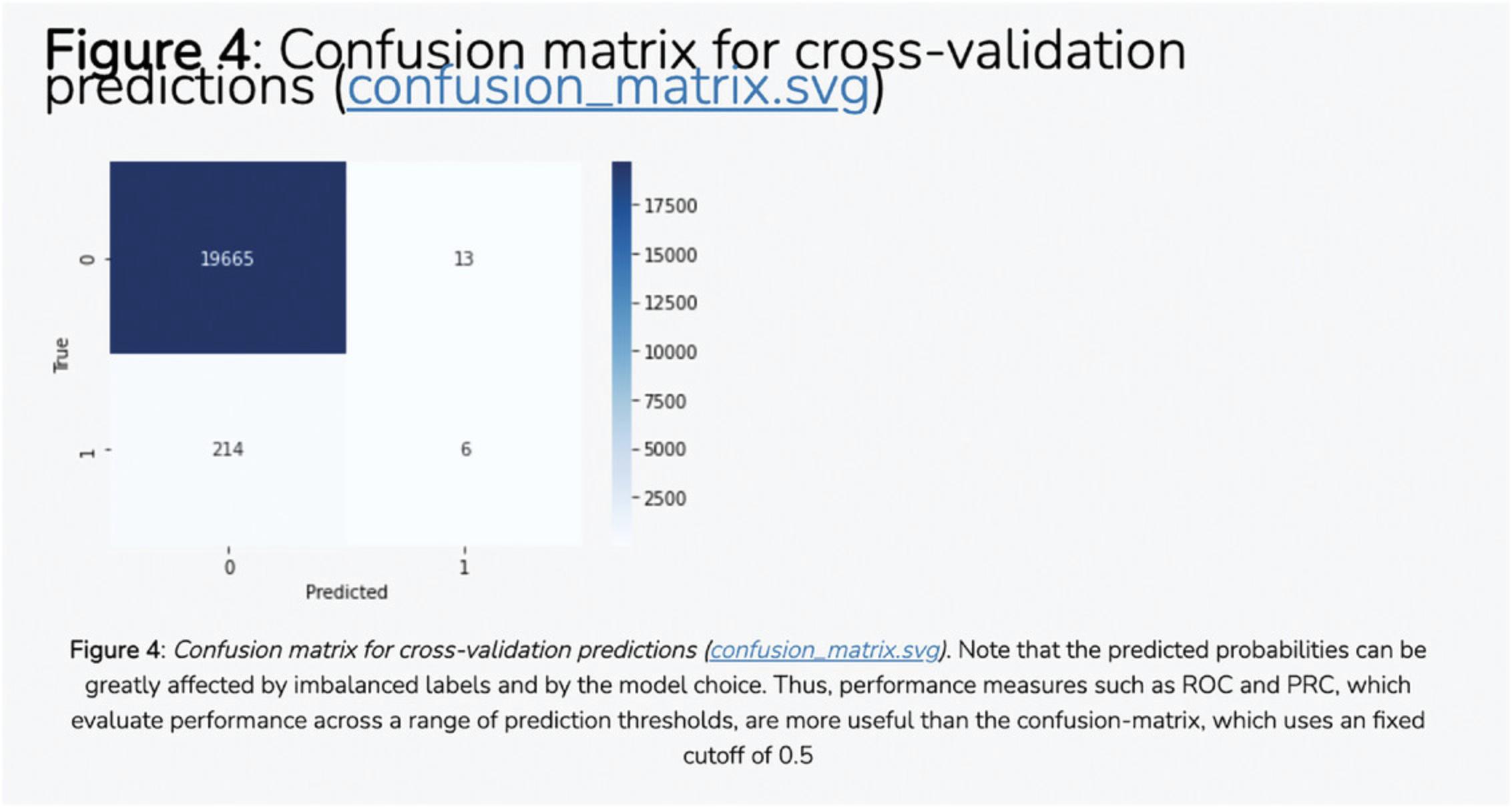
22.Navigate to the “Examine Predictions” section to view the predictions made by the model in addition to the distributions of mean probability estimates and t -statistics. Figures displaying the distribution of mean cross-validation predictions (Fig. 107), distribution of t -statistics (Fig. 108), a UMAP visualization of the feature space with overlaid predictions (Fig. 109), and a filterable table of the top predicted compounds (Fig. 110) are displayed. Click the hyperlinks in the figure and table headers to download the corresponding figure or table.
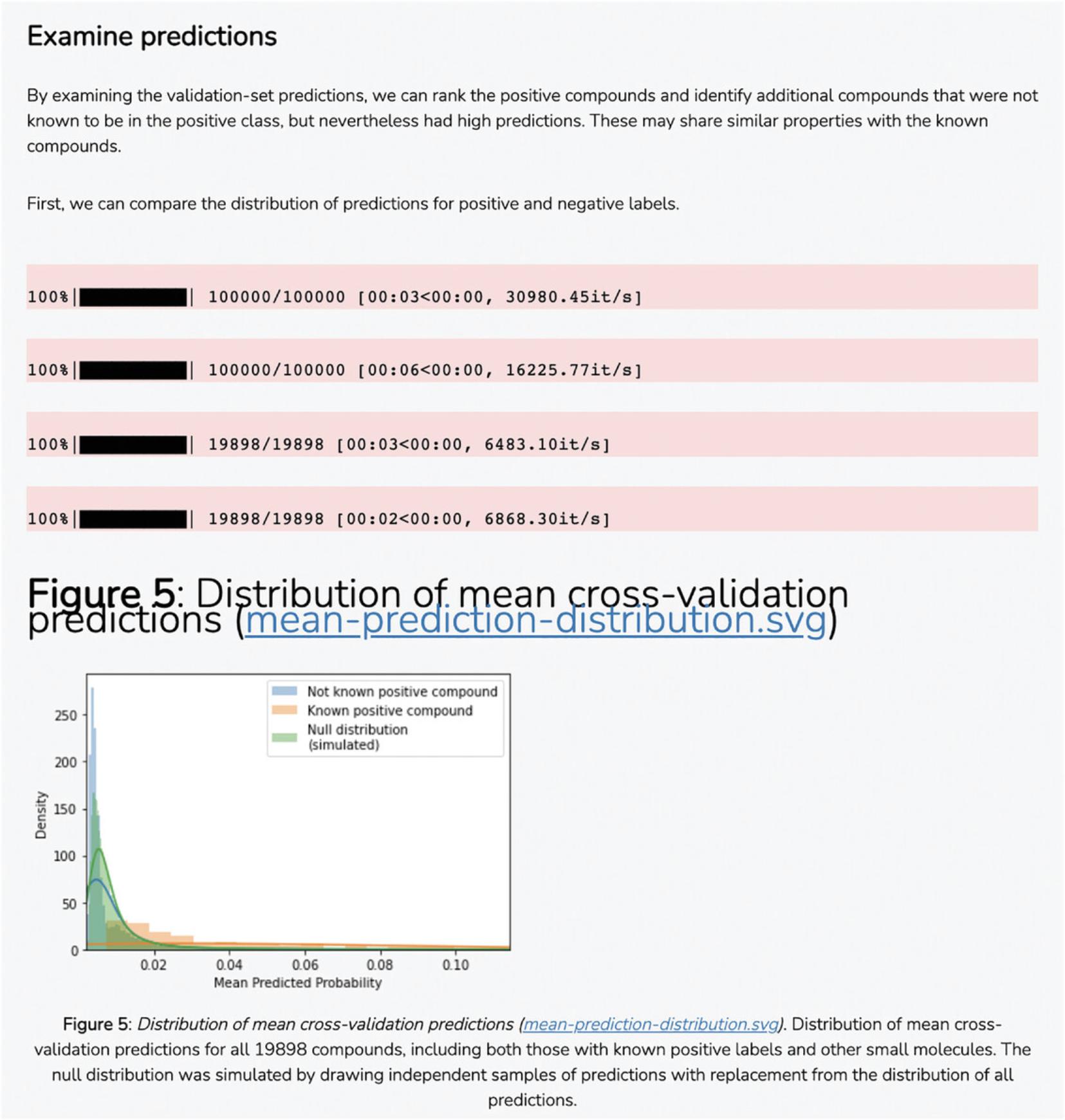
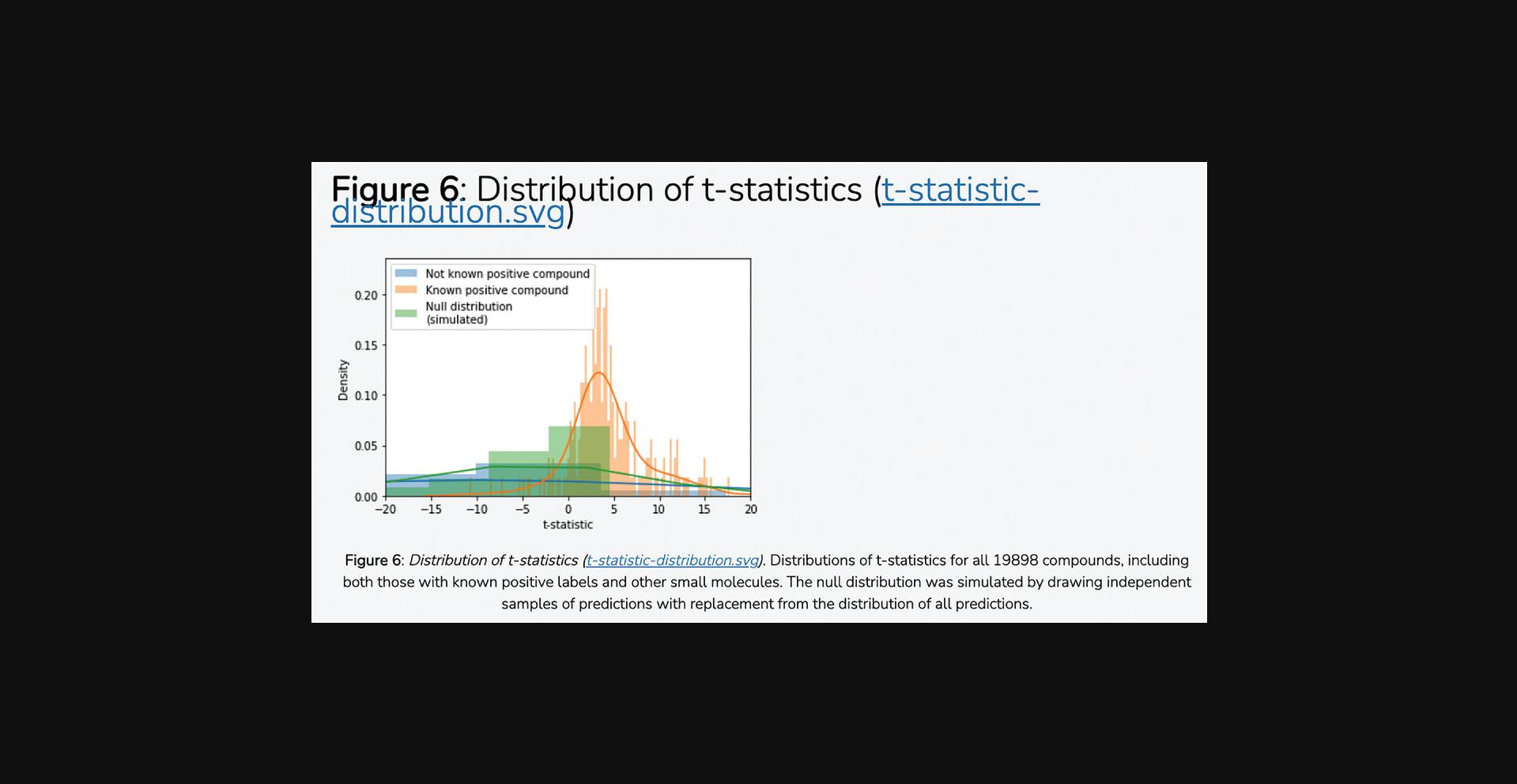
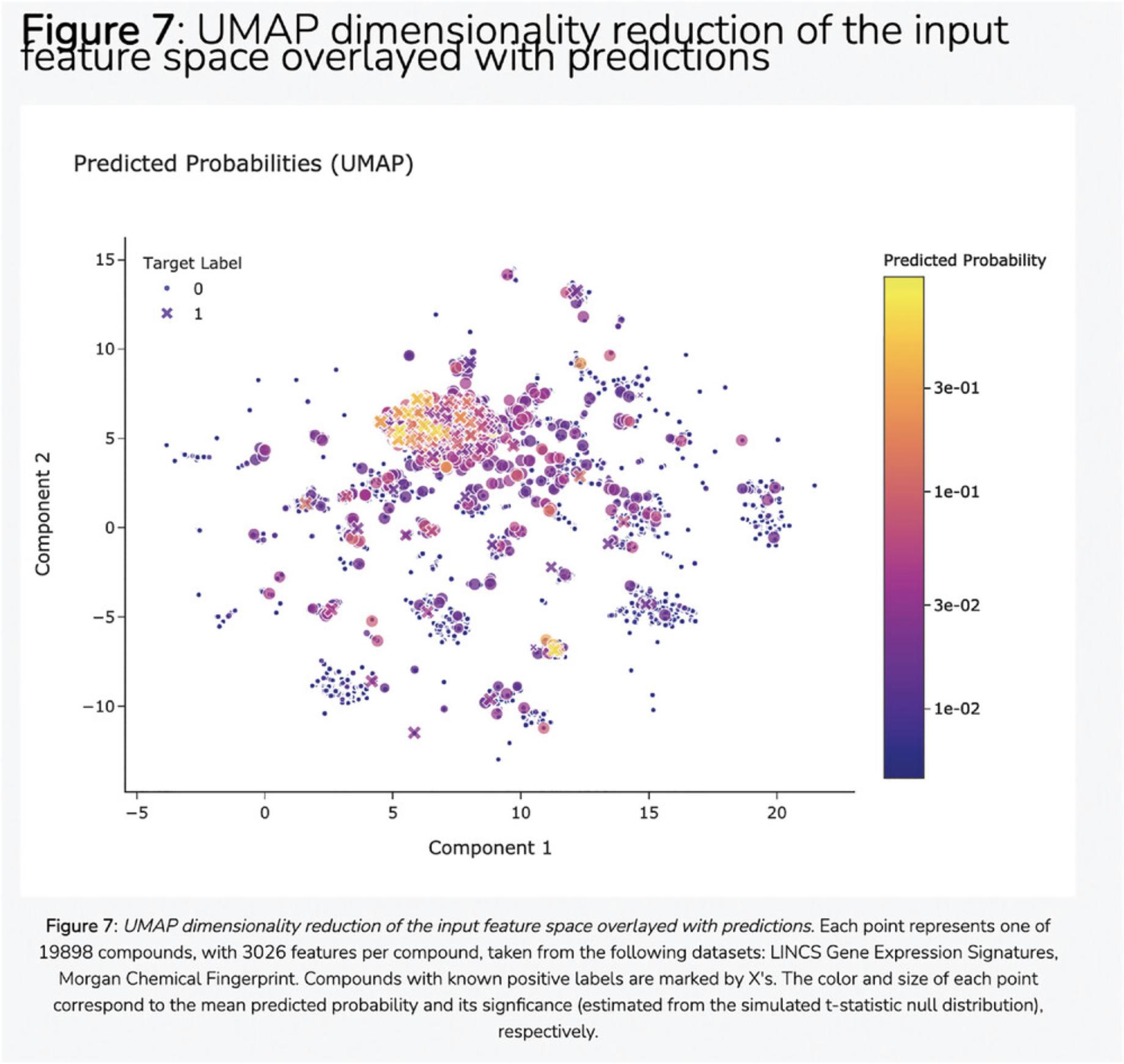
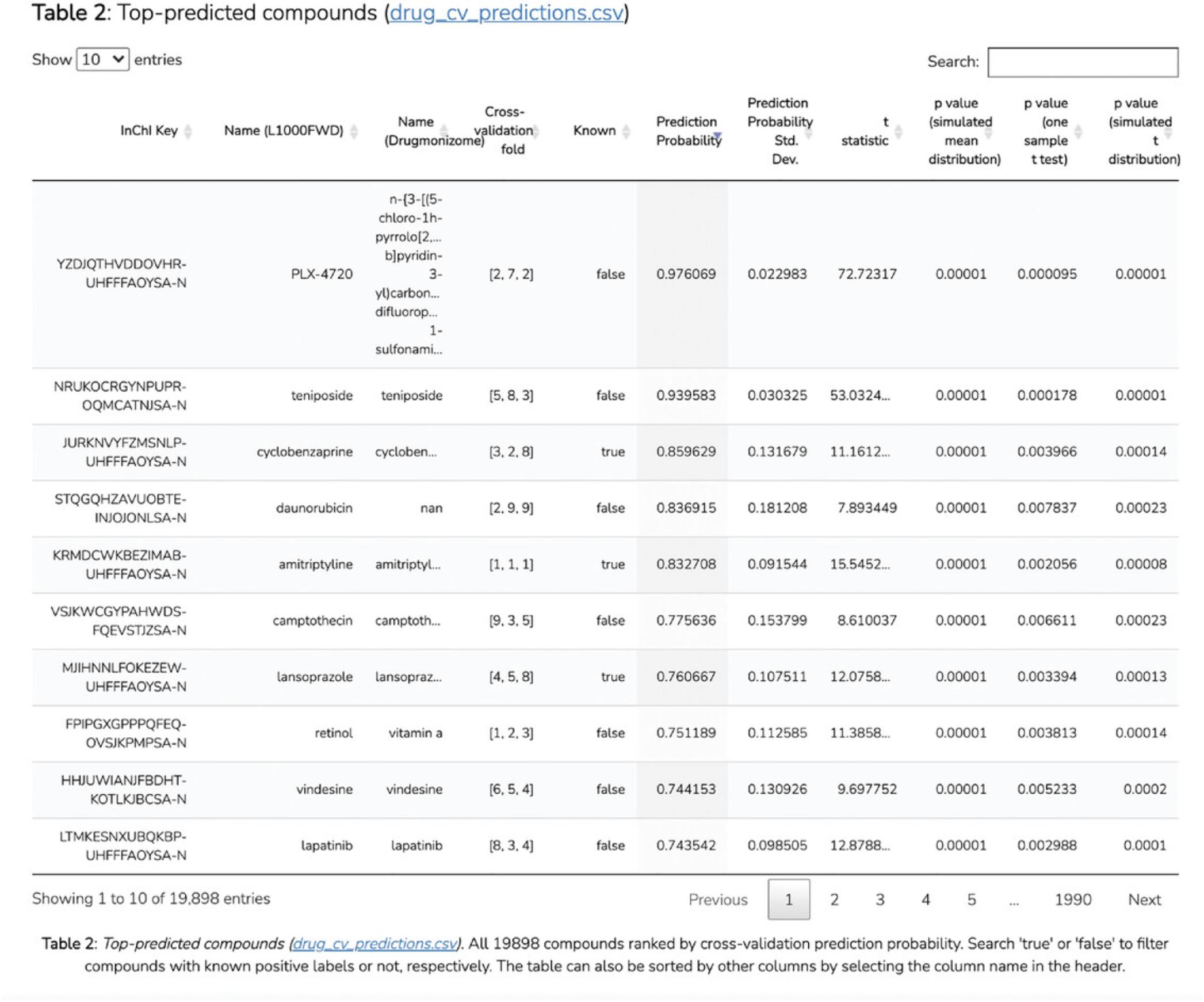
23.Navigate to the “Feature Importance” section to view the most important features from the input feature matrix that were used to make predictions. A table of the most important features used by the model to make predictions (Fig. 111), as well as a figure depicting the distributions of average and cumulative sum of feature importance (Fig. 112), are displayed. Click the hyperlinks in the figure and table headers to download the corresponding figure or table.
