2-step PCR mixture and conditions (Barcoded-head primers for seqs pooling)
Yin-Tse Huang, Tsu-Chun Hung
Abstract
PCR mixture and condition (PowerPol 2X PCR Mix)
Steps
Wear glove, clean up the working bench w. 1% bleach
For 1' PCR head-primers
Prepare 1' PCR master mixutre for head-primers ( prepare 1.2X of solutions for pipetting error if needed )
PCR mixture for head-primers for each reaction
| A | B | C | D |
|---|---|---|---|
| Component | Volume | Volume (1.2X) | Final conc. |
| Forward Primer (10 µM) | 0.5 μl | 1.2 μl | 0.2 µM |
| Reverse Primer (10 µM) | 0.5 μl | 1.2 μl | 0.2 µM |
| PowerPol 2X PCR Master Mix | 12.5 μl | 15 μl | - |
| ddH20 | 10.25 μl | 11.1 μl | - |
| Total volume | 23.75 μl | 28.5 μl | - |
Mix the 1' PCR master mixture gently by pippeting. Quick spin the tube.
Transfer 23.75µL 1' PCR master mixutre in 8-strip PCR tubes.
Add 1.25µLDNA template in 8-strip PCR tubes, resulting in a 25µL reaction mixture for 1' PCR.
Mix the reaction mixture gently by tapping the tubes. Quick spin the tubes.
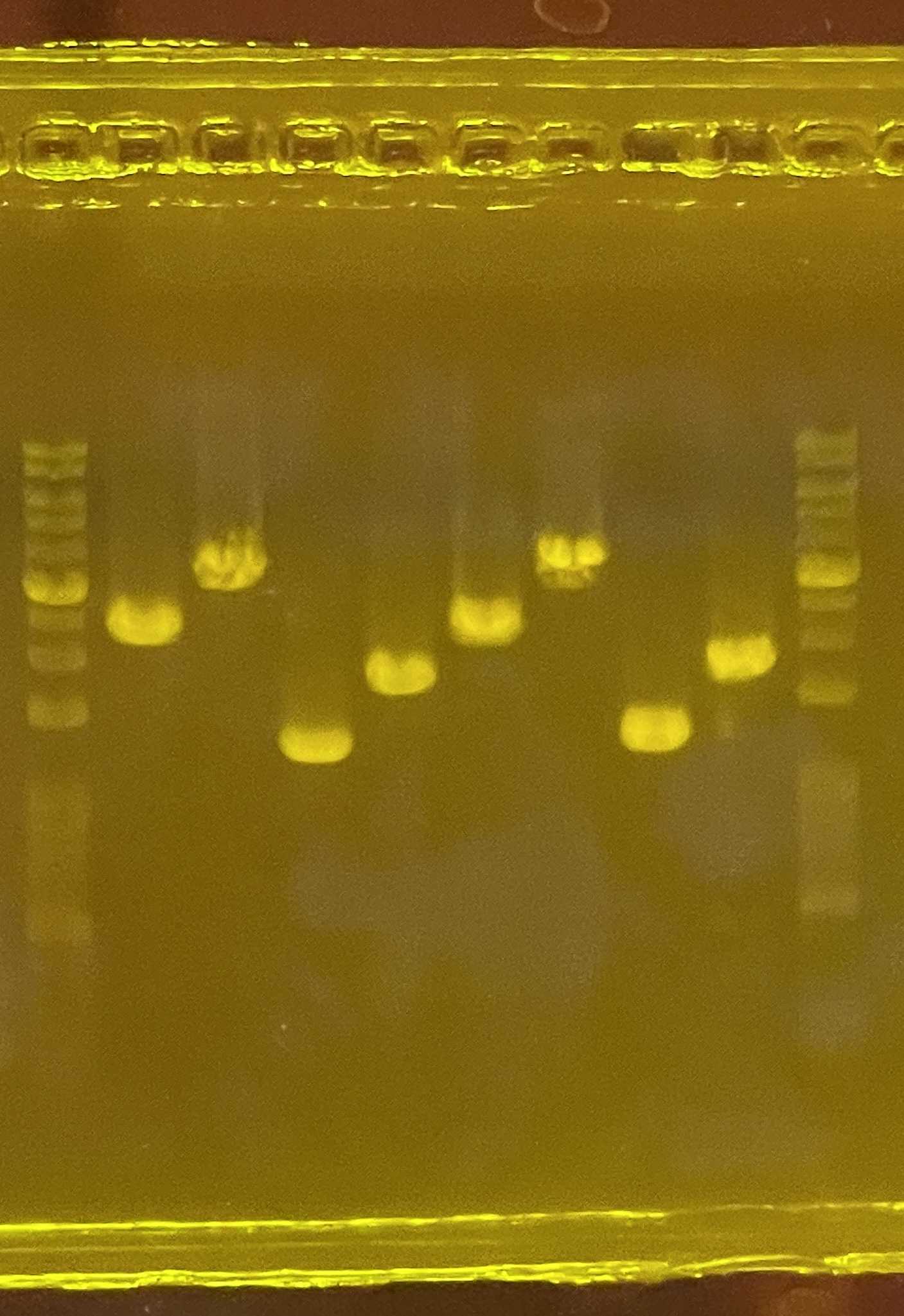
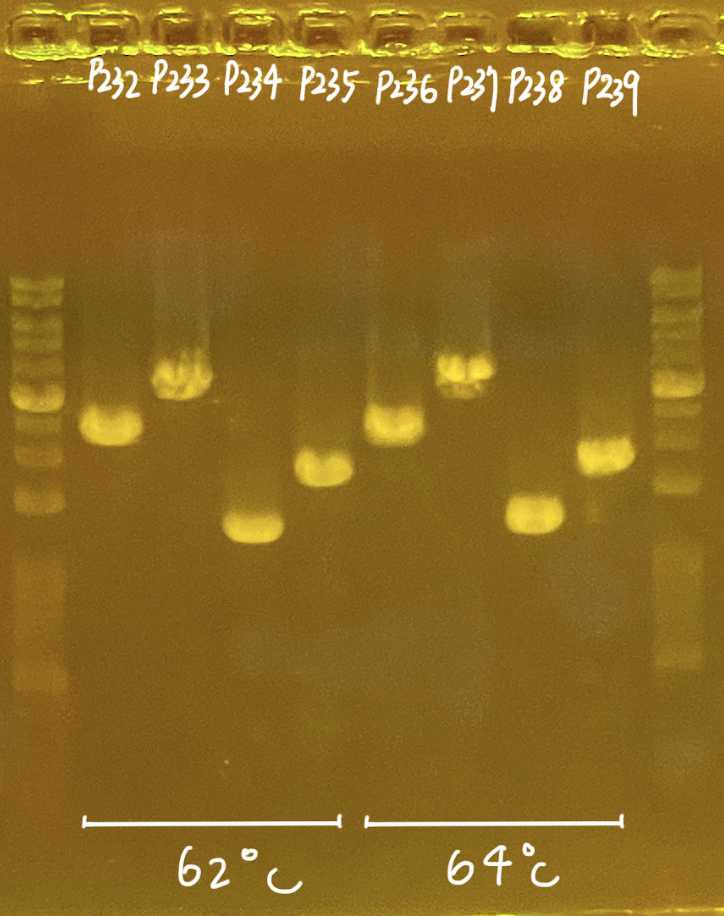
Carry out PCR using the following condition:
1' PCR condition for head-primers
| A | B | C | D |
|---|---|---|---|
| Step | Temp | Sec | Cycle |
| Initial denaturation | 98 ºC | 45 | |
| Denaturation | 98 ºC | 10 | 25 cycles |
| Annealing | 60-66 ºC varied (b) | 30 | |
| Extension | 72 ºC | 150 | |
| Final extension | 72 ºC | 300 | |
| Preservation | 4 ºC | ∞ |
b. Annealing varied, 60-66C is working; Refer to 1' PCR primers for annealing temperaturec. 1kb ~ 1min extension; enough time allow full extension of sequence