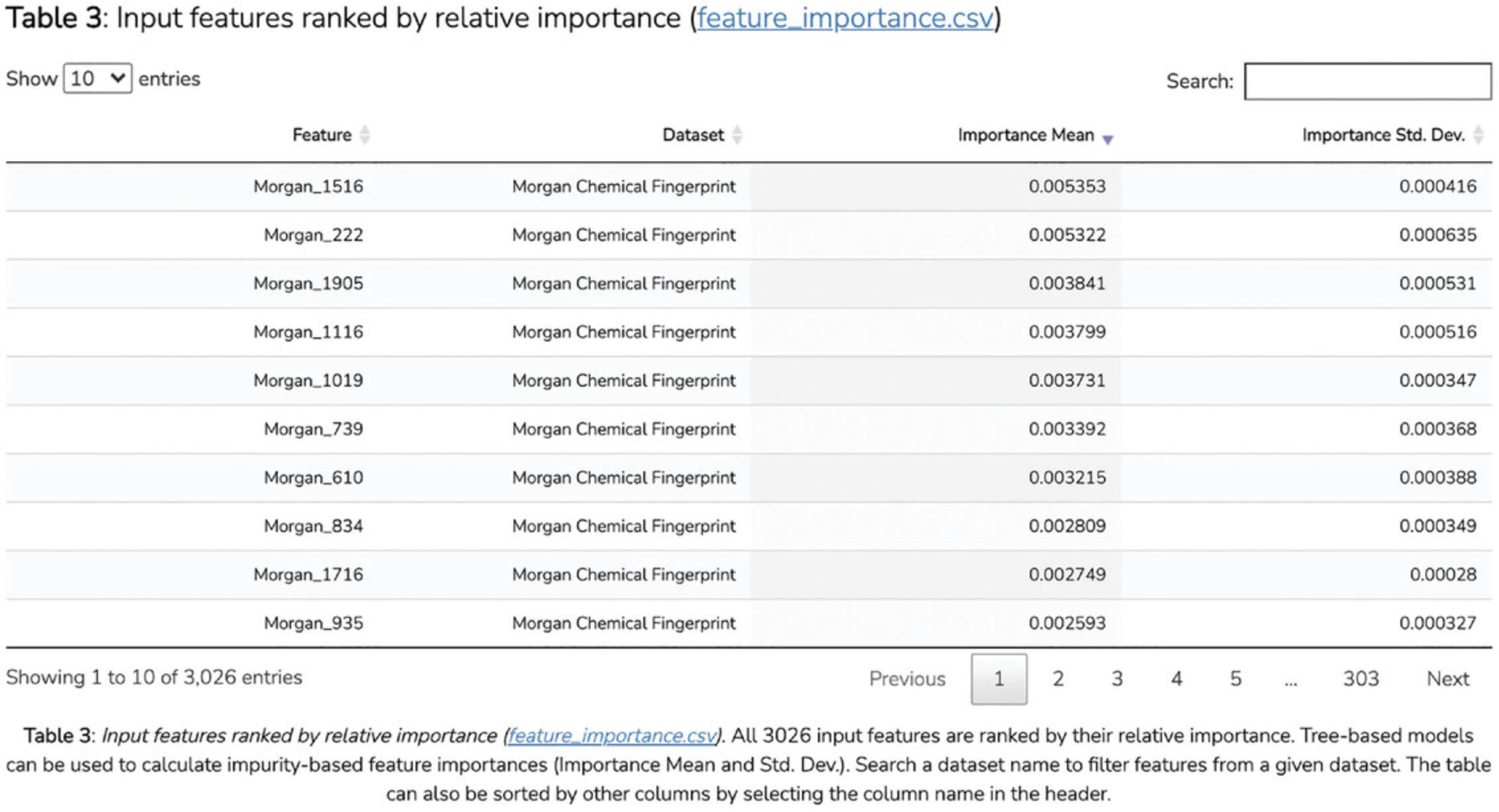
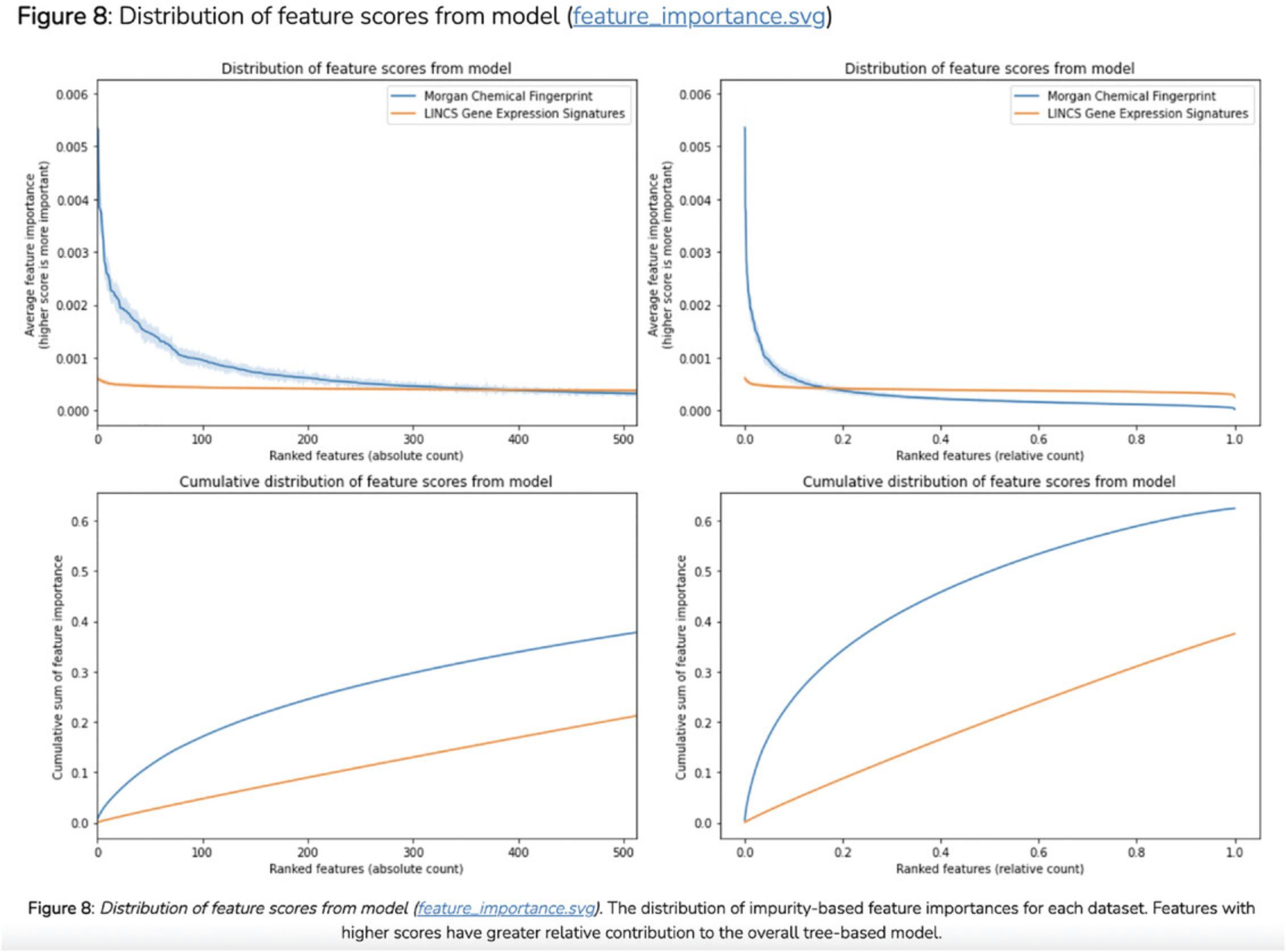
Basic Protocol 11: THE HARMONIZOME-ML APPYTER
Harmonizome (Rouillard et al., 2016) is a collection of processed datasets that abstract knowledge about genes and proteins. Using the processed data from Harmonizome, Harmonizome-ML enables interactive imputation of knowledge about the function and other properties of genes and proteins using machine learning. Combined with the user-friendly interface of an Appyter (Clarke et al., 2021)–a web-based software application enabling users to execute bioinformatics workflows without coding–the Harmonizome-ML Appyter can be used to build and evaluate machine learning pipelines with Harmonizome data in an accessible way. The Harmonizome-ML Appyter asks users to select or upload attributes for learning as well as specify a target vector to predict. Users also need to select from various machine learning algorithms and performance evaluation methods. Once these options are selected, the workflow is executed, and the results are presented as a Jupyter Notebook that is shareable and downloadable.
Necessary Resources
Hardware
- Desktop or a laptop computer, or a mobile device, with a fast Internet connection
Software
- An up-to-date web browser such as Google Chrome (https://www.google.com/chrome/), Mozilla Firefox (https://www.mozilla.org/en-US/firefox/), Apple Safari (https://www.apple.com/safari/), or Microsoft Edge (https://www.microsoft.com/en-us/edge)
Navigating the input page
1.Navigate to the Harmonizome-ML Appyter (https://appyters.maayanlab.cloud/#/harmonizome_ml). The input form is divided into two sections: “attribute and prediction class dataset selection” and “settings.”
2.In the “Attribute and Prediction Class Selection” section, select attributes by clicking on the check box to the left of an attribute of choice; a blue check mark indicates that an attribute has been selected. Users may opt to upload a custom attribute dataset using the “Browse” button as well. Target selection can be from Harmonizome or customized; click on the text for the target selection desired and customize the class in the text box below (Fig. 113).
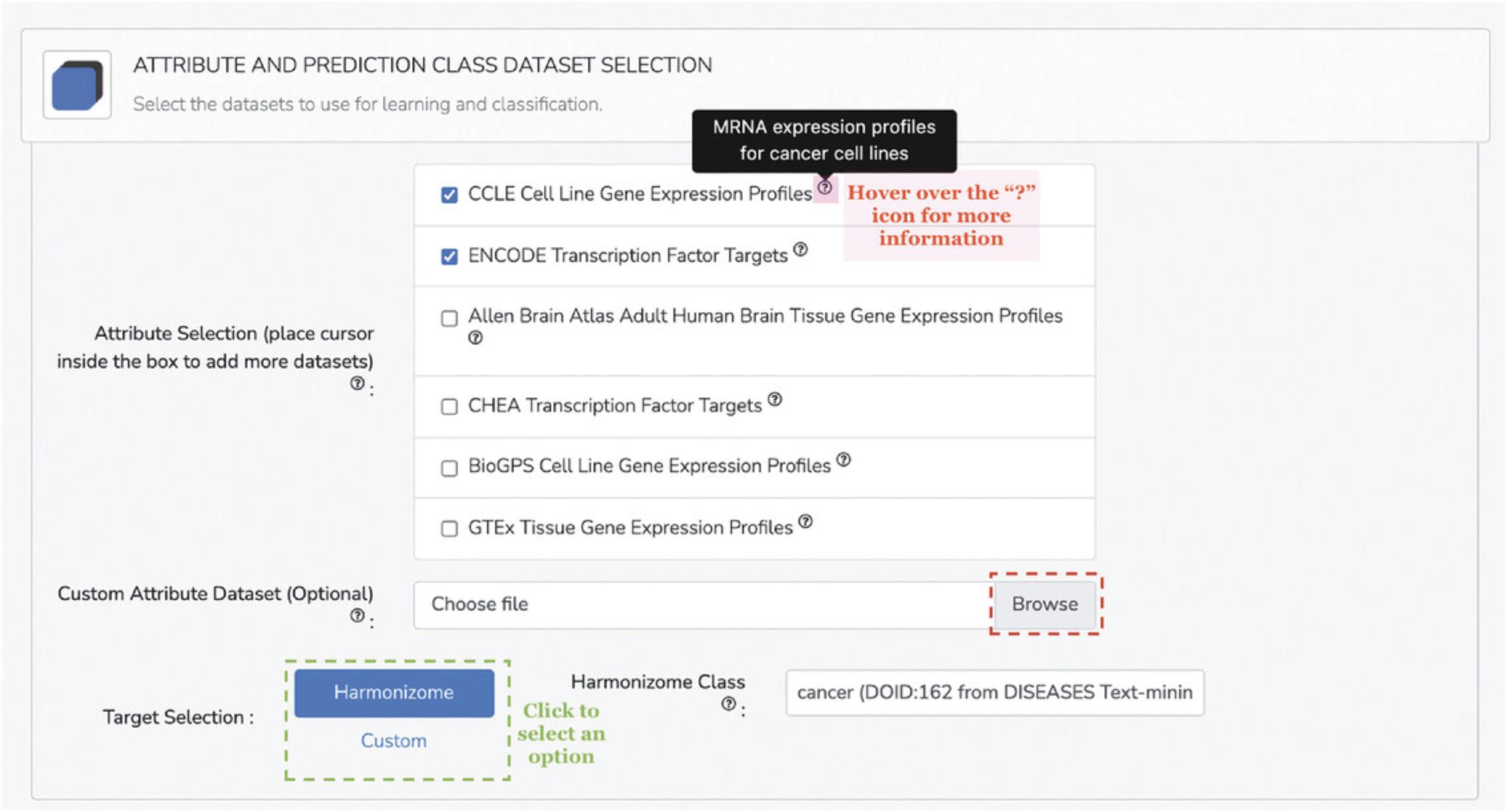
3.The “Settings” section includes settings for various algorithms (dimensionality reduction, manifold projection, ML feature selection, cross validation, ML algorithm, hyperparameter search type, evaluation metrics) that can be customized. Simply click on the drop-down menu below an algorithm to view and update the options. For example, clicking on the drop-down menu for “Dimensionality Reduction Algorithm” displays the following options: PCA, truncated SVD, incremental PCA, ICA, and Sparse PCA. Click on the desired algorithm to use it for dimensionality reduction (Fig. 114).
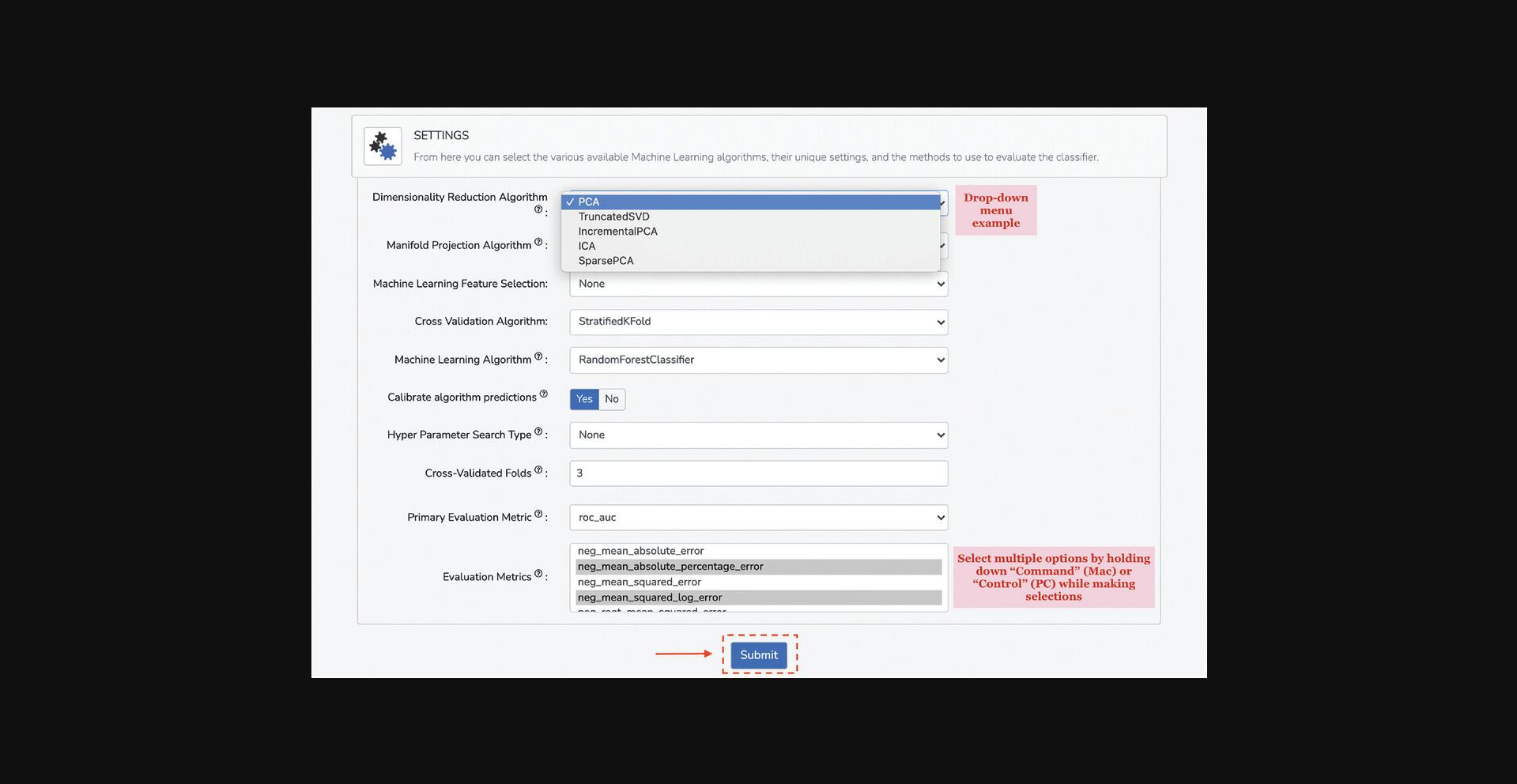
4.Once all selections have been made, click on the “Submit” button at the bottom of the page to run the analyses and generate the notebook.
Navigating the notebook
5.Each notebook generated by the Harmonizome-ML Appyter includes explanations followed by code, data, and figures (both static and interactive). To download the notebook, toggle notebook code, or run the notebook locally, select the appropriate button at the top of the page. The notebook is divided into three sections (which can be accessed through the table of contents on the left side of the page): Inputs, Dimensionality Reduction, and Machine Learning (Fig. 115).
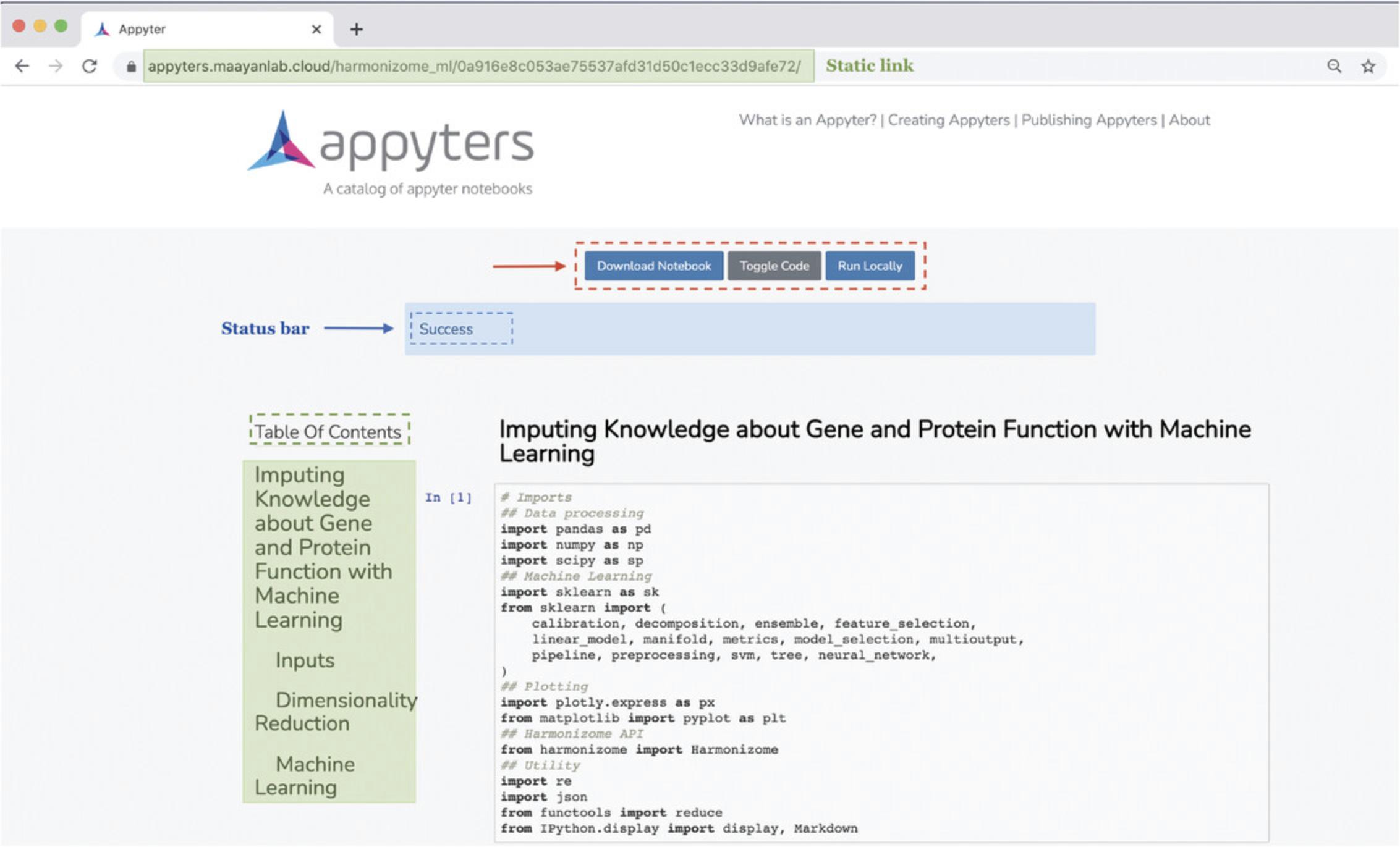
6.Navigate to the “Inputs” section to view the feature matrix Dataframe generated from the datasets selected in the input form (Fig. 116). Note that some Dataframes contain additional columns that can be explored by scrolling left to right. The first two Dataframes are individual datasets, whereas the final Dataframe displays the concatenated feature matrix that will be used for classification.
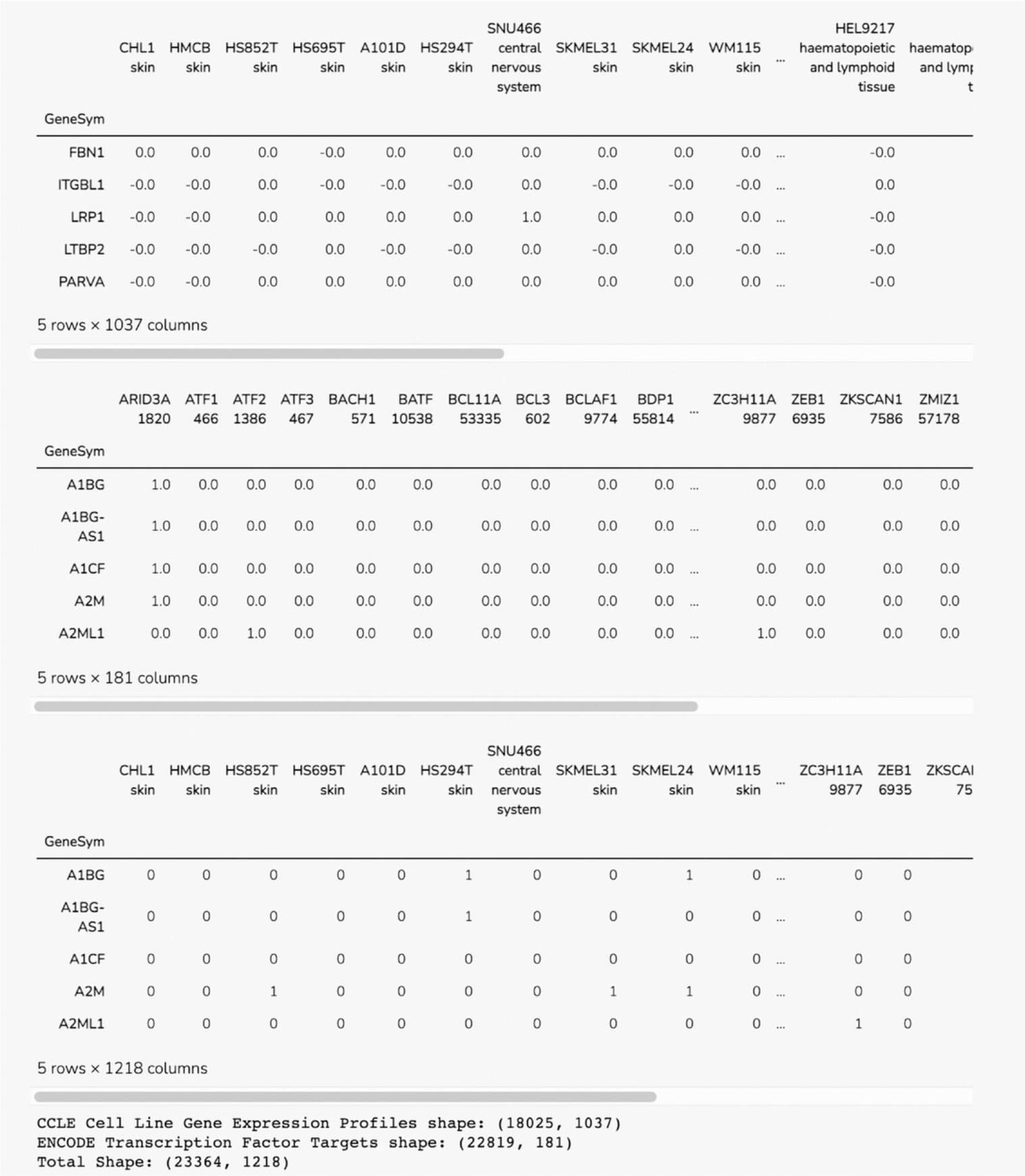
7.Scroll down to view the target array created from the dataset containing the class label to be predicted. Genes that are known to be associated with the class label are annotated with a 1, whereas genes not known to be associated with the class label are annotated with a 0 (Fig. 117).
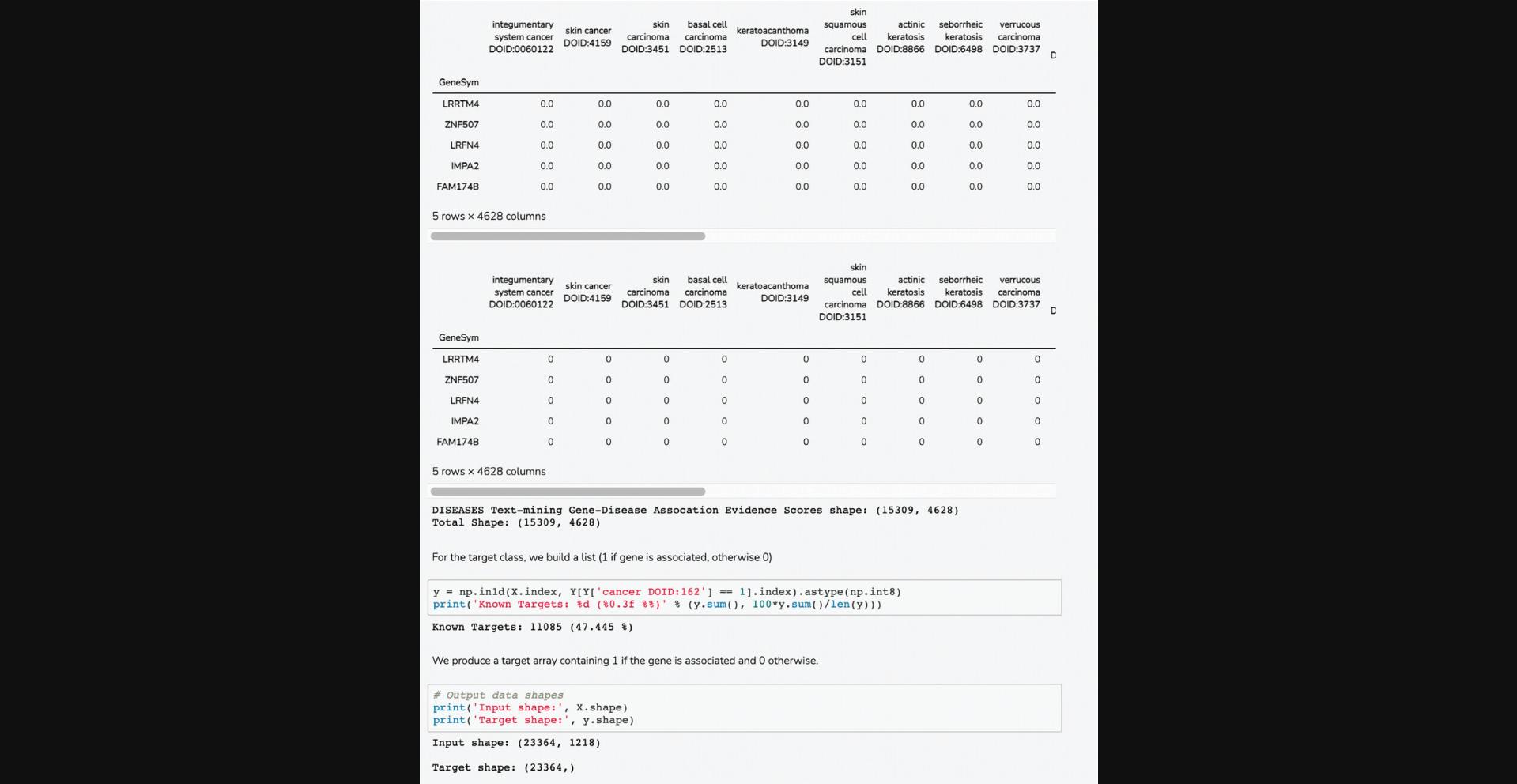
8.Navigate to the “Dimensionality Reduction” section. The process of dimensionality reduction involves transforming data from high-dimensional spaces to low-dimensional spaces without losing too much information. The input features are reduced using PCA and visualized in a 3D scatter plot (Fig. 118). The reduced features are also projected onto a manifold with T-SNE (Fig. 119).
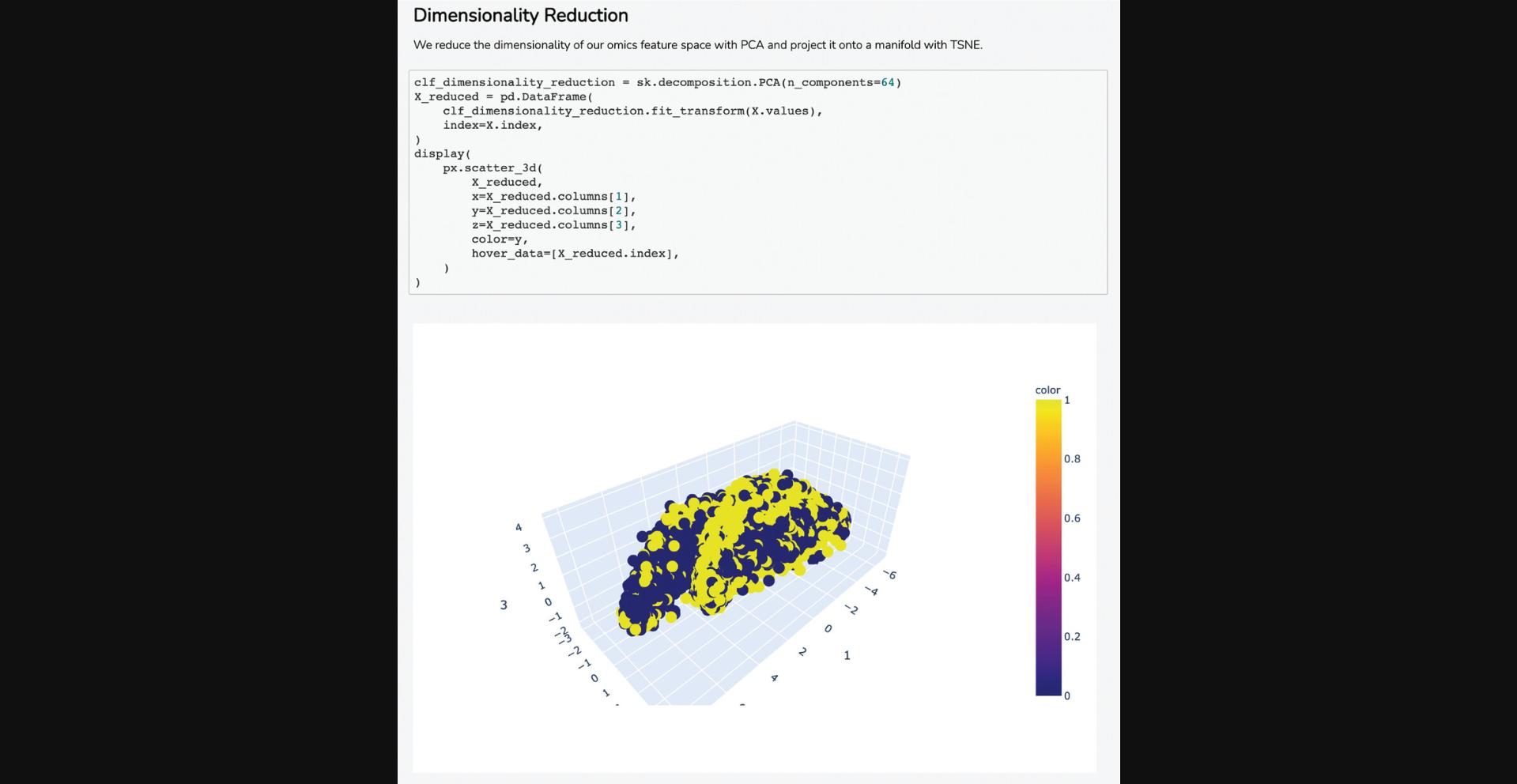
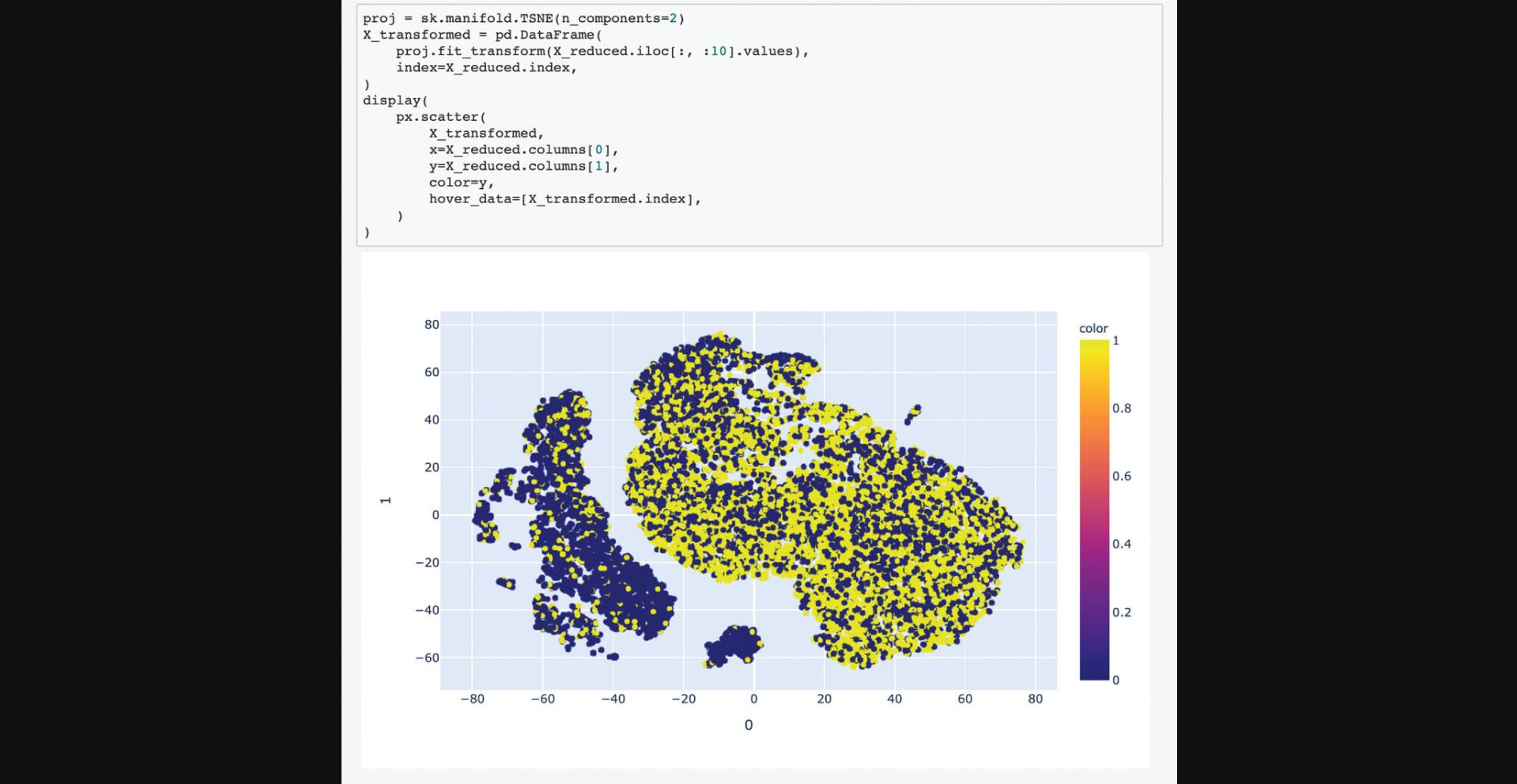
9.Navigate to the “Machine Learning” section which features the machine learning pipeline assembled from the input form submission. A model is generated and trained via the customized pipeline and then used to predict genes that are strongly correlated with the target attribute. General explanations for the model's performance are provided with ROC curves and a prediction matrix (Fig. 120).
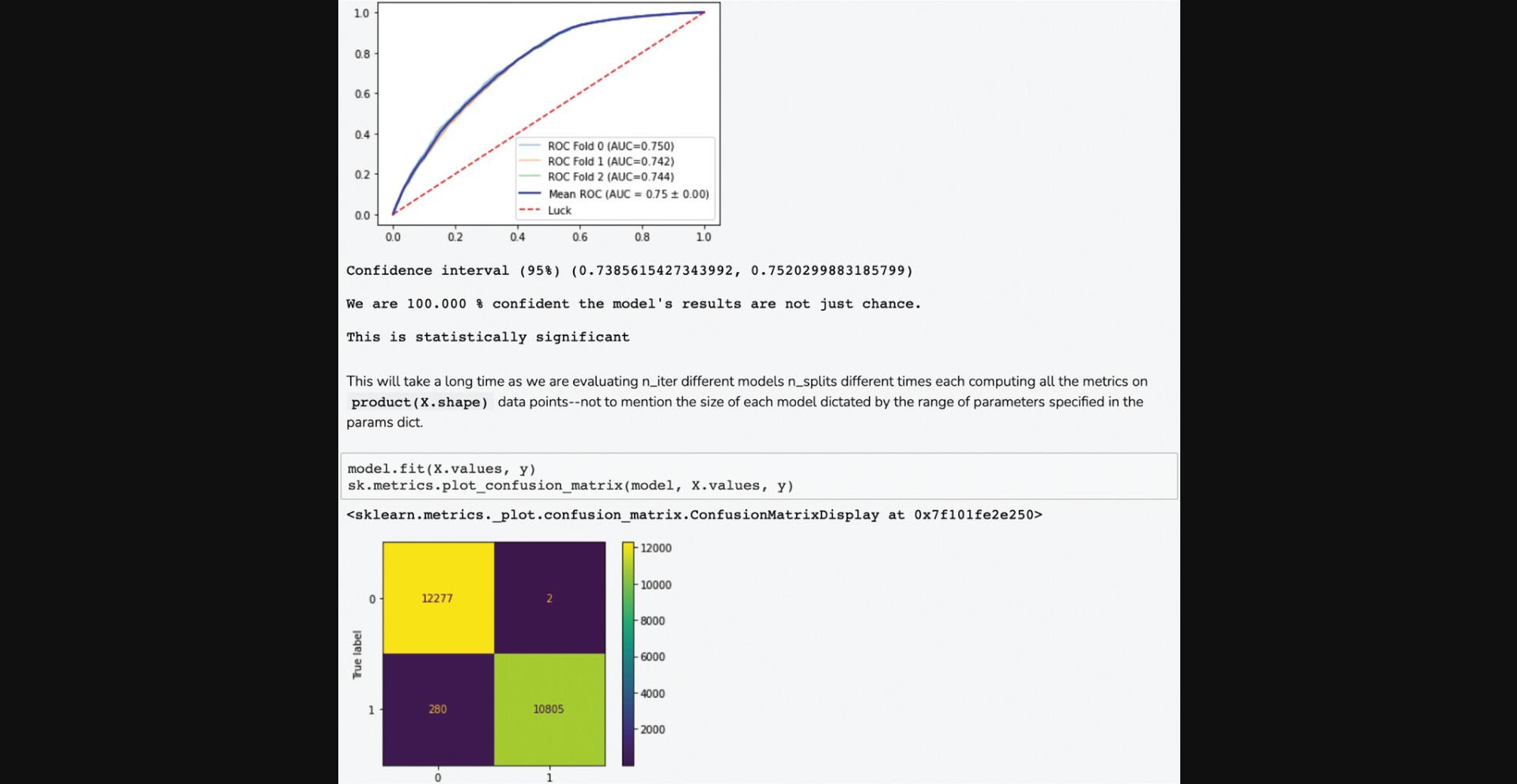
10.The prediction results are provided at the end of the pipeline and can be downloaded as a tab-separated (.tsv) file by clicking on results.tsv at the end of the notebook (Fig. 121).