For 2' PCR barcoded-head primers
Prepare 2' PCR master mixutre for barcoded-primers ( prepare 1.2X of solutions for pipetting error if needed )
PCR mixture for barcoded-primers for each reaction (NO PRIMERs at this point!!)
| A | B | C | D |
|---|---|---|---|
| Component | Volume | Volume (1.2X) | Final conc. |
| ZEJU PCR Master Mix | 7.5 µL | 9 µL | - |
| ddH20 | 5.55 µL | 6.66 µL | - |
| Total volume | 13.05 µL | 15.66 µL | - |
Mix the 2' PCR master mixture gently by pippeting. Quick spin the tube.
Transfer 13.05µL of the 2' PCR master mixture to 8-strip PCR tubes.
Add 1.2µL pre-mixed barcoded-head primers (Forward + Reverse) to each PCR tubes.
Add 0.75µL of 1' PCR product as template , resulting in 15µL reaction mixture for 2' PCR.
Negative control contains only 14.25µL master mixture and premixed barcoded-head primers but not DNA template
Mix gently by tapping the tubes. Quick spin the tubes.
Carry out 2' PCR using the following condition:
2' PCR condition for barcoded-head primers
| A | B | C | D |
|---|---|---|---|
| Step | Temp | Sec | Cycle |
| Initial denaturation | 98 ºC | 30 | |
| Denaturation | 98 ºC | 15 | 12 cycles |
| Annealing | 64-68 ºC varied (a) | 15 | |
| Extension | 72 ºC | 20 (b) | |
| Final extension | 72 ºC | 210 | |
| Preservation | Preservation | 4 ºC | ∞ |
a. Annealing varied, 65 C is working based on test on 220531; Refer 2' PCR primers for annealing temperatureb. 1kb ~ 1min extension; enough time allow full extension of sequence