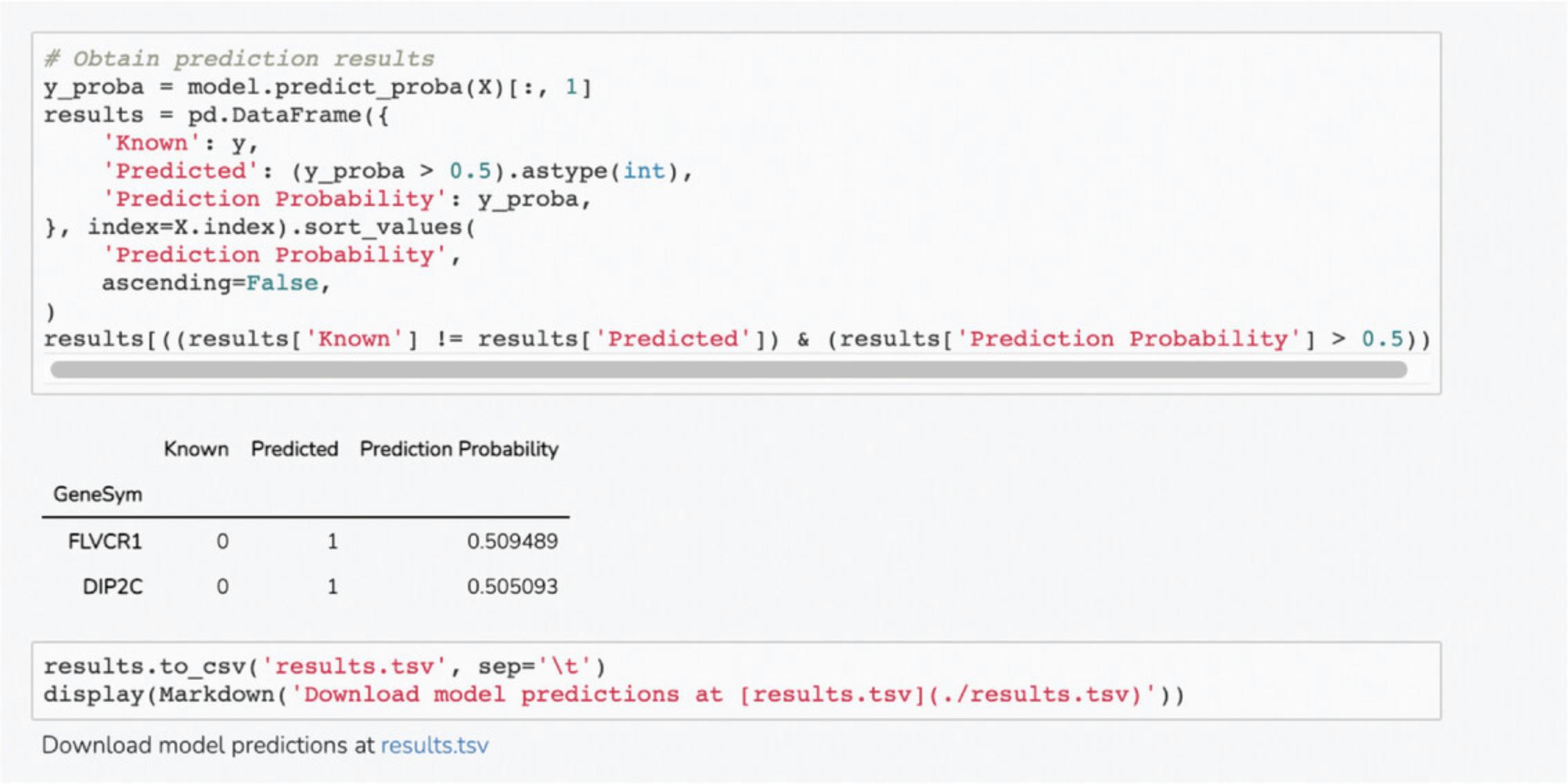
Basic Protocol 12: GWAS TARGET ILLUMINATION WITH TIGA
Target Illumination GWAS Analytics (TIGA; Yang et al., 2021) is a web application that facilitates drug target illumination by scoring and ranking protein-coding genes associated with traits from genome-wide association studies (GWAS). Similarly, TIGA can score and rank traits with the same gene-trait association metrics. Rather than a comprehensive analysis of GWAS for all biological implications and insights, this focused application provides a rational method by which GWAS findings can be aggregated and filtered for applicable, actionable intelligence, with evidence usable by drug discovery scientists to enrich prioritization of target hypotheses. TIGA derives its GWAS summary and metadata solely from the NHGRI-EBI GWAS Catalog and study-associated publications. Thus, TIGA traits are identified by Experimental Factor Ontology (EFO) terms.
Necessary Resources
Hardware
- Desktop or a laptop computer, or a mobile device, with a fast Internet connection
Software
- An up-to-date web browser such as Google Chrome (https://www.google.com/chrome/), Mozilla Firefox (https://www.mozilla.org/en-US/firefox/), Apple Safari (https://www.apple.com/safari/), or Microsoft Edge (https://www.microsoft.com/en-us/edge)
Navigating the input page
1.Navigate to the TIGA web app (https://unmtid-shinyapps.net/shiny/tiga/).
Trait to gene search
2.A trait query may be specified by browsing and selecting from the Traits (ALL) tab, or via the Trait query field.
3.To find genes associated with the EFO term "worry measurement" (EFO_0009589), begin typing "worry" in the Trait query field, and autosuggest will assist in selecting the trait, (Fig. 122).
<img src="https://static.yanyin.tech/literature_test/cpz1355-fig-0122-m.jpg" alt="TIGA gene plot for trait "worry measurement" (EFO_0009589)." loading="lazy" title="Details are in the caption following the image"/>
4.TIGA results will be displayed via the HitsTable tab and HitsPlot tab (Fig. 123).
<img src="https://static.yanyin.tech/literature_test/cpz1355-fig-0123-m.jpg" alt="TIGA gene hitlist for trait "worry measurement" (EFO_0009589)." loading="lazy" title="Details are in the caption following the image"/>
5.The HitsTable is ranked by meanRankScore as a measure of the strength and confidence of the inferred gene-trait association.
6.The HitsPlot displays hits with meanRankScore on the horizontal axis, and Effect on the vertical axis, either measured by odds ratio (OR) or N_beta (count of beta values).
7.Hits are annotated, either in the table as columns or as hover-tooltips, with several identifiers, measures, and variables, derived from the aggregated GWAS, or annotated from IDG. Target Development Levels (TDLs) are also color coded for ease of use, facilitating identification of well-known targets (Tclin) and under-studied targets (Tdark).
8.From the HitsTable, for a specific gene, the magnifying-glass icon links to the TIGA provenance for the corresponding gene-trait association. The provenance displays studies and publications supporting the association, with GWAS Catalog and PubMed link-outs, respectively, (Fig. 124).
<img src="https://static.yanyin.tech/literature_test/cpz1355-fig-0124-m.jpg" alt="TIGA provenance for trait "worry measurement" (EFO_0009589) associated gene Musculoskeletal embryonic nuclear protein 1 (MUSTN1), with two studies and associated publications, with GWAS Catalog and PubMed link-outs, respectively." loading="lazy" title="Details are in the caption following the image"/>
Gene to trait search
9.In Gene query mode, TIGA behaves much the same as in Trait query mode, but with traits as hits. Data that pertain to gene-trait associations will be the same, such as provenance, regardless of query mode.
10.TIGA genes are, as in the Catalog, identified by Ensembl Gene IDs. The Gene query field will autosuggest based on gene symbols. Thus, by typing "RAS", autosuggest will assist in selecting "RASA2", "Ras GTPase-activating protein 2."
11.As in Gene query mode, results will be via HitsTable and HitsPlot tabs.
Basic Protocol 13: PRIORITIZING KINASES FOR LISTS OF PROTEINS AND PHOSPHOPROTEINS USING KEA3
Kinase Enrichment Analysis 3 (KEA3) (Kuleshov et al., 2021) is a web-based server application that infers overrepresented upstream kinases whose putative substrates are present in a user-inputted list of differentially-phosphorylated proteins. To infer upstream kinases, KEA3 uses a collection of kinase-substrate libraries created from processing data from several online databases. Kinase enrichment analysis results are provided for each kinase-substrate library, as well as two integrated approaches to integrate all libraries: MeanRank and TopRank. The gene sets from the kinase-substrate libraries are compared to the user-inputted protein list, and Fisher's Exact Test is used to compute the significance of the overlap to prioritize kinases. The resulting ranked lists of kinases, as well as visualizations of the significant kinases as networks, are returned to the users as interactive and downloadable figures.
Necessary Resources
Hardware
- Desktop or a laptop computer, or a mobile device, with a fast Internet connection
Software
- An up-to-date web browser such as Google Chrome (https://www.google.com/chrome/), Mozilla Firefox (https://www.mozilla.org/en-US/firefox/), Apple Safari (https://www.apple.com/safari/), or Microsoft Edge (https://www.microsoft.com/en-us/edge)
- NOTE : There is a tutorial on navigating KEA3 results (https://maayanlab.cloud/kea3/templates/tutorial.jsp) from which some of the steps in this protocol have been paraphrased.
Submitting a gene set to KEA3
1.Navigate to the KEA3 homepage (https://maayanlab.cloud/kea3/).
2.Gene/protein sets may be submitted to KEA3 in two ways: by uploading the set as a plain text file or by pasting a list, one gene/protein name per line, into a text box. When submitting genes/proteins using the text box, a checklist below the text box denotes duplicates and confirms valid gene symbols in the input. Once uploaded or inputted, click on the “Submit” button to begin the analysis (Fig. 125).
Navigating KEA3 results
3.Scroll down to view the “Integrated results” tab, which includes bar charts, tables, subnetwork visualizations, and a clustergrammer visualization of integrated results across all KEA3 libraries using the MeanRank and TopRank methods (Fig. 126). The MeanRank method calculates the average rank, whereas the TopRank method calculates the best scaled rank of each kinase across all libraries containing the kinase. The tables can be downloaded in TSV format and visualizations can be downloaded in SVG and PNG format. Use the slider above each visualization to change the number of top results that are displayed.
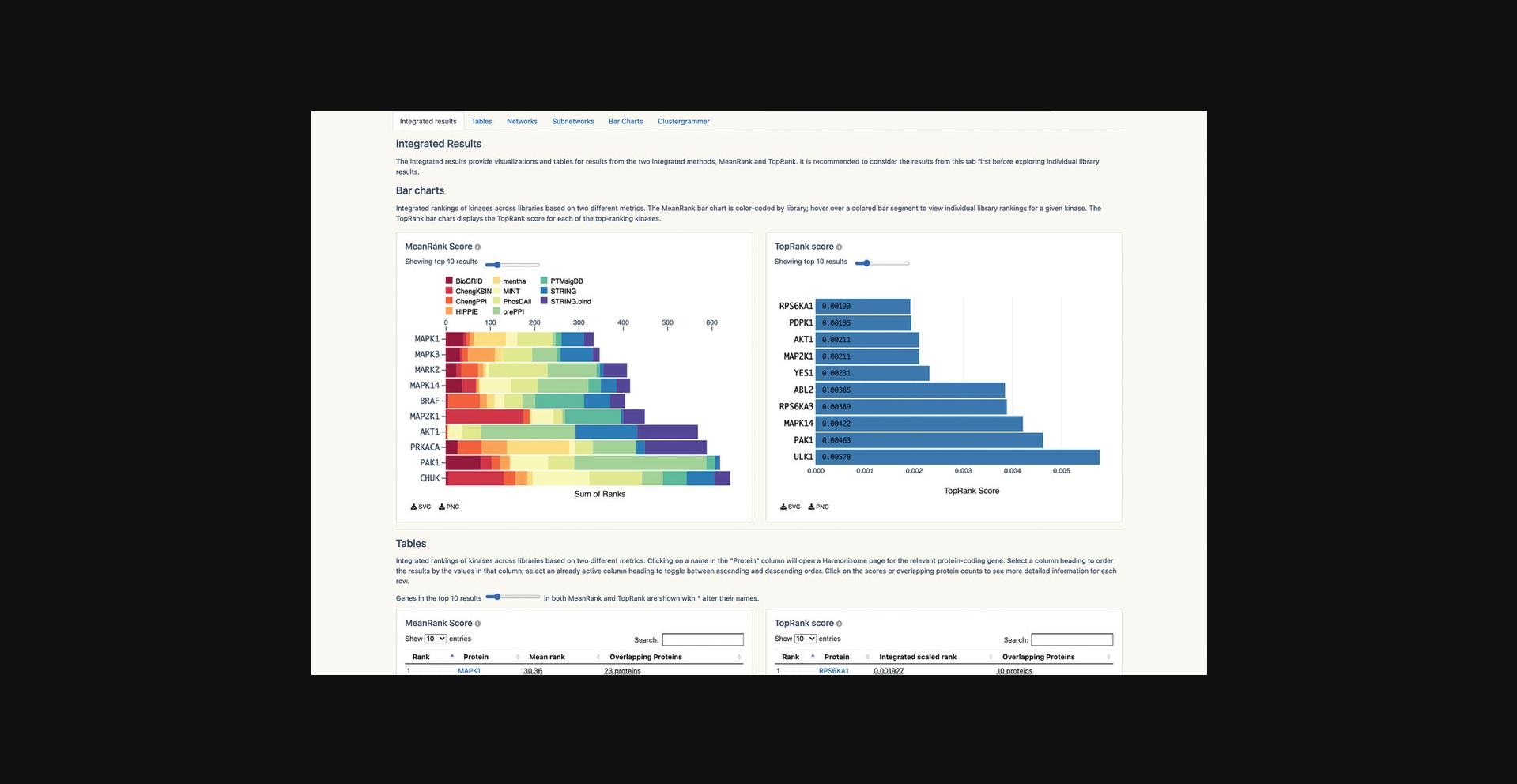
4.The Tables tab displays interactive tables of ranked kinases for each individual KEA3 library (Fig. 127). The tables are organized into kinase-kinase substrate interaction libraries, protein-protein interaction libraries, and libraries with all associations. Each table displays the top 10 ranked kinases using the Fisher's Exact Test p -value. Click on any of the table headers to re-sort the table. Clicking on any of the kinase names will redirect you to a single-gene landing page in Harmonizome. Access the complete list of kinases by downloading any table in TSV format using the download icon.
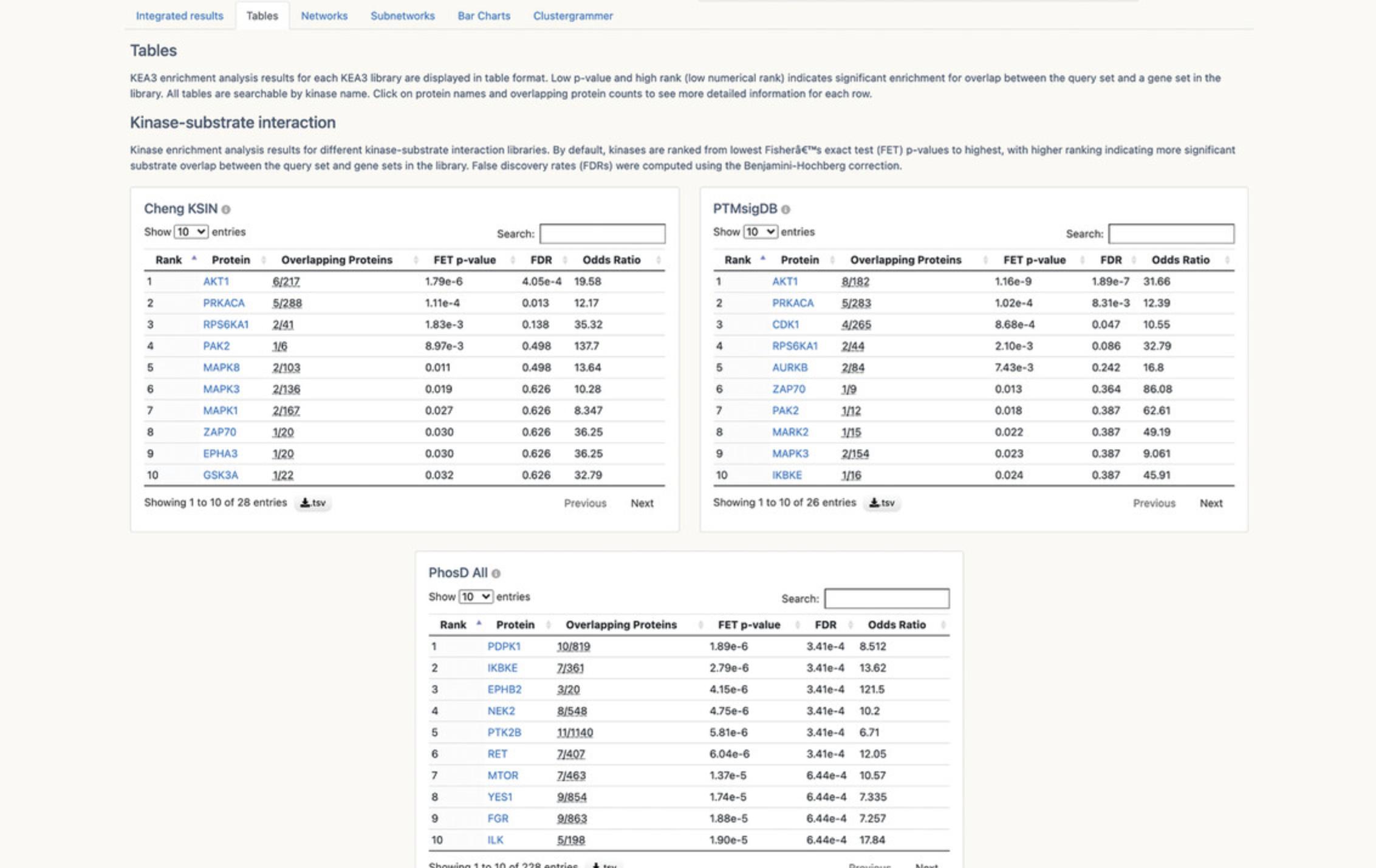
5.The Networks tab displays global kinase co-regulatory networks generated by applying Weighted Gene Co-expression Network Analysis (WGCNA; Langfelder & Horvath, 2008) to ARCHS4 (Lachmann et al., 2018), GTEx (Aguet et al., 2020), and TCGA (Tomczak, Czerwińska, & Wiznerowicz, 2015) data in order to visualize the top-ranked kinases in the context of the larger human phosphorylation network; the top-ranked kinases are highlighted in the network (Fig. 128). To choose the top-ranked kinases from a specific library, navigate to the “Select a library” drop-down menu and click on the desired library. Download each network as an SVG or PNG file by selecting the corresponding download button.
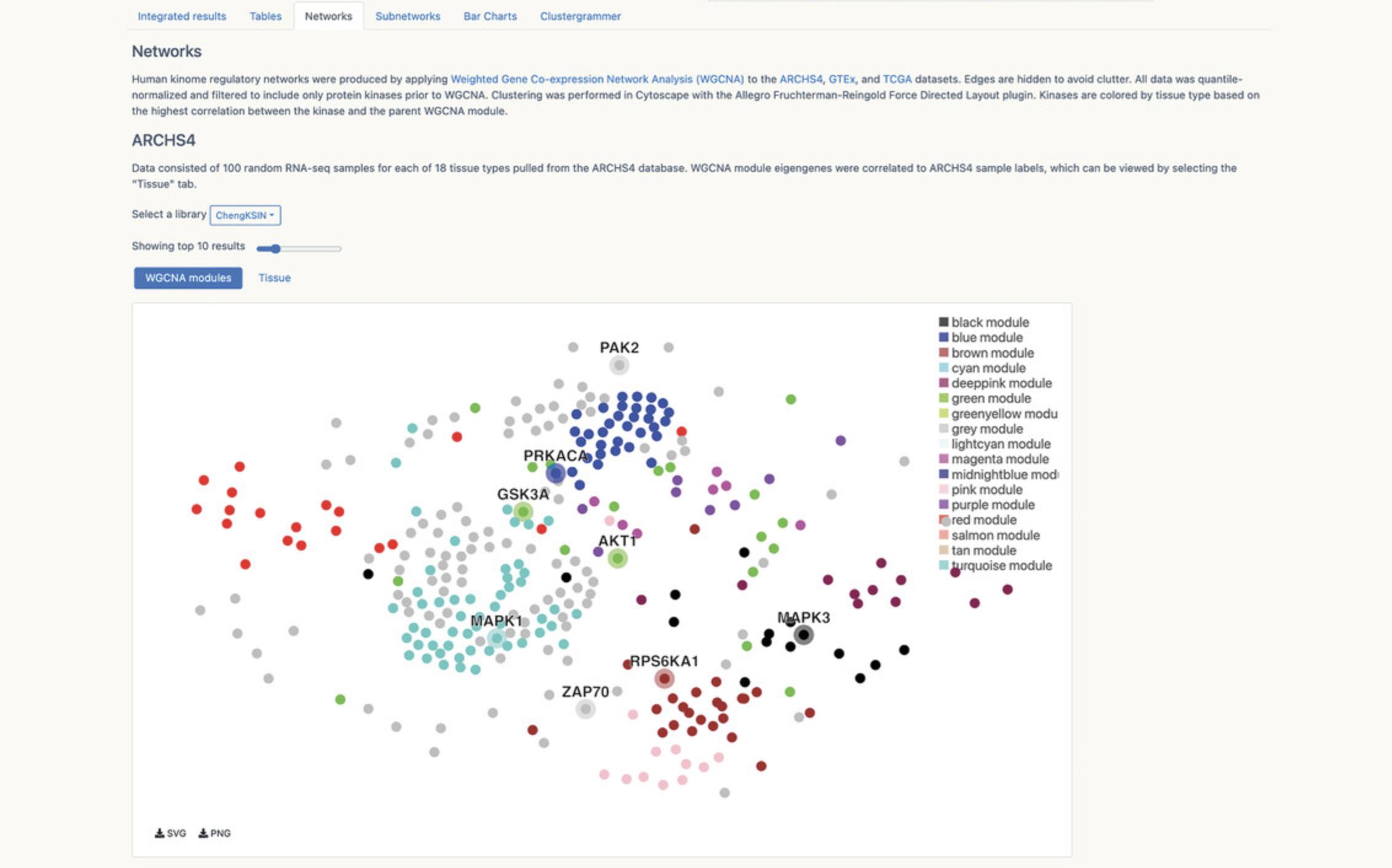
6.The Subnetworks tab displays kinase co-regulatory network visualizations which have been dynamically generated from the top-ranked kinases in each library (Fig. 129). An edge between two kinases indicates an interaction supported by library evidence from either a kinase-substrate interaction library (directed edge) or protein-protein interaction library (undirected edge). Hover over an edge to display the library evidence supporting the interaction. Download each network as an SVG or PNG by clicking the desired file type in the bottom left corner of the graph.
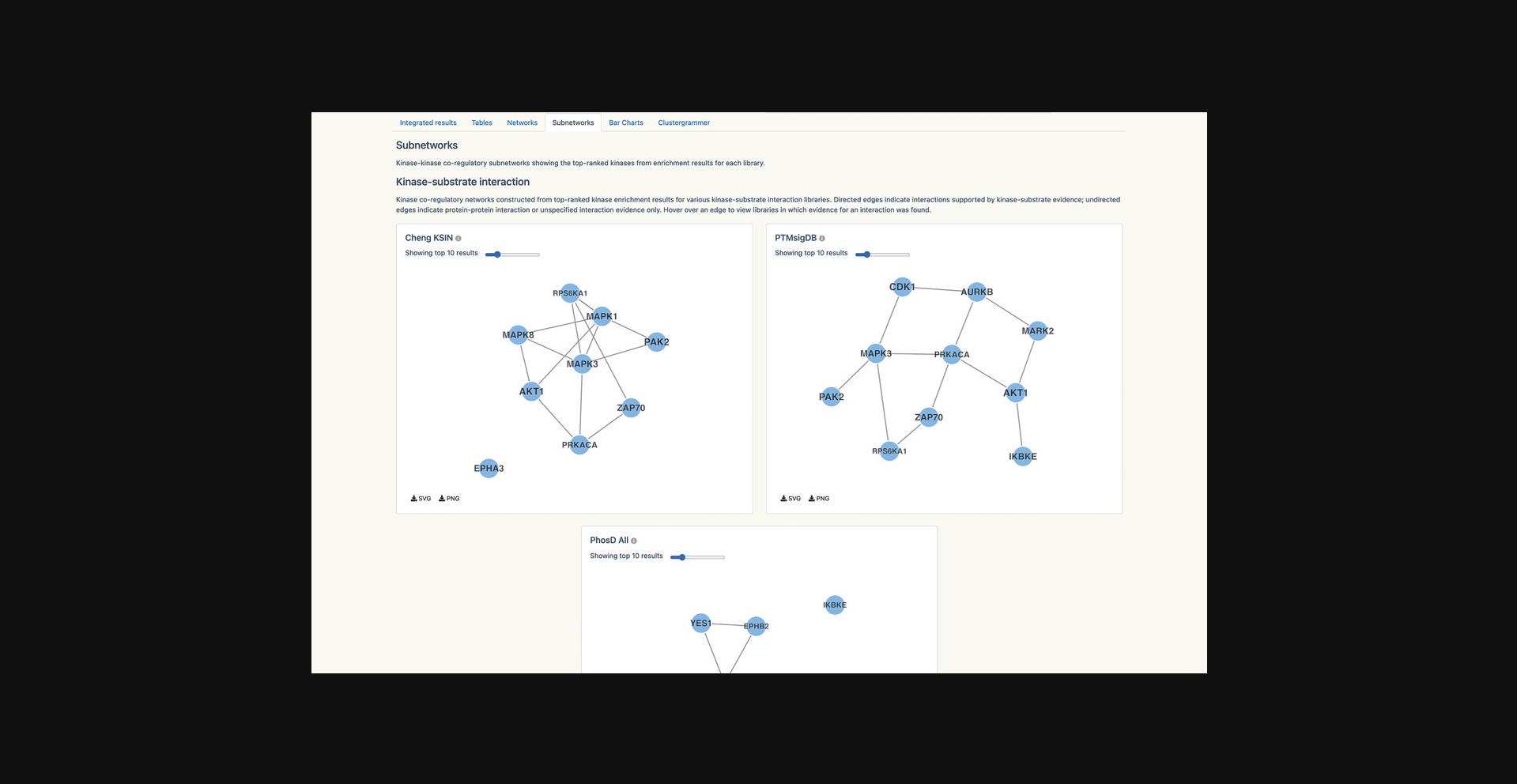
7.The Bar Charts tab provides bar charts showing the –log(p -value) of the top-ranked kinases for each individual library (Fig. 130). The bar charts are organized into kinase-kinase substrate interaction libraries, protein-protein interaction libraries, and libraries with all associations. Use the slider above each figure to change the number of top kinases within the figure. Download any given chart as an SVG or PNG by selecting the desired file type in the bottom left-hand corner of the chart.
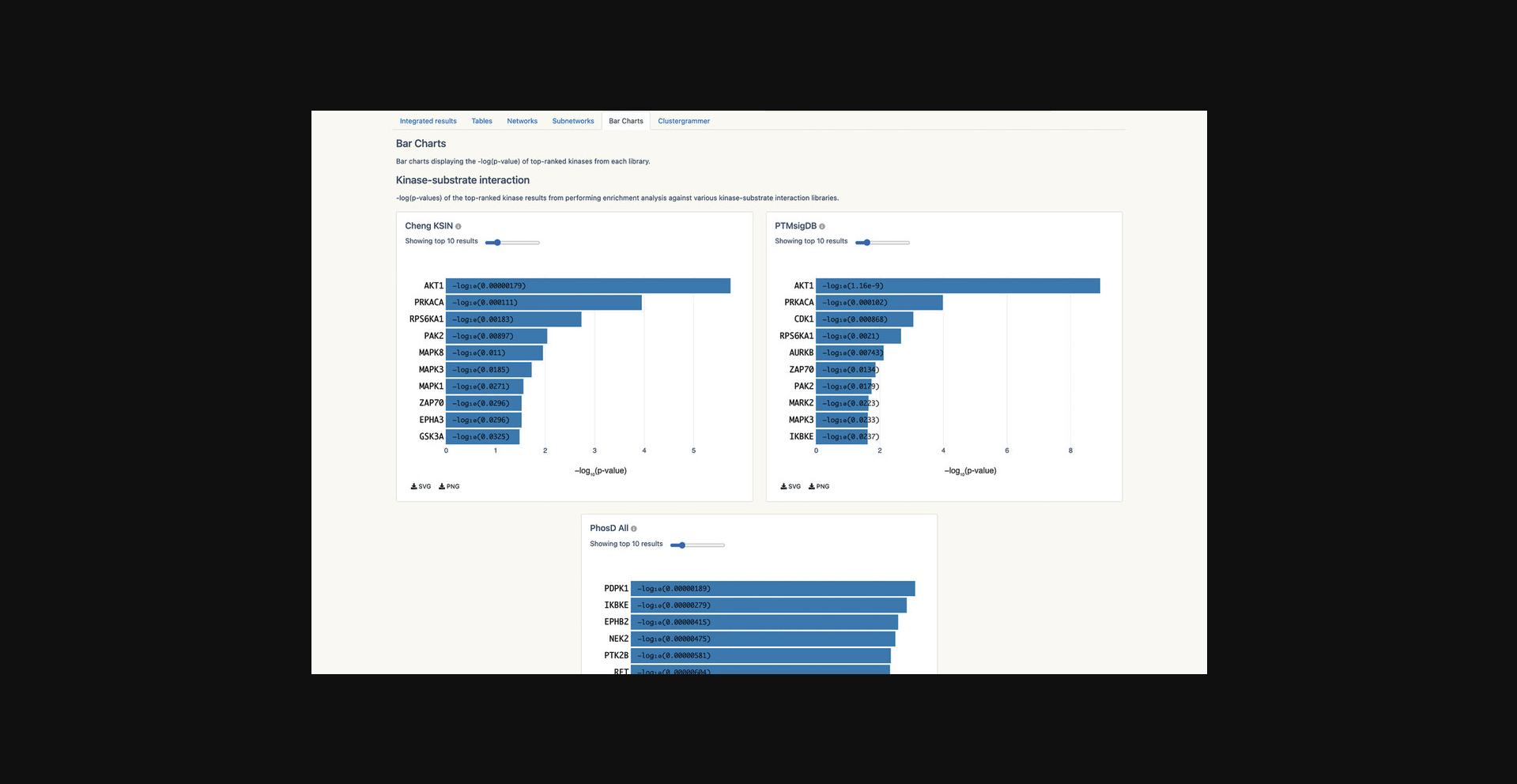
8.The Clustergrammer tab uses the Clustergrammer (Fernandez et al., 2017) application to provide an interactive clustergram of overlapping substrate targets between the input and the top library results (Fig. 131). Share, take a snapshot, download, or crop the clustergram matrix using the icons in the menu bar on the left side of the clustergram. Customize row order and column order by selecting one of the options (alphabetically, cluster, rank by sum, rank by variance) under “Row Order” and “Column Order,” respectively. Search for rows using the text search box. Adjust the dendrogram groups, which show clusters at different hierarchical levels and are represented by gray triangles and trapezoids along the bottom and right axes, using the gray triangular sliders on the right and bottom-left sides of the clustergram.
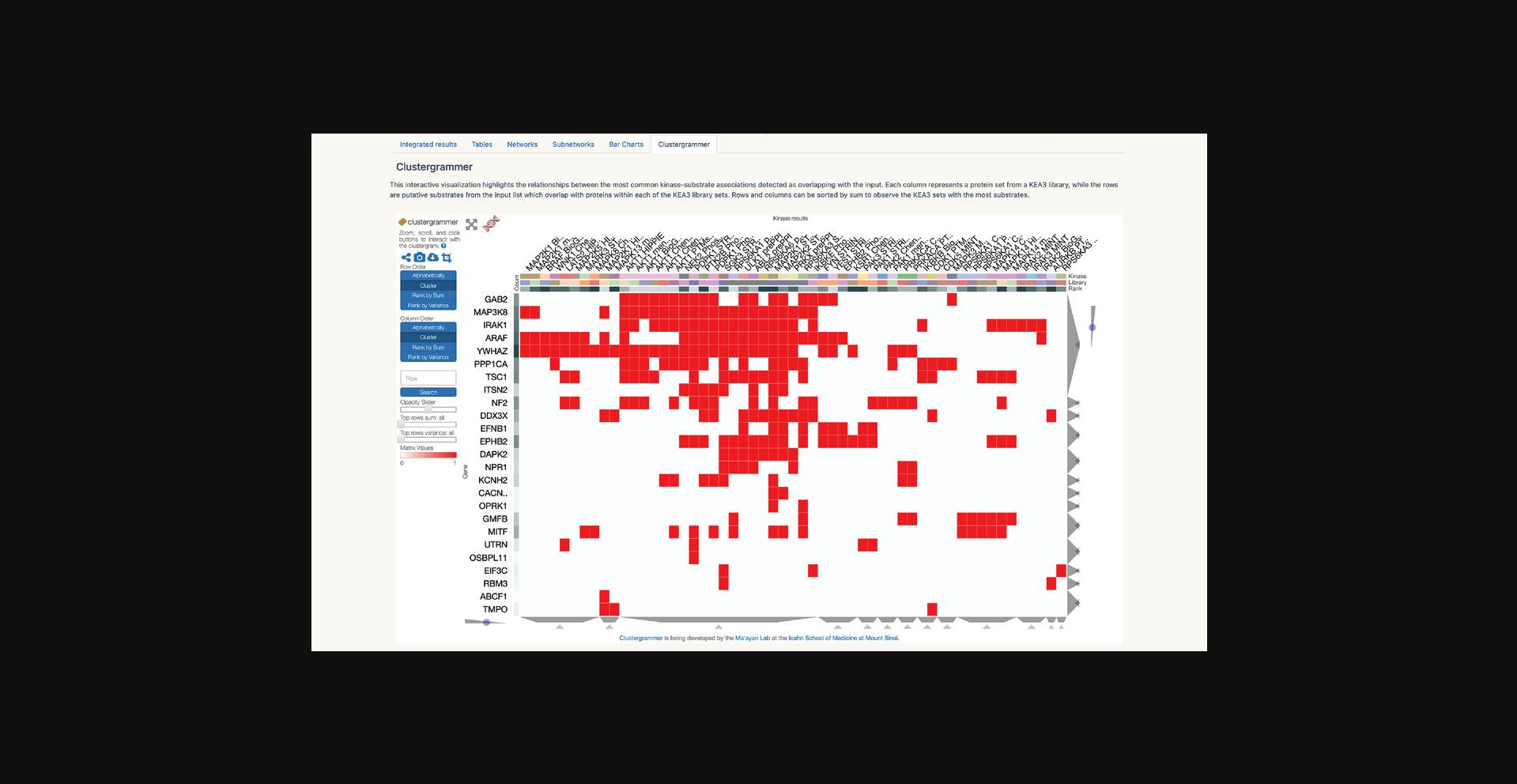
9.Open a new or existing Python code file. Import the JSON and requests libraries at the top of the file.
- import json
- import requests
10.Call the requests.post method to send a POST request to the URL. The payload variable contains the parameters that are sent to the API endpoint specified in KEA3_URL. In this case the endpoint is /enrich and the parameters are query_name, which specifies the name of the query, and gene_set, which specifies the query gene list to be enriched.
-
KEA3_URL = ˈhttps://maayanlab.cloud/kea3/api/enrich/ˈ
-
payload = {"query_name":"myQuery", "gene_set":["FOXM1","SMAD9","MYC","SMAD3","STAT1","STAT3"]}
-
response = requests.post(KEA3_URL, json=payload)
-
- data = json.loads(response.text)
-
print(data)
11.Use the json.loads method to view the response as a JSON object containing the top enrichment results from various libraries.
-
{
-
ˈIntegrated--meanRankˈ:
-
[{ˈQuery Nameˈ: ˈmyQueryˈ,
-
ˈRankˈ: ˈ1ˈ,
-
ˈTFˈ: ˈCDK4ˈ,
-
ˈScoreˈ: ˈ37.73ˈ,
-
ˈLibraryˈ: ˈSTRING.bind,20;ChengPPI,2;PhosDAll,39;BioGRID,4;HIPPIE,13;ChengKSIN,29;STRING,107;MINT,59;mentha,2;prePPI,137;PTMsigDB,3ˈ,
-
ˈOverlapping_Genesˈ: ˈSMAD3,STAT1,MYC,STAT3,SMAD9,FOXM1ˈ},
-
{ˈQuery Nameˈ: ˈmyQueryˈ,
-
ˈRankˈ: ˈ2ˈ,
-
ˈTFˈ: ˈPDGFRAˈ,
-
ˈScoreˈ: ˈ48.38ˈ,
-
ˈLibraryˈ: ˈSTRING.bind,11;ChengPPI,7;PhosDAll,59;BioGRID,110;HIPPIE,2;STRING,61;mentha,8;prePPI,129ˈ,
-
ˈOverlapping_Genesˈ: ˈSMAD3,STAT1,MYC,STAT3,SMAD9,FOXM1ˈ},
-
}
NOTE : More detailed instructions, as well as examples from the command line and in R, can be found at https://maayanlab.cloud/kea3/templates/api.jsp.
Basic Protocol 14: CONVERTING PubMed SEARCHES TO DRUG SETS WITH THE DrugShot APPYTER
PubMed contains millions of publications that co-mention drugs with other biomedical terms such as genes or diseases. DrugShot is an Appyter (Clarke et al., 2021) that enables users to enter any biomedical search term into an input form to receive ranked lists of drugs and small molecules based on their relevance to the search term. DrugShot then deploys a Jupyter Notebook in the cloud to display ranked lists of drugs. To achieve this, DrugShot cross-references returned PubMed IDs with DrugRIF, a curated resource of drug-PMID associations, to produce an associated compound list where each compound is ranked according to the total co-mentions with the search term from shared PubMed IDs. Additionally, lists of compounds predicted to be associated with the search term are generated based on drug-drug co-occurrence in the literature, and drug-drug co-expression correlations computed from L1000 drug-induced gene expression profiles. Through its search functionality and abstraction of drug sets from different sources, DrugShot facilitates hypothesis generation by suggesting small molecules related to any searched biomedical term.
Necessary Resources
Hardware
- Desktop or a laptop computer, or a mobile device, with a fast Internet connection
Software
- An up-to-date web browser such as Google Chrome (https://www.google.com/chrome/), Mozilla Firefox (https://www.mozilla.org/en-US/firefox/), Apple Safari (https://www.apple.com/safari/), or Microsoft Edge (https://www.microsoft.com/en-us/edge)
Query biomedical term
1.Navigate to the DrugShot Appyter (https://appyters.maayanlab.cloud/DrugShot/). The Appyter input form includes options to query a biomedical term to retrieve a prioritized list of small molecules that is augmented using drug-drug similarity matrices, or to submit a list of small molecules to be augmented using drug-drug similarity matrices.
2.Input a biomedical term into the “Biomedical Term” field. The default string used for this demonstration is “Lung Cancer.” Input an integer ranging from 20 to 200 in the “Associated Drug Set Size” field; this value is used to determine the size of the unweighted drug set that is used to predict related compounds. The larger the value selected, the broader the resulting predictions will be (Fig. 132).
3.Click submit on the Appyter input form and a Jupyter Notebook with the input parameters will be launched in the cloud.
4.The first output element of the notebook is a table of “Top Associated Compounds” (Fig. 133). This table provides the top-ranked drug and compound names associated with the query term (Index Column), the count of PubMed publications associating each drug with the search term (Column 1), and the fraction of the publications associating the drug and search term divided by the total number of publications related to the drug regardless of search term (Column 2). Click on the hyperlinked filename below the table title to download a .csv file listing all the associated compounds. This file also includes a Score column containing values that are the product of the first two columns.
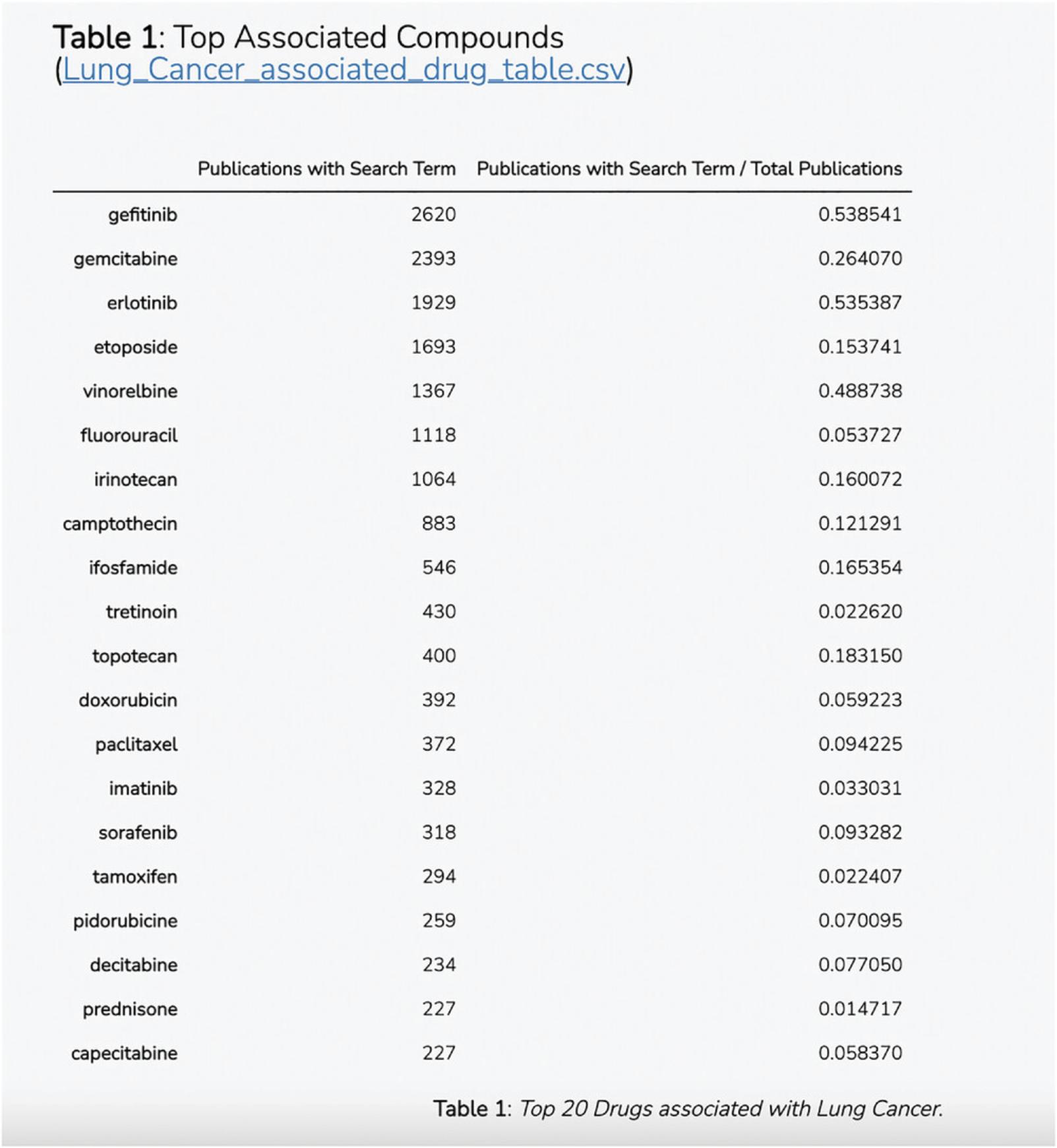
5.The second output component of this notebook is a scatter plot (Fig. 134) of the values from the table of “Top Associated Compounds.” The x axis displays the integer counts of Publications with Search Term, and the y axis shows the fraction of Publications with Search Term/Total Publications. Hover over any point on this plot to display the compound's name and its corresponding x and y values.
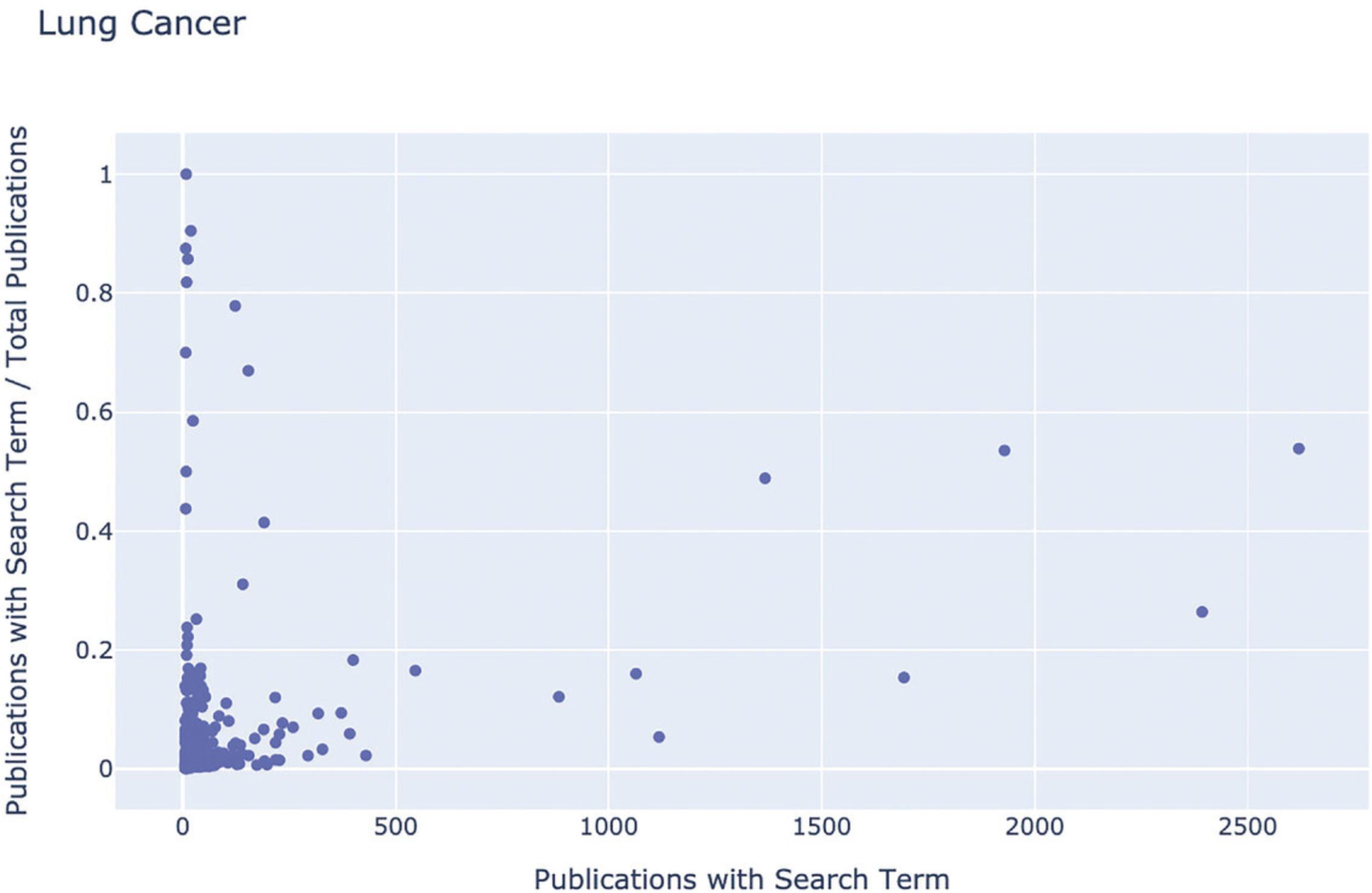
6.An unweighted drug set is created through ranking small molecules from the association table by the product of the total associated publications and their normalized fraction.
Querying a list of small molecules
7.Alternatively, submit a newline-separated .txt file of small molecule names using the input form, thereby omitting steps 2-6.The submitted small molecules will be used as the unweighted drug set that will be used in subsequent steps (Fig. 135).
Literature co-mentions predictions
8.A receiver operating characteristic (ROC) curve that describes the ranking of associated compounds in the DrugRIF literature co-mentions matrix is output (Fig. 136). This plot shows the True Positive Rate on the y -axis and the False Positive Rate on the x -axis. The predicted compounds are computed using average co-mention counts of PubMed IDs between the unweighted drug set, and other drugs and small molecules within DrugRIF. The area under the curve (AUC) is shown to the right of the plot, and hovering over any point on the curve displays the associated x and y values.
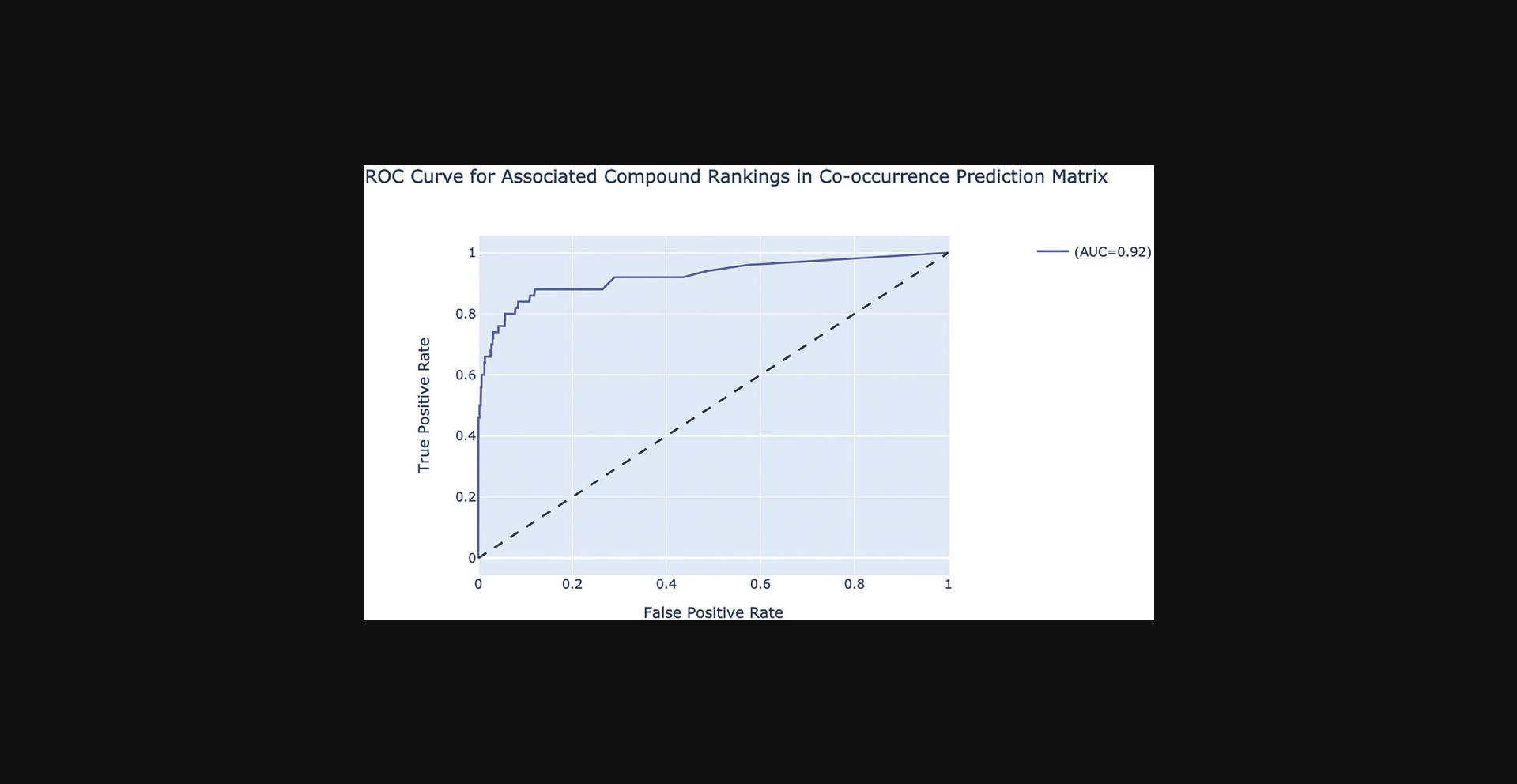
9.The literature co-mentions prediction matrix is seeded with the unweighted drug set, and the top predicted compounds are ranked by their average co-mentions with the small molecules in the unweighted drug set. The “average co-mentions” values are provided in a table that displays the top 20 predicted compounds (Fig. 137). Click on the hyperlinked filename below the Table 2 header to download the table as a .csv file.
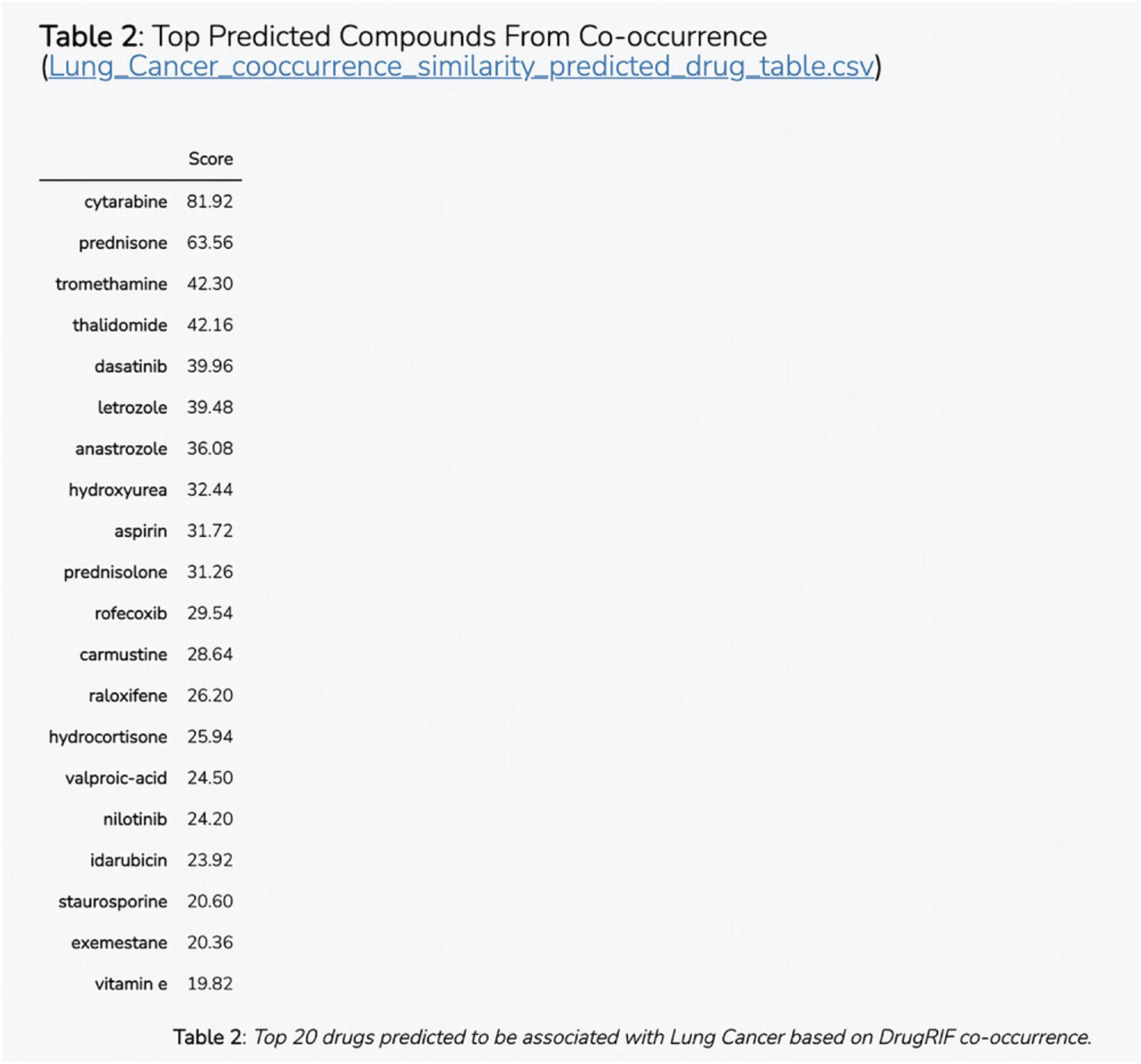
10.The top 50 co-occurrence-predicted compounds are queried using the DrugEnrichr API for drug set enrichment analysis. The top 10 enriched terms from the down-regulated and up-regulated GO Biological Processes drug set libraries and the SIDER drug set library are displayed as bar plots (Fig. 138). Click the link below the bar plots to be directed to the DrugEnrichr enrichment results page (Fig. 139).
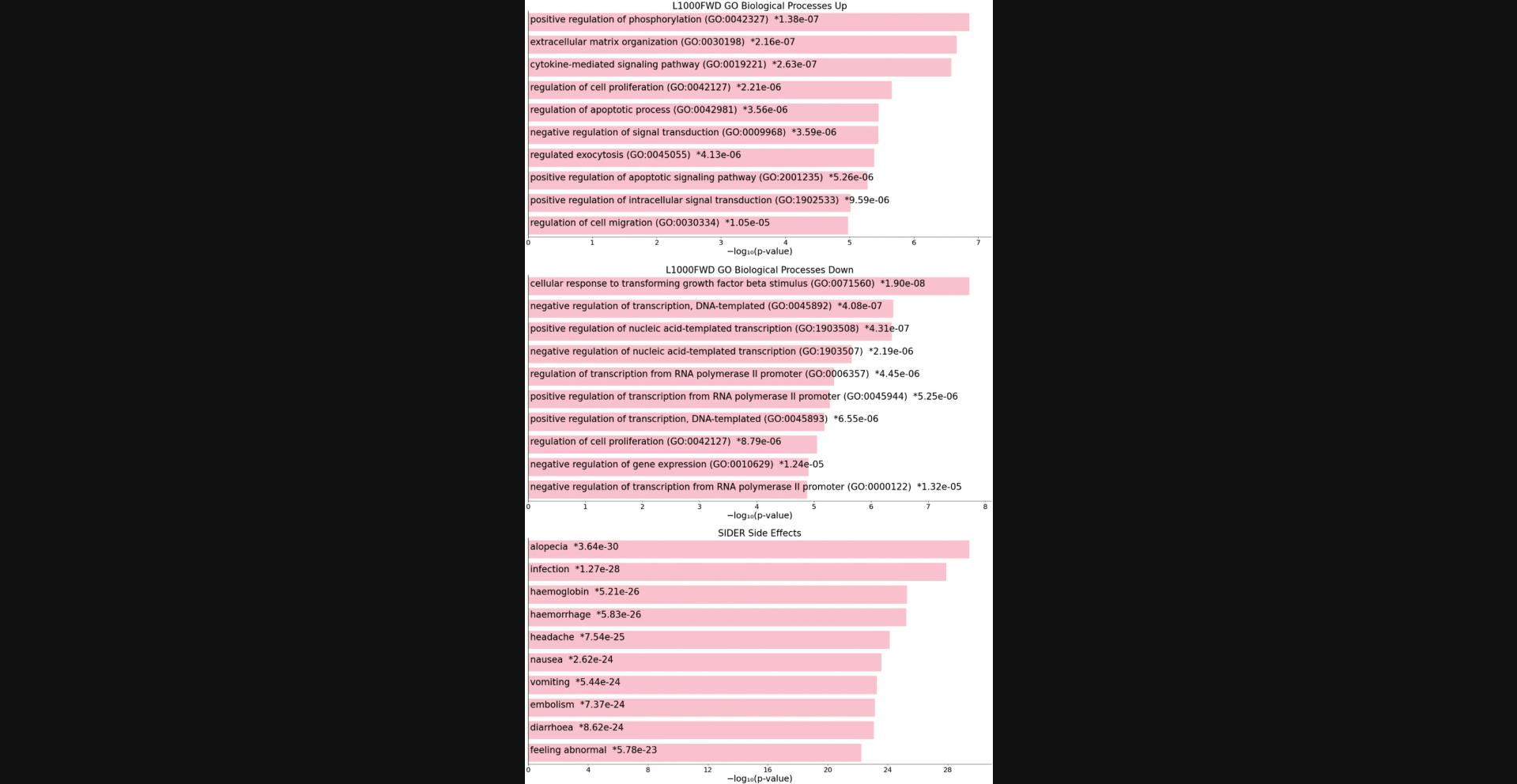
Signature similarity predictions
11.A receiver operating characteristic (ROC) curve that describes the ranking of associated compounds in the L1000 signature similarity matrix is output (Fig. 140). This plot shows the True Positive Rate on the y -axis and the False Positive Rate on the x -axis. The predicted compounds are computed using average cosine similarity of drug-induced gene expression signatures between the unweighted drug set and other drugs and small molecules within the co-expression prediction matrix. The area under the curve (AUC) is shown to the right of the plot, and hovering over any point on the curve displays the associated x and y values.
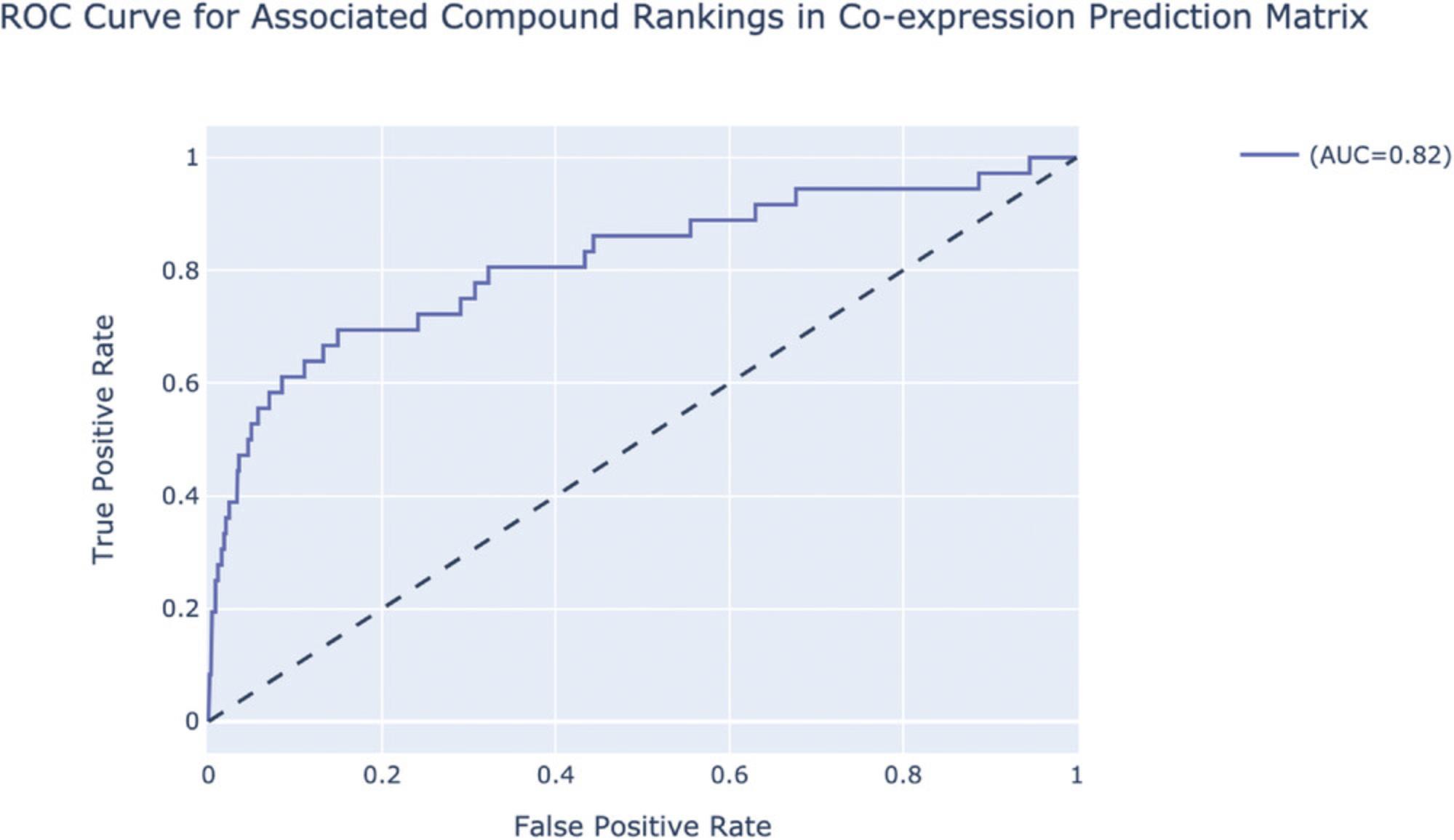
12.The signature similarity prediction matrix is seeded with the unweighted drug set, and the top predicted compounds are ranked by their average cosine similarity to the small molecules in the unweighted drug set. The “average cosine similarity” values are provided in a table that displays the top 20 predicted compounds (Fig. 141). Click on the hyperlinked filename below the table header to download the table as a .csv file.
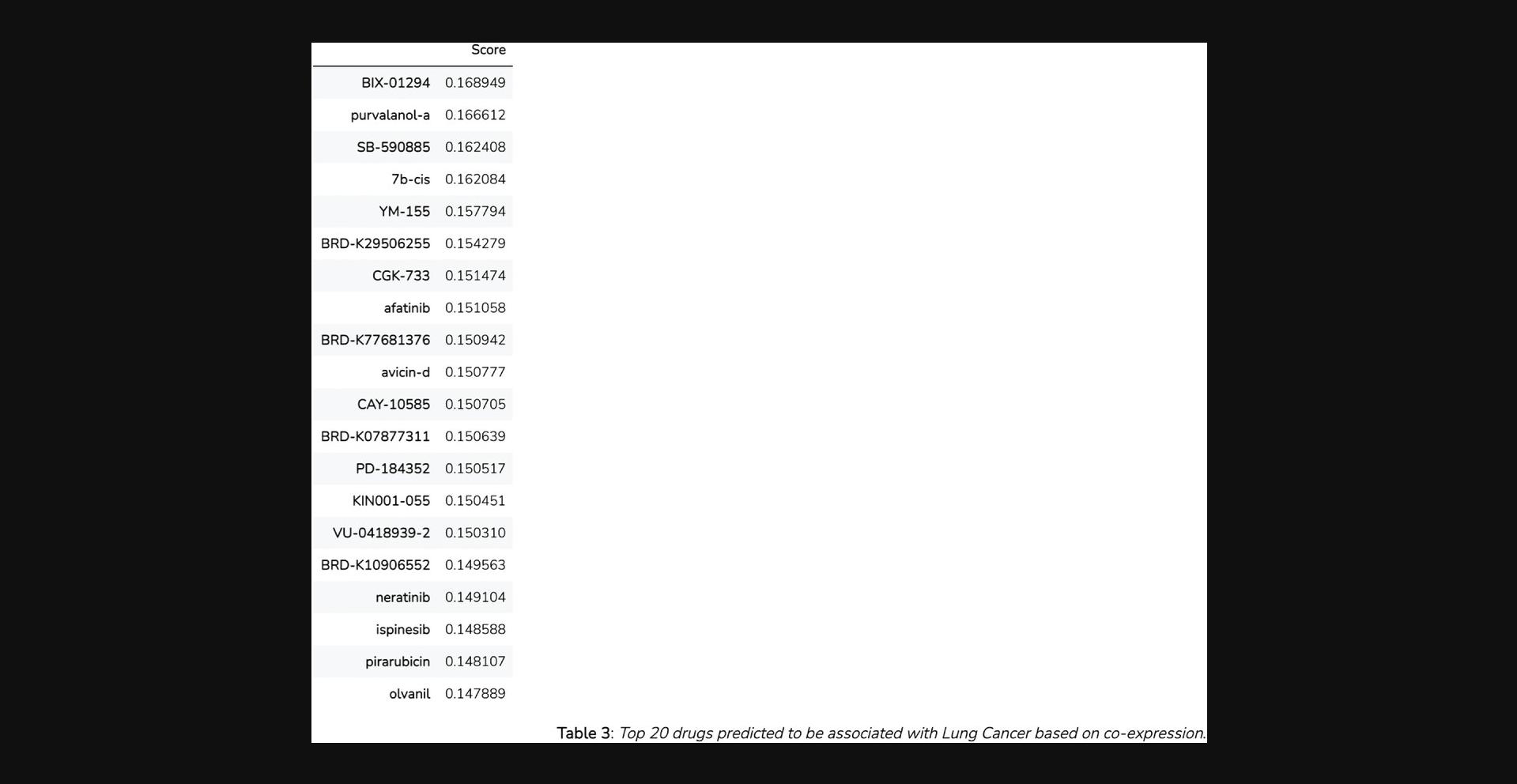
13.The top 50 signature-similarity predicted compounds are queried using the DrugEnrichr API for drug set enrichment analysis. The top 10 enriched terms from the down-regulated and up-regulated GO Biological Processes drug set libraries and the SIDER drug set library are displayed as bar plots (Fig. 142). Click the link to be directed to the DrugEnrichr enrichment results page (Fig. 143).

COMMENTARY
Background Information
The IDG consortium has generated several different resources that are available to the research community. These resources include experimental data, tools, and reagents from the Data and Resource Generating Centers (DRGCs) covering the IDG-highlighted protein families. These proteins are investigated by compound library screening (in vitro and in silico), antibody development, function and activation state profiling, and mouse expression profiling. Moreover, illuminating the druggable GPCR-ome is achieved by a two-pronged approach of experimental screening of drugs followed by computational screening against modeled structures of the GPCR to produce optimized lead compounds. This work has led to the discovery of several novel compounds, for example, the small molecule "ogerin" binds to GPR68 (Huang et al., 2015). Much of the success of identifying such novel GPCR binding compounds is due to development of a novel screening assay, PRESTO-Tango (Kroeze et al., 2015), which enables simultaneous investigation of every non-olfactory G protein-coupled receptor in the human genome. Additionally, the DRGCs recently gained insight into new potential therapeutics to help treat circadian rhythm disorders via the melatonin receptors MT1 and MT2 (Stein et al., 2020). The DRGCs also illuminate ion channels by utilizing CRISPR technology to map expression profiles, assess channel activities, develop antibodies, and generate new mouse lines. This work recently elucidated TMEM16C and its involvement in thermoregulation and protection from febrile seizures in rodent pups (Wang et al., 2021). Furthermore, discovering the function of the under-studied druggable kinome includes using Multiplex Inhibitor Beads (MIB)/Mass Spectrometry (MS) to identify kinase activation status in response to perturbagens. This approach is applied to model cell lines and patient-derived xenografts. These data, along with other data collection efforts, are incorporated into the Dark Kinase Knowledgebase (DKK) which provides gene-by-gene and network-level information on the dark kinome and its interaction with other signal transduction regulatory networks (Berginski et al., 2021). For example, recently, the kinase CDC42BPA/MRCKα has been identified as a potential target for brain, ovarian, and skin cancers (East & Asquith, 2021). Moreover, the Kinase Chemogenomic Set (KCGS) is the most highly annotated set of selective kinase inhibitors available to researchers for use in cell-based screens. Recently, the NIH IDG initiative nominated 162 dark kinases to develop chemical and biological tools to seed research on these under-studied proteins. Currently, KCGS contains data of 37 inhibitors from the IDG dark kinases, which may be helpful and improve initial chemical tools to study these kinases (Wells et al., 2021).
Congruently, the IDG Knowledge Management Center (IDG-KMC) develops bioinformatics tools and other digital assets, enabling users to query and visualize the data produced by the DRGCs and other sources. The IDG-KMC gathers knowledge covering the entire human genome and expanding to model systems, including GWAS studies, expression data, compound binding, and patent information via ChEMBL (Mendez et al., 2019). Furthermore, the IDG-KMC incorporates associated information related to human protein-coding genes, diseases, mouse phenotypes, small molecules, and approved drugs (perturbagens) that modulate these proteins/genes and diseases. Utilizing these collected and annotated databases generates opportunities for machine learning–ready platforms. For example, using these tools (i.e., combining data on genes, proteins, and RNA molecules from fourteen databases and publications), the IDG-KMC developed a machine learning algorithm that prioritizes targets for human genes associated with 17 unique types of pain, and identified thirteen potential IDG family drug targets for migraine drug development and four for rheumatoid arthritis (Jeon, Jagodnik, Kropiwnicki, Stein, & Ma'ayan, 2021). Here we provide a collection of step-by-step get-started protocols to gain initial access to the resources created by the IDG-KMC. We hope that these protocols will facilitate experimental and computational biologists to further engage with the unique opportunities offered by the IDG program toward accelerating drug and target discovery.
Critical Parameters
There are several libraries and data sources that IDG-KMC web applications rely on. PubMed (https://pubmed.ncbi.nlm.nih.gov/) and DrugCentral (Avram et al., 2021) play an important role in several of the protocols. PubMed and DrugCentral are used by IDG-KMC web applications as both sources of data and also as external references that users can reach from within some IDG-KMC web applications.
The Target Central Resource Database (TCRD) is the central resource behind the Illuminating the Druggable Genome Knowledge Management Center (IDG-KMC) (Sheils et al., 2021). TCRD contains information about human targets and emphasizes four families of targets central to the NIH IDG initiative: GPCRs (note that olfactory GPCRs are treated as a separate family), kinases, and ion channels. A unique aim of the KMC is to classify the development/druggability level of targets via Target Development Level (TDLs). TDLs are currently categorized into four development/druggability levels: Tclin , Tchem , Tbio , and Tdark. Tclin targets have activities in DrugCentral with a known mechanism of action. Tchem targets have activities in ChEMBL (Mendez et al., 2019), Guide to Pharmacology (Armstrong et al., 2019), or DrugCentral that satisfy the activity thresholds, but no approved drugs. Tbio targets do not have known drug or small molecule activities that satisfy the activity thresholds and satisfy one or more of the following criteria: target is above the cutoff criteria for Tdark, or the target is annotated with a Gene Ontology Molecular Function or Biological Process (The Gene Ontology Consortium, 2019) leaf term(s) with an Experimental Evidence code. Tdark targets have limited information or knowledge about them. Moreover, TDark currently includes ∼31% of the human proteins that were manually curated at the primary sequence level in UniProt, but do not meet any of the Tclin , Tchem , or Tbio criteria.
Each of the datasets in Harmonizome are compiled from various resources that contain information regarding gene-attribute associations. Gene-attribute associations can range from chemical perturbations that induce differential expression in select genes (Subramanian et al., 2017) to specific genes differentially expressed in cell lines (Barretina et al., 2012; Cowley et al., 2014). The evidence for these associations depends on the resource and can be from text mining, high-throughput -omics data, and other methods.
The ARCHS4 resource, and by extension the PrismEXP Appyter, depend on FASTQ files generated from RNA-seq experiments deposited in the Gene Expression Omnibus (GEO) (Edgar, Domrachev, & Lash, 2002).
Geneshot relies on knowledge about under-studied targets from GeneRIF (Osborne et al., 2007) and AutoRIF (Lachmann et al., 2019), association files that catalog gene-PubMed ID co-mentions. AutoRIF is larger and more comprehensive than GeneRIF, but potentially less accurate due to its automated creation. Furthermore, Geneshot generates predictions from gene-gene similarity matrices compiled from AutoRIF, GeneRIF, ARCHS4, Enrichr (Kuleshov et al., 2016), and Tagger (Pletscher-Frankild & Jensen, 2019).
For TIN-X, Drug Target Ontology (Lin et al., 2017) is used to establish associations between drug targets and disease states. TIN-X allows the user to browse diseases based on TDL, IDG Family, as well as user-supplied search terms for drug targets associated with the disease being considered.
Drugmonizome depends upon drug-attribute associations compiled from various resources. These drug-attribute associations are stored as drug set libraries, collections of drug sets that describe relationships between biomedical terms and sets of drugs. The drug set libraries are categorized into distinct categories that include: drug targets and associated genes; side effects, adverse events, and phenotypes; gene ontology (GO) and pathway terms; chemical structure and sub-structure motifs; and modes of action.
Several of the protocols (namely Basic Protocols 4, 10, 11, and 15) mention Appyters. Appyters turn Jupyter Notebooks into functional standalone web applications for bioinformatics workflows (Clarke et al., 2021). Each Appyter presents a unique workflow tied to an input form that can be modified by the user. Once the user submits the input form options, a Jupyter Notebook is executed in the cloud and populated with the selected options. These notebooks contain various analyses and publication-ready figures that can be shared and downloaded by the research community.
GWAS target illumination depends upon GWAS summary and metadata from the NHGRI-EBI GWAS Catalog with study-associated publications.
TIGA traits are identified by Experimental Factor Ontology (EFO) terms.
The prioritization of kinases for lists of proteins and phosphoproteins with KEA3 makes use of individual libraries generated from kinase-substrate interactions and protein-protein interactions, plus two integrated libraries, MeanRank and TopRank.
When converting PubMed searches to drug sets with the DrugShot Appyter, DrugRIF is used as a background database of drug-PMID associations. Furthermore, drug-drug similarity matrices generated from pairwise drug co-mentions from DrugRIF and pairwise cosine similarity of drug-induced gene expression profiles from SEP-L1000 (Wang et al., 2016) are used to predict novel drug-term associations.
Acknowledgments
This work was partially supported by NIH grants U24CA224260, U54HL127624, R01DK131525, OT2OD030160 U24CA224370, U24TR002278, U01CA239108, and OT2OD030546.
Author Contributions
Eryk Kropiwnicki : software, data curation, writing original draft, writing review and editing; Jessica L. Binder : writing original draft, writing review and editing; Jeremy J. Yang : data curation, software, visualization; Daniel J. B. Clarke : methodology, software, validation, visualization; Jayme Holmes : data curation, resources, software; Alexander Lachmann : formal analysis, investigation, methodology, software, validation; Vincent T. Metzger : software, writing review and editing; Timothy Sheils : methodology, software, validation, visualization; Keith J. Kelleher : data curation, software; Cristian G. Bologa : data curation, methodology, software, writing review and editing; Tudor I. Oprea : conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, project administration, resources, software, supervision, validation, visualization, writing original draft, writing review and editing; Avi Ma'ayan : conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, project administration, resources, software, supervision, validation, visualization, writing original draft, writing review and editing.
Conflict of Interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
Literature Cited
- Aguet, F., Anand, S., Ardlie, K. G., Gabriel, S., Getz, G. A., Graubert, A., … Kashin, S. (2020). The GTEx Consortium atlas of genetic regulatory effects across human tissues. Science , 369, 1318-1330.
- Armstrong, J. F., Faccenda, E., Harding, S. D., Pawson, A. J., Southan, C., Sharman, J. L., … Davies, J. A. (2019). The IUPHAR/BPS Guide to PHARMACOLOGY in 2020: Extending immunopharmacology content and introducing the IUPHAR/MMV Guide to MALARIA PHARMACOLOGY. Nucleic Acids Research , doi: 10.1093/nar/gkz951.
- Avram, S., Bologa, C. G., Holmes, J., Bocci, G., Wilson, T. B., Nguyen, D.-T., … Oprea, T. I. (2021). DrugCentral 2021 supports drug discovery and repositioning. Nucleic Acids Research , 49, D1160–D1169. doi: 10.1093/nar/gkaa997.
- Avram, S., Curpan, R., Halip, L., Bora, A., & Oprea, T. I. (2020). Off-patent drug repositioning. Journal of Chemical Information and Modeling , 60, 5746–5753. doi: 10.1021/acs.jcim.0c00826.
- Barretina, J., Caponigro, G., Stransky, N., Venkatesan, K., Margolin, A. A., Kim, S., … Garraway, L. A. (2012). The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature , 483, 603–607. doi: 10.1038/nature11003.
- Benet, L. Z., Broccatelli, F., & Oprea, T. I. (2011). BDDCS applied to over 900 drugs. The AAPS Journal , 13, 519–547. doi: 10.1208/s12248-011-9290-9.
- Berginski, M. E., Moret, N., Liu, C., Goldfarb, D., Sorger, P. K., & Gomez, S. M. (2021). The Dark Kinase Knowledgebase: An online compendium of knowledge and experimental results of understudied kinases. Nucleic Acids Research , 49, D529–D535. doi: 10.1093/nar/gkaa853.
- Bhattacharyya, S. B. (2016). Overview of SNOMED CT. In Introduction to SNOMED CT (pp. 1–2). Singapore: Springer Singapore.
- Bocci, G., Bradfute, S. B., Ye, C., Garcia, M. J., Parvathareddy, J., Reichard, W., … Oprea, T. I. (2020). Virtual and in vitro antiviral screening revive therapeutic drugs for COVID-19. ACS Pharmacology & Translational Science, 3, 1278–1292.
- Cai, D.-C., Fonteijn, H., Guadalupe, T., Zwiers, M., Wittfeld, K., Teumer, A., … Hagoort, P. (2014). A genome-wide search for quantitative trait loci affecting the cortical surface area and thickness of Heschl's gyrus. Genes, Brain and Behavior , 13, 675–685. doi: 10.1111/gbb.12157.
- Cannon, D. C., Yang, J. J., Mathias, S. L., Ursu, O., Mani, S., Waller, A., … Oprea, T. I. (2017). TIN-X: Target importance and novelty explorer. Bioinformatics , 33, 2601–2603. doi: 10.1093/bioinformatics/btx200.
- Clarke, D. J. B., Jeon, M., Stein, D. J., Moiseyev, N., Kropiwnicki, E., Dai, C., … Ma'ayan, A. (2021). Appyters: Turning Jupyter Notebooks into data-driven web apps. Patterns , 2, 100213.
- Contrera, J. F., Matthews, E. J., Kruhlak, N. L., & Benz, R. D. (2004). Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose. Regulatory Toxicology and Pharmacology , 40, 185–206. doi: 10.1016/j.yrtph.2004.08.004.
- Cowley, G. S., Weir, B. A., Vazquez, F., Tamayo, P., Scott, J. A., Rusin, S., … Hahn, W. C. (2014). Parallel genome-scale loss of function screens in 216 cancer cell lines for the identification of context-specific genetic dependencies. Scientific Data , 1, 140035. doi: 10.1038/sdata.2014.35.
- East, M. P., & Asquith, C. R. M. (2021). CDC42BPA/MRCKα: A kinase target for brain, ovarian and skin cancers. Nature Reviews: Drug Discovery , 20, 167. doi: 10.1038/d41573-021-00023-9.
- Edgar, R., Domrachev, M., & Lash, A. E. (2002). Gene expression omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Research , 30, 207–210. doi: 10.1093/nar/30.1.207.
- Egaña, L. A., Cuevas, R. A., Baust, T. B., Parra, L. A., Leak, R. K., Hochendoner, S., … Torres, G. E. (2009). Physical and functional interaction between the dopamine transporter and the synaptic vesicle protein synaptogyrin-3. Journal of Neuroscience , 29, 4592–4604. doi: 10.1523/JNEUROSCI.4559-08.2009.
- Fernandez, N. F., Gundersen, G. W., Rahman, A., Grimes, M. L., Rikova, K., Hornbeck, P., & Ma'ayan, A. (2017). Clustergrammer, a web-based heatmap visualization and analysis tool for high-dimensional biological data. Scientific Data , 4, 170151. doi: 10.1038/sdata.2017.151.
- Hopkins, A. L., & Groom, C. R. (2002). The druggable genome. Nature Reviews. Drug Discovery , 1, 727–730. doi: 10.1038/nrd892.
- Huang, L., Zalkikar, J., & Tiwari, R. C. (2011). A likelihood ratio test based method for signal detection with application to FDA's drug safety data. Journal of the American Statistical Association , 106, 1230–1241. doi: 10.1198/jasa.2011.ap10243.
- Huang, X.-P., Karpiak, J., Kroeze, W. K., Zhu, H., Chen, X., Moy, S. S., … Roth, B. L. (2015). Allosteric ligands for the pharmacologically dark receptors GPR68 and GPR65. Nature , 527, 477–483. doi: 10.1038/nature15699.
- Jeon, M., Jagodnik, K. M., Kropiwnicki, E., Stein, D. J., & Ma'ayan, A. (2021). Prioritizing pain-associated targets with machine learning. Biochemistry , 60, 1430–1446. doi: 10.1021/acs.biochem.0c00930.
- Johns, M. A., Russ, A., & Fu, H. A. (2012). Current drug targets and the druggable genome. Chemical Genomics , 320–331.
- Kc, G. B., Bocci, G., Verma, S., Hassan, M. M., Holmes, J., Yang, J. J., … Oprea, T. I. (2021). A machine learning platform to estimate anti-SARS-CoV-2 activities. Nature Machine Intelligence , 3, 527–535. doi: 10.1038/s42256-021-00335-w.
- KC, G., Bocci, G., Verma, S., Hassan, M., Holmes, J., Yang, J., … Oprea, T. I. (2020). REDIAL-2020: A suite of machine learning models to estimate anti-SARS-CoV-2 activities. ChemRxiv , Available at https://chemrxiv.org/articles/preprint/REDIAL-2020_A_Suite_of_Machine_Learning_Models_to_Estimate_Anti-SARS-CoV-2_Activities/12915779.
- Kroeze, W. K., Sassano, M. F., Huang, X.-P., Lansu, K., McCorvy, J. D., Giguère, P. M., … Roth, B. L. (2015). PRESTO-Tango as an open-source resource for interrogation of the druggable human GPCRome. Nature Structural & Molecular Biology, 22, 362–369.
- Kropiwnicki, E., Evangelista, J. E., Stein, D. J., Clarke, D. J. B., Lachmann, A., Kuleshov, M. V., … Ma'ayan, A. (2021). Drugmonizome and Drugmonizome-ML: Integration and abstraction of small molecule attributes for drug enrichment analysis and machine learning. Database , 2021, baab017. doi: 10.1093/database/baab017.
- Kuleshov, M. V., Jones, M. R., Rouillard, A. D., Fernandez, N. F., Duan, Q., Wang, Z., … Ma'ayan, A. (2016). Enrichr: A comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Research , 44, W90–7. doi: 10.1093/nar/gkw377.
- Kuleshov, M. V., Xie, Z., London, A. B. K., Yang, J., Evangelista, J. E., Lachmann, A., … Ma'ayan, A. (2021). KEA3: Improved kinase enrichment analysis via data integration. Nucleic Acids Research , 49, W304–W316. doi: 10.1093/nar/gkab359.
- Lachmann, A., Rizzo, K., Bartal, A., Jeon, M., & Clarke, D. J. B. (2021). PrismExp: Predicting Human gene function by partitioning massive RNA-seq Co-expression data. bioRxiv. Available at: https://www.biorxiv.org/content/10.1101/2021.01.20.427528v1.abstract.
- Lachmann, A., Schilder, B. M., Wojciechowicz, M. L., Torre, D., Kuleshov, M. V., Keenan, A. B., & Ma'ayan, A. (2019). Geneshot: Search engine for ranking genes from arbitrary text queries. Nucleic Acids Research , 47, W571–W577. doi: 10.1093/nar/gkz393.
- Lachmann, A., Torre, D., Keenan, A. B., Jagodnik, K. M., Lee, H. J., Wang, L., … Ma'ayan, A. (2018). Massive mining of publicly available RNA-seq data from human and mouse. Nature Communications , 9, 1366. doi: 10.1038/s41467-018-03751-6.
- Langfelder, P., & Horvath, S. (2008). WGCNA: An R package for weighted correlation network analysis. BMC Bioinformatics , 9, 559. doi: 10.1186/1471-2105-9-559.
- Lin, Y., Mehta, S., Küçük-McGinty, H., Turner, J. P., Vidovic, D., Forlin, M., … Schürer, S. C. (2017). Drug target ontology to classify and integrate drug discovery data. Journal of Biomedical Semantics , 8, 50. doi: 10.1186/s13326-017-0161-x.
- Lipinski, C. A., Lombardo, F., Dominy, B. W., & Feeney, P. J. (2001). Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Advanced Drug Delivery Reviews , 46, 3–26. doi: 10.1016/s0169-409x(00)00129-0.
- Lombardo, F., Berellini, G., & Obach, R. S. (2018). Trend analysis of a database of intravenous pharmacokinetic parameters in humans for 1352 drug compounds. Drug Metabolism and Disposition , 46, 1466–1477. doi: 10.1124/dmd.118.082966.
- Maglott, D., Ostell, J., Pruitt, K. D., & Tatusova, T. (2011). Entrez Gene: Gene-centered information at NCBI. Nucleic Acids Research , 39, D52–7. doi: 10.1093/nar/gkq1237.
- Mendez, D., Gaulton, A., Bento, A. P., Chambers, J., De Veij, M., Félix, E., … Leach, A. R. (2019). ChEMBL: Towards direct deposition of bioassay data. Nucleic Acids Research , 47, D930–D940. doi: 10.1093/nar/gky1075.
- Miller, M. B., Yan, Y., Machida, K., Kiraly, D. D., Levy, A. D., Wu, Y. I., … Mains, R. E. (2017). Brain region and isoform-specific phosphorylation alters kalirin SH2 domain interaction sites and calpain sensitivity. ACS Chemical Neuroscience , 8, 1554–1569. doi: 10.1021/acschemneuro.7b00076.
- Milletti, F., Storchi, L., Goracci, L., Bendels, S., Wagner, B., Kansy, M., & Cruciani, G. (2010). Extending pKa prediction accuracy: High-throughput pKa measurements to understand pKa modulation of new chemical series. European Journal of Medicinal Chemistry , 45, 4270–4279. doi: 10.1016/j.ejmech.2010.06.026.
- Nguyen, D.-T., Mathias, S., Bologa, C., Brunak, S., Fernandez, N., Gaulton, A., … Guha, R. (2017). Pharos: Collating protein information to shed light on the druggable genome. Nucleic Acids Research , 45, D995–D1002. doi: 10.1093/nar/gkw1072.
- Oprea, T. I., Bologa, C. G., Brunak, S., Campbell, A., Gan, G. N., Gaulton, A., … Zahoránszky-Köhalmi, G. (2018). Erratum: Unexplored therapeutic opportunities in the human genome. Nature Reviews Drug Discovery , 17, 377–377. doi: 10.1038/nrd.2018.52.
- Osborne, J. D., Lin, S., Kibbe, W. A., Zhu, L., Danila, M. I., & Chisholm, R. L. (2007). GeneRIF is a more comprehensive, current and computationally tractable source of gene-disease relationships than OMIM. Bioinformatics Core, Northwestern University Technical Report , Available at: https://www.academia.edu/download/37808069/geneRIFDO16.pdf.
- Pletscher-Frankild, S., & Jensen, L. J. (2019). Design, implementation, and operation of a rapid, robust named entity recognition web service. Journal of Cheminformatics , 11, 19. doi: 10.1186/s13321-019-0344-9.
- Rouillard, A. D., Gundersen, G. W., Fernandez, N. F., Wang, Z., Monteiro, C. D., McDermott, M. G., & Ma'ayan, A. (2016). The harmonizome: A collection of processed datasets gathered to serve and mine knowledge about genes and proteins. Database: The Journal of Biological Databases and Curation , 2016, baw100. doi: 10.1093/database/baw100.
- Russ, A. P., & Lampel, S. (2005). The druggable genome: An update. Drug Discovery Today , 10, 1607–1610. doi: 10.1016/S1359-6446(05)03666-4.
- Santos, R., Ursu, O., Gaulton, A., Bento, A. P., Donadi, R. S., Bologa, C. G., … Overington, J. P. (2017). A comprehensive map of molecular drug targets. Nature Reviews. Drug Discovery , 16, 19–34. doi: 10.1038/nrd.2016.230.
- Schriml, L. M., Mitraka, E., Munro, J., Tauber, B., Schor, M., Nickle, L., … Greene, C. (2019). Human Disease Ontology 2018 update: Classification, content and workflow expansion. Nucleic Acids Research , 47, D955–D962. doi: 10.1093/nar/gky1032.
- Sheils, T. K., Mathias, S. L., Kelleher, K. J., Siramshetty, V. B., Nguyen, D.-T., Bologa, C. G., … Oprea, T. I. (2021). TCRD and Pharos 2021: Mining the human proteome for disease biology. Nucleic Acids Research , 49, D1334–D1346. doi: 10.1093/nar/gkaa993.
- Sheils, T., Mathias, S. L., Siramshetty, V. B., Bocci, G., Bologa, C. G., Yang, J. J., … Oprea, T. I. (2020). How to illuminate the druggable genome using Pharos. Current Protocols in Bioinformatics , 69, e92.
- Si, L., Bai, H., Rodas, M., Cao, W., Oh, C. Y., Jiang, A., … Ingber, D. E. (2021). A human-airway-on-a-chip for the rapid identification of candidate antiviral therapeutics and prophylactics. Nature Biomedical Engineering , 5, 815–829. doi: 10.1038/s41551-021-00718-9.
- Stein, R. M., Kang, H. J., McCorvy, J. D., Glatfelter, G. C., Jones, A. J., Che, T., … Dubocovich, M. L. (2020). Virtual discovery of melatonin receptor ligands to modulate circadian rhythms. Nature , 579, 609–614. doi: 10.1038/s41586-020-2027-0.
- Subramanian, A., Narayan, R., Corsello, S. M., Peck, D. D., Natoli, T. E., Lu, X., … Golub, T. R. (2017). A next generation connectivity map: L1000 platform and the first 1,000,000 profiles. Cell , 171, 1437–1452.e17. doi: 10.1016/j.cell.2017.10.049.
- The Gene Ontology Consortium (2019). The gene ontology resource: 20 years and still GOing strong. Nucleic Acids Research , 47, D330–D338. doi: 10.1093/nar/gky1055.
- Tomczak, K., Czerwińska, P., & Wiznerowicz, M. (2015). The Cancer Genome Atlas (TCGA): An immeasurable source of knowledge. Contemporary Oncology , 19, A68–77.
- Ursu, O., Holmes, J., Bologa, C. G., Yang, J. J., Mathias, S. L., Stathias, V., … Oprea, T. (2019). DrugCentral 2018: An update. Nucleic Acids Research , 47, D963–D970. doi: 10.1093/nar/gky963.
- Ursu, O., Holmes, J., Knockel, J., Bologa, C. G., Yang, J. J., Mathias, S. L., … Oprea, T. I. (2017). DrugCentral: Online drug compendium. Nucleic Acids Research , 45, D932–D939. doi: 10.1093/nar/gkw993.
- Venter, J. C., Adams, M. D., Myers, E. W., Li, P. W., Mural, R. J., Sutton, G. G., … Zhu, X. (2001). The sequence of the human genome. Science , 291, 1304–1351. doi: 10.1126/science.1058040.
- Wang, T. A., Chen, C., Huang, F., Feng, S., Tien, J., Braz, J. M., … Jan, L. Y. (2021). TMEM16C is involved in thermoregulation and protects rodent pups from febrile seizures. Proceedings of the National Academy of Sciences of the United States of America , 118, e2023342118. doi: 10.1073/pnas.2023342118.
- Wang, Z., Clark, N. R., & Ma'ayan, A. (2016). Drug-induced adverse events prediction with the LINCS L1000 data. Bioinformatics , 32, 2338–2345. doi: 10.1093/bioinformatics/btw168.
- Weininger, D. (1988). SMILES, a chemical language and information system. 1. Introduction to methodology and encoding rules. Journal of Chemical Information and Computer Sciences , 28, 31–36.
- Wells, C. I., Al-Ali, H., Andrews, D. M., Asquith, C. R. M., Axtman, A. D., Dikic, I., … Drewry, D. H. (2021). The Kinase Chemogenomic Set (KCGS): An open science resource for kinase vulnerability identification. International Journal of Molecular Sciences , 22, 566. doi: 10.3390/ijms22020566.
- Witoelar, A., Jansen, I. E., Wang, Y., Desikan, R. S., Gibbs, J. R., Blauwendraat, C., … International Parkinson's Disease Genomics Consortium NABEC and United Kingdom Brain Expression Consortium I (2017). Genome-wide pleiotropy between Parkinson disease and autoimmune diseases. JAMA Neurology , 74, 780–792. doi: 10.1001/jamaneurol.2017.0469.
- Yang, J. J., Grissa, D., Lambert, C. G., Bologa, C. G., Mathias, S. L., Waller, A., … Oprea, T. I. (2021). TIGA: Target illumination GWAS analytics. Bioinformatics , btab427. doi: 10.1093/bioinformatics/btab427.
Internet Resources
EBI Web Team ChEBI.
Disease Ontology—Institute for Genome Sciences at University of Maryland.
MeSH Browser.
National Drug File (NDFRT) reference terminology source information (2018).
Orange Book: Approved drug products with therapeutic equivalence evaluations.
RxNorm.
WHO Collaborating Centre for Drug Statistics Methodology (WHOCC): ATC/DDD Index.
Citing Literature
Number of times cited according to CrossRef: 3
- Abdolhakim Ghanbarzehi, Ali Sepehrinezhad, Nazanin Hashemi, Minoo Karimi, Ali Shahbazi, Disclosing common biological signatures and predicting new therapeutic targets in schizophrenia and obsessive–compulsive disorder by integrated bioinformatics analysis, BMC Psychiatry, 10.1186/s12888-023-04543-z, 23 , 1, (2023).
- Wenjing Xu, Nathan P. Nelson-Maney, László Bálint, Hyouk-Bum Kwon, Reema B. Davis, Danielle C. M. Dy, James M. Dunleavey, Brad St. Croix, Kathleen M. Caron, Orphan G-Protein Coupled Receptor GPRC5B Is Critical for Lymphatic Development, International Journal of Molecular Sciences, 10.3390/ijms23105712, 23 , 10, (5712), (2022).
- Sorin Avram, Thomas B Wilson, Ramona Curpan, Liliana Halip, Ana Borota, Alina Bora, Cristian G Bologa, Jayme Holmes, Jeffrey Knockel, Jeremy J Yang, Tudor I Oprea, DrugCentral 2023 extends human clinical data and integrates veterinary drugs, Nucleic Acids Research, 10.1093/nar/gkac1085, 51 , D1, (D1276-D1287), (2022).

